The most well-known function of lipids is providing the barrier that marks the boundaries of the cell. In 1925, Gorter & Grandel first proved that lipids were present in the cellular membrane. The other function of lipids is that they are the primary energy storage sources for high energy-consuming organs such as heart. They are also responsible for the production of specialized lipid mediators involved in important intracellular events (eg. Phospholipase C and Phospholipid A2). They form the structure of some important substances such as steroid hormones and vitamin D in the body.
After showing that the lipids are present in the cellular membrane, for many years it has been though that they do not have an important function but they just exist there for structural purposes. With the discovery of acetylsalicylic acid (aspirin), interest in lipids has increased. It has been shown that acetylsalicylic acid inhibits cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) enzymes involved in the formation of eicosanoids in the lipid anabolic pathway. Later on, COX-2 inhibitors have been more prominent in the treatment of elderly patients with arthritis, but when these drugs appeared to cause myocardial infarction due to thrombogenic effect, they were banned. The use of lipid cholesterol as a marker (biomarker) of plasma levels in the diagnosis of ischemic heart diseases has increased the interest in lipids. Finally, the definition of lipidomics introduced in the early 2000s has opened a new era in the use of lipids.
lipids, lipid peroxidation, oxidative lipidomics
The general definition of lipid peroxidation is the degradation of membrane lipids due to the oxidative stress. In other words, the unsaturated bonds of cholesterol and fatty acids in cell membranes react with free radicals and peroxidation products occur. Often this happens in two ways:
a) Enzymatic pathways: In these pathways, active enzyme groups are consisted of lipoxygenases (LOX), cyclooxygenases (COX), cytochromes (Cytochrome P450, Cytochrome C, etc).
b) Non-enzymatic pathways: In non-enzymatic pathways, transition metals, especially with Haber-Weiss or Fenton reactions, react with reactive oxygen radicals and free radicals are formed. In addition, lipid-hydroperoxides (L-O-O-H) and their secondary metabolites can function as signaling molecules in various levels of lipid peroxidation (initiation, propagation, termination).
Lipid peroxidation is known to play a key role in the pathophysiology of critical diseases. Up to date, it has often been focused on the end products of lipid peroxidation, which may have caused a delay in our understanding of early mediators. However, it was estimated that the metabolites active in the early period were involved in chronic diseases such as in hypertension, atherosclerosis, inflammation of traumas, sepsis and head trauma recovery processes. To summarize the relationship between lipid peroxidation and oxidative lipidomics, it allows us to understand the mechanisms of emergence of lipid peroxidation products, time of emergence and synergistic functions with other events in the body. All this information suggests the question of whether oxidative lipidomics is enlightening in the course and treatment of diseases.
What is “Omics” Science (Technique)? (Genomics, Transcriptomics, Metabolomics, Lipidomics)
In 1865, Gregor Mendel showed the distinction between autosomal and recessive dominancy and the first gene concept was introduced in 1909. The Human Genome Project, which took place between 1900 and 2003, was completed with a very surprising result. Contrary the exceptions, the structural differences of genes in humans was found only as 0.1% [1]. In light of this information, Nature Magazine published in 2004 that the number of genes in the human body is not 100.000, but between 20-25.000. All these developments have led to the abandonment of one gene-one enzyme hypothesis and raised the question whether the hidden information is in the postgenomic area, while starting the individual genomic medicine period [2]. The researchers have then focused on RNA, transcription molecules, proteins, protein-protein interactions and lipid analysis. In 2003, Tyers et al. have made an important statement and claimed that the information that could be gathered from the metabolites and proteins was more than the genomic information. After this important explanation, all the information determining the clinical phenotype was found to be hidden in the metabolites formed in the cell [3]. As a result, it can be suggested that a study only covering the genomics and the proteomics would be inadequate and metabolomic studies should be involved for a more comprehensive and accurate analysis [4,5].
According to this dogma, lipidomics are biomolecular structures which may be the most suitable biomarkers in human due to their proximity to phenotype, close relationship to diseases and the biological events, unlike other omic groups. Lipidomes cooperate closely with proteins in the fulfillment of cellular functions. The most prominent example of this situation is the intense cooperation of eicosanoids with lipids in inflammatory and immune system reactions.
When we examine the pathophysiology of the diseases we struggle with, we can see that the situation depends on many factors. Therefore, the use of genomics, transcriptomics, proteomics, lipidomics technologies in the diagnosis of diseases enables us to understand the multifactorial structure of the diseases and to make the right approach for treatment. In fact, the relationship between the lipids and chronic diseases is well-known, and the purpose of lipidomics is to carry the diagnosis and treatment one step further. One feature of the lipidomes is that they are susceptible to intracellular and extracellular biochemical changes as they are found in cell membrane structures. This leads to changes in normal hemostasis, i.e., early biochemical abnormalities.
Mass spectrometry is the most commonly used method in lipidomic analysis. The others are nuclear magnetic resonance (NMR), fluorescence spectroscopy, column chromatography, microfluidic devices, respectively. The mass spectrometer operates mainly on the principle of high-energy electron bombardment. As the positive ions pass through two opposing charged plates, the mass of the molecule is determined by looking at the magnitude of the deviation.
Lipidomics
Lipidomics was first described in 2003 and widely used through the development of mass spectrometry. The aim of lipidomics is to determine the quantitative and qualitative measurement of lipidomes by bioinformatics, determine the biomarkers, as well as personalized drug design and targeted treatments. Although its definition was made in these years, lipidomics has been studied previously as a subgroup of metabolomics. An example of this is the randomized, prospective study performed in 1994 in 69 pregnant women. In this study, it was evaluated whether there was a correlation between the rates of urinary prostacyclin (PGI2) / thromboxane (TXA2) and pregnancy-induced hypertension between 16-20 weeks of gestation until 6 weeks postpartum period. As a result, it was determined that the ratio of prostacyclin (PGI2) / thromboxane (TXA2) in the urine of patients developing pregnancy-induced hypertension was decreased, i.e. a correlation was found [6].
Oxidative lipidomics and literature review
Oxidative lipidomics are oxidized modified lipids at a much more molecular level, which are subsequently added to the lipidomes. Generally, animal experiments are intense and at a molecular level, combined with various branches of the omic technique.
An animal study conducted in 2010 demonstrated the role of oxidative lipidomics in hyperoxic lung injury. As is known, pulmonary endothelium is the most common site of early functional and structural changes in hyperoxic lung injury. As the pathways in the early stages of apoptosis are not completely clarified, the treatment of acute lung injury is unknown.
In the study, the mouse lung was administered with 100% O2 for 72 hours and apoptosis (programmed cell death) in the pulmonary endothelium was established. Apoptosis induced in the hypoxic pulmonary endothelium has been shown with increased levels of caspase-3 and caspase-7, histochemical staining of the endothelium (with dUTP) and increase in phospholipid hydroperoxides (Figure 1).
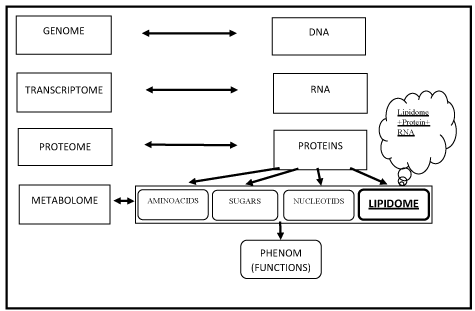
Figure 1. The new scientific dogma of the organism
Metabolome: Total name of metabolites present in cell, tissue or organism
Lipidome: The name of the total number of lipids in the cell and the subgroup of the metabolome
Lipidomic: Investigation of the lipids and mediators originating from lipids in the organism and their functions in the biological system
Phenom: A collection of phenotypes expressed by a cell, tissue or organism
In measuring molecular species of mitochondrial cardiolipin (C18:2,C20:4) and phosphatidylserine (C22:5,C22:6) of normal and hyperoxic mouse lungs using mass spectrometry, both quantitative values of anionic oxidized phospholipids were shown to be decreased. In addition, levels of oxidized cardiolipin and phosphatidylserine groups were decreased in the hypoxic mouse lung when compared to the normal mouse lung after administration of the mitochondrial targeted electron collecting nitroxide (XJB-5-131), which prevents the reproduction of reactive oxygen species (ROS). As a result, it has been suggested that cardiolipin and phosphatidylserine play a role in signaling of apoptosis and apoptosis regression which cause hyperoxic lung injury. XJB-5-131 inhibits oxidative stress-dependent apoptosis in vivo. In the future, hyperoxia-induced lung injuries can be maintained by using these strategies and thus hyperoxic lung can be preserved [7].
An animal study has been conducted to investigate the reactions of oxidative lipidomics according to time in traumatic brain injury. It is known that the levels of lipid peroxidation increase, anti-oxidants decrease and oxidative stress develops in traumatic brain injury. Cardiolipin (C22: 6) is an early and specific target for oxidative stress due to traumatic brain injury. The main unknowns are the molecular targets, the peroxidized phospholipids and their signaling functions. In rats, craniotomy has been performed and traumatic brain injury has been established. The hypothesis of the study is to investigate whether cardiolipin oxidation products are early markers of apoptosis in rat brain and if they may be the target of treatment. In the study, 17-day-old rats were used and it was stated that this is similar to the neuronal development of a child. After traumatic brain injury, various phospholipid hydroxiperoxides (cardiolipin, phosphatidylethanolamine, phosphatidylcholine, phosphatidylserine, phosphatidylinositol, sphingomyelin, lysophosphatidylcholine) obtained from the cerebral cortex were evaluated.
At the end of the evaluation, cardiolipin was observed to be oxidized at 3 hours, which is the earliest and most accurate time for detection after brain injury, but at the same time scale there was no difference in other evaluated phospholipids. In the 24th hour after traumatic brain injury, in addition to cardiolipin, a significant oxidation in phosphatidylserine and a slight difference in other phospholipids have been observed. However, it has been seen that caspase activity, which is a serious marker of apoptosis, has not increased at the 3rd hour, which is the time cardiolipin is elevated, but has rather increased at 24 hours, significantly. Consequently, cardiolipin hydroperoxides can be used as a marker of apoptosis in brain trauma patients. It is thought that early detection of cardiolipin oxidation may provide more positive results in the treatment and post-traumatic injury period [8].
A study has been conducted to evaluate the role of bioactive lipid mediators in different phases of influenza infection and an infected mouse lung (Mouse influenza model) has been established by influenza virus application. In the study, 141 kinds of lipid metabolites have been listed by bronchoalveolar lavage, and 52 were studied (COX, LOX, Cyp450, Non-Enzymatic, Linoleic acid, Linolenic acid, DHA, EPA). Similarly, lipidomes which were obtained from human nasopharyngeal lavage during the influenza epidemic have been studied. In chronic diseases, it is known that the values of hydroxylated linoleic acid 13-HODE and 9-HODE (hydroxyoctadecadieneoic acid) increase. In order to avoid individual differences, the ratios of these two linoleic acids have been preferred. The concentrations of lipoxygenase (LOX) and linoleic acid metabolites is noteworthy in both mice and humans, especially in those with intensive symptomatic clinic. As a result, it has been emphasized that hydroxylated linoleic acid metabolites can be used as markers during influenza infection and they can give important information on the severity and recovery process of the disease [9].
The function of oxidative lipidomics is not only to act as markers in inflammatory processes. They may also play an active role in detection of new lipid mediators during the inflammation process. One of the examples is Dioxolane A3, (DXA3) (8-hydroxy-9,11-dioxolane eicosatetraenoic acid), a lipid derivative generated by COX-1, which has been identified in the platelets by using oxidative lipidomics. It has been found that this newly discovered oxidative lipidomic activates neutrophils and plays a role in innate immunity and acute inflammation [10]. In a different study on the detection of lipid mediators with the help of oxidative lipidomics, new maresins (22-hydroxy- MAR1 and 14-oxo-MAR1) were found to be involved in the resolution of inflammation in E. Coli infective exudate, similar to mediators (lipoxins, protectins, maresins, resolvins) involved in the resolution phase of sepsis [11].
Oxidative lipidomics also plays a major role in the understanding of various biological mechanisms. Molecular structures found in breast milk increase both immunity and development in newborn babies. In a study conducted on this subject, special mediators (RvD1, RvD2, RvD3, AT-RvD3, RvD4, PD1, MaR1, RvE1, RvD2, RvE3, LXA4, LXB4) have been discovered in the mother's milk and further analysis of this milk have revealed that these mediators reduce the maximum neutrophil count and resolution time by 54% in peritonitis-induced mice [12].
The most important limitation in this regard is that, a low amount of oxidative lipidomics which is hard to detect may be activating an important metabolic pathway.
In conclusion, oxidative lipidomics provide an understanding of lipid peroxidation products and their pathophysiological functions related to the critical diseases. Understanding the lipid mediators at different stages of the injury can change our approach to treatment. Knowing other molecules that interfere with the interaction and function of lipidomics, enables us to develop new individual treatment approaches. The aim is to understand all biological events in the system by collecting information from different groups (lipidomic, genomic, etc.) and achieving success in the individual approach to the treatment of complex diseases. Perhaps, from a molecular point of view, in the future diseases will be reclassified according to lipidomics.
- Venter JC (2003) A part of the human genome sequence. Science 299: 1183-1184. [Crossref]
- Tyers M, Mann M (2003) From genomics to proteomics. Nature 422: 193-197. [Crossref]
- Bren L (2005) Metabolomics: working toward personalized medicine. FDA Consum 39: 28-33. [Crossref]
- Fiehn O (2001) Combining genomics, metabolome analysis, and biochemical modelling to understand metabolic networks. Comparative and Functional Genomics 2: 155-168.
- Ibanez C, Valdes A, Garcia Canas V, Simo C, Celebier M, et al. (2012) Global Foodomics strategy to investigate the health benefits of dietary constituents. J Chromatogr 1248: 139-153.
- Klockenbusch W, Somville T, Hafner D, Strobach H, Schrör K (1994) Excretion of prostacyclin and thromboxane metabolites before, during, and after pregnancy-induced hypertension. Eur J Obstet Gynecol Reprod Biol 57: 47-50. [Crossref]
- Tyurina YY, Tyurin VA, Kaynar AM, Kapralova VI, Wasserloos K, et al. (2010) Oxidative lipidomics of hyperoxic acute lung injury: mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Am J Physiol Lung Cell Mol Physiol. 299: 73-85.
- Bayir H, Tyurin VA, Tyurina YY, Viner R, Ritov V, et al. (2007) Selective early cardiolipin peroxidation after traumatic brain injury: an oxidative lipidomics analysis. Ann Neurol 62: 154-169. [Crossref]
- Tam VC, Quehenberger O, Oshansky CM, Suen R, Armando AM, et al. (2013) Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell 154: 213-227. [Crossref]
- Hinz C, Aldrovandi M, Uhlson C, Marnett LJ, Longhurst HJ, et al. (2016) Human platelets utilize cycloxygenase-1 to generate dioxolane a3, a neutrophil-activating eicosanoid. The Journal of biological chemistry 291: 13448-13464.
- Colas RA, Dalli J, Chiang N, Vlasakov I, Sanger JM, et al. (2016) Identification and Actions of the Maresin 1 Metabolome in Infectious Inflammation. J Immunol 197: 4444-4452. [Crossref]
- Arnardottir H, Orr SK, Dalli J, Serhan CN (2016) Human milk proresolving mediators stimulate resolution of acute inflammation. Mucosal Immunol 9: 757-766.

