Abstract
Aim: Nigella sativa (N. sativa, black seeds, NS) has been traditionally used for many years as a food and as a complementary drug. Pre-clinical pharmacological investigations disclosed potential clinical therapeutic effects of NS for diverse diseases including cancer, diabetes, hyperlipidemia, inflammation, seizures, multiple sclerosis, infectious diseases, gastroduodenal ulcers, hypertension, and asthma. The aims of this communication to review the evidence-based human investigations of NS therapeutic properties.
Materials and Methods: Primary PubMed literature searches and secondary Medline searches were conducted to permit a retrospective narrative review of the NS published human studies. The studies reviewed were controlled clinical investigations which investigated the therapeutic actions of NS in patients and in healthy human subjects.
Results: The controlled studies were limited in scope and none were sponsored by pharmaceutical or commercial manufacturers. Briefly, in patients with uncontrolled type-2 diabetes mellitus (DM), the adjuvant uses of NS significantly improved glycemic control, lipid levels and oxidative stress markers. In patients with Hashimoto’s thyroiditis, adjuvant administration of NS significantly improved thyroid functions, serum lipids and anthropometric features. Adjuvant use of NS exhibited anticonvulsant effect in children with intractable seizures. Several studies established anti-asthmatic effects of NS. Two studies have shown the efficacy of NS for the treatment of rheumatoid arthritis in patients receiving disease-modifying anti-rheumatic drugs (DMARDs). One study established NS efficacy for the eradication of Helicobacter pylori in patients with non-ulcer dyspepsia and improved its symptoms. In type-2 DM patients with concurrent hepatitis-C infection, a study disclosed that NS decreased the viral load, improved oxidative stress markers and glycemic control. Two studies conducted in elderly and adolescent healthy human subjects, disclosed that NS significantly enhanced memory, attention and cognition. In all studies reviewed, NS was well tolerated and had no major adverse reactions when given continuously for one year.
Conclusions: The clinical therapeutic efficacy of NS in man is consistent with its broad pre-clinical pharmacological properties. However, appropriate pharmaceutical dosage forms containing stable formulations of NS should be developed to permit defining specific use in human therapeutics in comparison with standard therapies.
Keywords
asthma; black seed; black seed oil; cognition; diabetes; dyspepsia; endocrinology; gastroenterology; hashimoto thyroiditis; helicobacter pylori; hyperlipidemia; infections; memory; mechanisms; neurology; nigella sativa; pharmaceutical preparations; pulmonary medicine; rheumatology; rheumatoid arthritis; seizures; thyroid.
Introduction
Nigella sativa (sometimes known by several other names such as the blessed seed by the Arabs, black cumin in the Holy Bible, black caraway and Kalonji in South Asia) has been used by various cultures in Asia, Africa, Europe, the Middle and Far East to flavor food and as a natural remedy for several diseases. N. sativa is a plant from the Ranunculaceae (buttercup) family. The seeds have been traditionally used to treat variety of ailments related to respiratory health, digestive complaints, diabetes, infections, rheumatism, skin disorder, circulatory and immune system support [1].
The earliest written reference to NS is thought to be in the book of Isaiah in the Old Testament, where the reaping of Nigella and wheat is contrasted (Isaiah 28: 25, 27; Wikipedia, the Free Encyclopedia). The Easton's Bible dictionary states that the Hebrew word ketsah refers to N. sativa. The many interesting uses of the NS seeds have given it the Arabic approbation Habbatul Baraka, meaning the "seed of blessing" (Wikipedia, the Free Encyclopedia). Figure 1 presents photos of the Nigella sativa plant, flower and seeds.

Figure 1. Photographs of the Nigella sativa plant, flower and seeds. This figure was reproduced from Ahmad, et al. [1] publication on the basis of noncommercial license
Recently reviewed preclinical studies disclosed that NS possesses broad pharmaco-logical actions which include: anti-cancer, anti-diabetic, anti-inflammatory, analgesic, anti-convulsant, anti-microbial, anti-hyperlipidemic, anti-asthmatic and anti-hypertensive properties [1,2]. Its mode of action is mediated by anti-oxidant, immunomodulating, cytoprotective and inhibitory effects on mediators of inflammation [2]. N. sativa also inhibits tumor cell proliferation through modulation of apoptosis signaling, the inhibition of angiogenesis and cell cycle arrest [3].
Despite the very large number of published pre-clinical studies, there are only few controlled clinical studies which investigated the actions of NS in human therapeutics. It is the objective of this communication is to provide a narrative review of the published, evidence-based, human investigations of NS seeds and its components.
Search Methods
A primary search of the published literature was carried out using online PubMed searches, which covered the period from January 1960 to May 2018. Secondary searches for older studies were also performed using Medline. Only studies written in the English language and with experiments that were properly designed and well-controlled were considered for inclusion in this review. The keywords in these searches included all the search items listed by PubMed under the heading of Nigella sativa. In addition, sub-searches included the following additional key words: Alpha-Hederin, Black Seeds, Black Cumin Seeds, Black Seed Oil, Human Investigations, Thymoquinone, Therapeutics and Toxicology. These keywords were used both singly and in combination. Truncation and the use of adjacent searches were also conducted. In addition, some of the references of the articles uncovered in the primary search were also obtained and examined because PubMed and Medline search engines do not index some foreign journals.
Results
The PubMed searches disclosed a total of 136 articles investigated the human therapeutic actions of N. sativa seeds, its oil, its various extracts and one of its active component thymoquinone. Many articles were published in peer-reviewed biomedical journals. Access to the complete articles was either obtained from the journals, or from the authors or from regional libraries, whenever possible. Therefore, access to the complete articles was of significant importance to this research investigation.
All the published research specifically cited in this review adhered to our selection criteria, especially with respect to controlled investigations, good experimental design and general conduct of the studies. However, the investigations were concerned principally with clinical characterization of NS but not with its potential drug development consideration needed for regulatory submissions essential for clinical practice guidelines. All of the studies reviewed were conducted at a single clinical research center (medical school clinics and their affiliated hospitals) and no studies comprised multi-clinic investigations. However, the population sample size for the clinical investigation was calculated, a priori, to establish statistical support for the intervention at P<0.05. Furthermore, all of the clinical investigations were financed either by the authors or by their medical institutions, or by government grants, but not by any commercial sponsors or pharmaceutical companies.
Chemical Composition and the Dosage Forms
The NS seeds contain numerous esters of unsaturated fatty acids with terpene alcohols, the alkaloids nigellidine and nigellimin and saponin [4,5]. The biologically active substances in NS seeds include thymol, thymoquinone, and dithymoquinone (Figure 2). It was recently reported that the controlled heating of the seeds (roasting at 50-150 degree Centigrade for ten minutes) prior to their milling significantly enhanced their anti-proliferative action when compared with unroasted seeds [6]. However, none of the reviewed studies had roasted the seeds prior to their milling or prior to the preparation of the NS oil or aqueous extracts used in the preparation of the pharmaceutical dosage forms given to the study subjects.
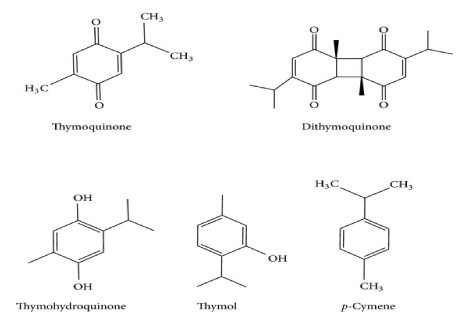
Figure 2. Chemical Structures of Thymoquinone and Related Compounds. This figure was reproduced from the Rahmani, et al. [64] publication on the basis of noncommercial license
The NS oil is usually prepared by a cold press procedure of the seeds and contains fixed oil and about 1.1% of volatile constituents [7]. Extensive chemical analyses performed by Kolahdooz, et al. [7] established that the fatty acids components of the fixed oil (non-volatile oil) are linoleic acid (58%), oleic acid (22%) and palmitic acid (13%). The main components of the volatile oil are p-cymene (52%), thymoquinone (15%) and alpha-thujene (14%). Many of the active components of NS are chemically unstable and the pharmaceutical dosage forms were not standardized across the diverse clinical studies.
The oral pharmaceutical dosage forms employed in the clinical studies comprised of either capsule filled with the NS ground seeds, or its black seed oil, or thymoquinone, or aqueous seed extract consumed as a tea. The dosage forms did not conform to established good manufacturing practices (GMP) common with commercial sponsors, with respect to the assay of the active contents and to ensure that there was no significant degradation of the pharmacologically active substances during the conduct of the studies. The United States Federal Food and Drug Administration (FDA) regulations mandates that pharmaceutical sponsors must adhere to the standards of the Chemistry and Manufacturing Controls (CMC) which chemically characterizes the active drug(s), its stability and the manufacturing of the dosage forms. None of the studies reviewed complied with the FDA regulations regarding the CMC provisions. Therefore, the pharmaceutical dosage forms in the NS studies are probably not optimum preparations.
Overview of the Clinical Investigations
All controlled clinical investigations were conducted in consenting subjects and the ethics of the studies were approved by the Institutional Review Boards for human investigations for their respective institutions. Furthermore, like the United States and other developed countries, some but not all of the clinical trials, were also registered in their countries of origin. The selection of the doses used in the clinical studies was essentially based on folklore complementary medicine practices and experiences. However, one dose-ranging study conducted in type-2 DM patients established that crushed seeds administered at the 2 gm/day and given in two daily doses showed clinical efficacy. A brief description of the clinical trials and their therapeutic outcomes are provided below:
Diabetes Mellitus Type-2: Three controlled studies investigated the adjuvant use of NS in type-2 DM [8,9,10]. The Bamosa, et al. study [8] was a 12-weeks dose-ranging pilot study and the Kaatabi, et al. [9] study was a one-year treatment study. Since the Bamosa, et al. and the Kaatabi, et al. studies were conducted at the same institution and had a similar experimental design and analytical assessments, the studies will be reviewed together. The protocols for both studies were reviewed and approved by the Research and Ethical Committee of the University Of Dammam College of Medicine in Saudi Arabia. Written informed consent was obtained from each subject. Subjects were included if they were using concomitant oral hypoglycemic drugs (glibenclamide, metformin, rosiglitazone) and had uncontrolled type-2 DM, based on two successive readings, three months apart, of glycated hemoglobin A1C (HbA1C) of >7%. Subjects were excluded if they had HbA1C >9%, insulin therapy, body mass index >40, coronary artery disease, valvular heart disease, heart failure, uncontrolled hypertension, renal failure, hepatic failure and patients with less than 90% compliance or changed their standard medications during the one-year study.
All study subjects were supplied with self-monitoring glucometers of the same make (AccuCheck, Roche Diagnostic GMBH, Germany). Calibration of the glucometers against standards was carried out before handing out the equipment to the subjects and then at each follow-up visit. After an orientation with the recording method, the subjects were asked to check their blood sugar level once weekly, first after 8 hours fast and then 2 hours after a meal (postprandial). The mean of all the weekly recordings for a month was considered for the monthly average. In the Bamosa, et al. study, the subjects (N=94) were randomized to one of three NS dosage treatment groups: 1 gm/day, 2 gm/day and 3 gm/day taken at the frequency of twice daily. Capsules, each containing 500 mg of the powdered seeds, were continuously administered for 12 weeks. The capsules were prepared by a contract manufacturing laboratory (Bioextract, Sri Lanka) who milled the seeds and filled the capsules. However, there was no information provided with regard to the milling process, particle size, the assay of the NS active components, or the stability of the filled capsules over the course of the study.
Glycemic control was assessed at four-week intervals by the measurement of fasting blood glucose, blood glucose 2-hours postprandial (2Hr-PG), glycated hemoglobin (HbA1C) and C-peptide levels. Insulin resistance and Beta cell functions were calculated using computer software HOMA2 (Homeostasis Model Assessment calculator, released by Oxford University, UK in 2004 utilizing fasting blood glucose and C-peptide level).
Nigella sativa, administered at the daily dosages of 2 g/day and 3 g/day, was associated with statistically significant reductions in fasting blood glucose, the 2-hours postprandial glucose and HbA1C levels. Fasting blood glucose was reduced by an average of 45, 62 and 56 mg/dL at 4, 8 and 12 weeks, respectively. At the 12-week treatment period, insulin resistance was significantly (P<0.01) reduced and the B-cells function was significantly increased (P<0.02). The lowest NS tested dosage (1 g/day) showed a trend but not a statistically significant change of the measured parameters. The NS highest dosage of 3 g/day did not demonstrate greater therapeutic benefits when compared with the intermediate dosage of 2 gm/day.
The NS treatment appeared to be well tolerated and was not associated with any adverse renal or hepatic functions throughout the study period. The investigators concluded that NS, administered twice daily at dosage of 2 g/day, appeared to be an optimum dosage as an adjuvant therapy when given with oral hypoglycemics in uncontrolled type-2 DM patients.
The Kaatabi, et al. [9] study investigated the long-term (one year) adjuvant use of NS (2 gm/day) in uncontrolled type-2 DM patients who were maintained on standard oral hypoglycemic drugs. The trial was a randomized, placebo-controlled, participant-blinded study conducted in 114 patients. The clinical trial was registered in India. The inclusion and exclusion criteria were similar to their previous study.8 The control group (N=57) received activated charcoal capsules and the NS group (N=57) received a total daily NS dose of 2 g/day taken twice daily for one year in addition to their standard hypoglycemic medications. Fasting blood glucose, HbA1C, C-peptide, total antioxidant capacity (TAC), superoxide dismutase (SOD), catalase (CAT), glutathione and thiobarbituric acid reactive substances (TBARS) at baseline, and every 3 months thereafter, were determined. Insulin resistance and Beta-cell activity was calculated using HOMA 2 calculator.
The NS powdered seeds capsules (500 mg) were manufactured by the contract laboratory Bioextract Private Limited, Sri Lanka. The placebo capsules consisted of activated powder charcoal (260 mg) similar in size, appearance and color to the NS capsules and were prepared by Arkopharma Pharmaceutical Laboratories, Carros, France. Subjects were instructed to take either NS or placebo with their prescribed hypoglycemic drugs taken in two divided daily doses for one year. The subjects were not aware of their treatment assignment (placebo or NS).
As is evident from Table 1, fasting blood glucose was significantly reduced to the same extent in each of the observation intervals of 3, 6, 9 and 12-months treatment period indicating that were no pharmacological tolerance to the hypoglycemic effect of NS. Likewise, the HbA1C levels were also significantly reduced at all of the time intervals examined (Table 2). The NS treatment increased glutathione concentration and decreased levels of peroxidation index (thiobarbituric acid reactive substances (TBARS) indicating that it has clinical anti-oxidant effects in patients with type-2 DM (Table 3).
Table 1.Fasting Blood Glucose Values in Uncontrolled Type-2 Diabetes Mellitus Patients Receiving Adjuvant Nigella sativa crushed seeds with Their Standard Oral Hypoglycemic Drugs. Values represent Mean ± Standard Error of the Mean [9]
Test Interval (Months) |
Fsting Blood Glucose level (mg/dL) |
P Value |
Control Group |
N. sativa Group |
Baseline |
180 ± 5.75 |
195 ± 6.57 |
- |
3 months |
184 ± 5.81 |
163 ± 6.31 |
0.002* |
6 Months |
185 ± 5.59 |
164 ± 5.97 |
0.000* |
9 Months |
183 ± 5.41 |
176 ± 6.59 |
0.021* |
12 Months |
180 ± 5.59 |
172 ± 5.83 |
0.017* |
|
Consenting (N=114) uncontrolled diabetics (based on two successive readings taken three months apart of HbA1C of >7%) were randomized to treatment to either N. sativa capsules (2g/day) or its matching placebo. The test drugs were administered in two divided daily doses in conjunction with their standard oral hypoglycemic drugs (glibenclamide, metformin, rosiglitazone) as adjusted by their physicians. Blood was withdrawn after 12 hours fast and analyzed for glucose.
(*) Statistically significant difference. |
Table 2.Effect of Adjuvant Use of Nigella sativa Crushed Seeds Co-administered with Standard Oral Hypoglycemic Drugs on Glycated Hemoglobin A1C (HbA1C) in Patients with Uncontrolled Type-2 Diabetes Mellitus. Values represent Mean ± Standard Error of the Mean [9]
Test Interval (Months) |
HbA1C (%) |
P Value |
Control Group |
N. sativa Group |
Baseline |
8.2 ± 0.12 |
8.6 ± 0.13 |
- |
3 Months |
8.3 ± 0.12 |
7.9 ± 0.18 |
0.000* |
6 Months |
8.3 ± 5.59 |
7.8 ± 0.22 |
0.000* |
9 Months |
8.5 ± 0.15 |
7.9 ±0.99 |
0.022* |
12 Months |
8.5 ± 0.14 |
8.2 ± 0.14 |
0.010 |
|
Consenting uncontrolled diabetics (based on two successive readings three months apart of HbA1C of > 7%) subjects (N=114) were randomized to treatment by either crushed N. sativa capsules (2g/day) or its matching placebo. The test drugs were administered in two divided daily doses in conjunction with their standard oral hypoglycemic drugs (glibenclamide, metformin, rosiglitazone) as adjusted by their physicians. Blood withdrawal and analysis were determined after 12 hours fast.
(*) Statistically significant difference. |
Table 3.Effect of Adjuvant Nigella sativa Crushed Seeds on Total Anti-oxidant Capacity (TAC) in Subjects with Uncontrolled Type-2 Diabetes Mellitus Receiving Oral Hypoglycemic Drugs. Values represent Means ± Standard Error of the mean [9]
Treatment Duration |
Total Anto-oxidant Capacity (mM) |
P Value |
Control Group |
N. sativa Group |
Baseline |
2.5 ± 0.15 |
2.1 ± 0.17 |
0.32 |
3 Months |
2.69 ± 0.13 |
2.81 ± 0.15 |
0.56 |
6 Months |
2.47 ± 0.16 |
2.76 ± 0.14 |
0.17 |
9 Months |
2.18 ± 0.13 |
2.79 ± 0.18 |
0.01* |
12 Months |
2.33 ± 0.19 |
2.93 ± 0.12 |
0.01* |
Consenting uncontrolled diabetics (based on two successive readings three months apart of HbA1C of >7 %) subjects (N=114) were randomized to treatment with either crushed N. sativa (NS) capsules (2g/day) or its matching placebo. The test treatments were administered in two divided daily doses in conjunction with standard oral hypoglycemic drugs (glibenclamide, metformin, rosiglitazone) as adjusted by their physicians. Although data are not shown in the table, glutathione levels were significantly increased but the lipid peroxidation index (thiobarbituric acid reactive substances-TBARS) values were significantly decreased in response to the N. sativa treatment.
(*) Statistically significant difference. |
The authors did not report any adverse reactions during the one-year treatment with NS or placebo. These results indicate that the adjuvant use of NS improves the glycemic control, decreases the insulin resistance, improves beta-cells function and ameliorate the oxidative stress in uncontrolled type-2 DM patients.
Hosseini, et al. [10] investigated the adjuvant hypoglycemic effect of Nigella sativa oil in type-2 DM patients (N=70) using a randomized, double-blind, placebo-controlled clinical study. The study protocol was reviewed and approved by the Institutional Review Board of Baqiyatallah University Medical Sciences in Iran. The trial was registered in the Iranian Registry of Clinical trials. Consenting subjects were included in the study if they had: fasting blood glucose between 140 and 180 mg/dL, with a disease duration of 2 to 8 years, weighing between 55 to 75 kg, aged between 34 to 63 years, normal blood pressure and lipid levels and taking no more than two 500 mg metformin tablets and two 5 mg glyburide tablets every day. Subjects were excluded if they were: receiving insulin therapy; history of cardiac, renal, hepatic and hematological diseases, history of gallstones or gallbladder surgery, or using estrogens, steroids, beta-blockers and thiazides, pregnant or breast-feeding women, alcohol consumption and cigarette smoking. Nigella sativa oil was prepared by a cold press procedure (Barig Essence Company, Kashan City, Iran). The placebo consisted of mineral oil which had chlorophyll and red chili extract added to render similar appearance and taste to the NS oil. The trial subjects were given bottles containing 150 ml of either the active or placebo oil treatments. The subjects were randomly assigned to two groups (N=35 each) and each subject was instructed to take 2.5 ml of the NS oil taken twice daily for 3 months. Fasting and 2-hours postprandial blood glucose, HbA1c, body mass index (BMI), lipid tests, liver and renal function tests were determined at baseline and after three months of treatment. All treatments were well tolerated. The NS treated group showed statistically significant decreases in blood levels of fasting glucose, postprandial blood glucose, HbA1c and BMI (Table 4).
Table 4.Effect of Adjuvant Use of Nigella sativa Oil Co-administered with Standard Oral Hypoglycemic Drugs on Blood Glucose, Glycated Hemoglobin A1C (HbA1C) in Patients with Type-2 Diabetes Mellitus. Values represent Mean (Standard Deviation) [10]
Parameters (Months) |
Baseline |
3-Month Post Treatment |
Placebo Oil |
Nigella s. oil |
Placebo Oil |
Nigella s. Oil |
Fasting Glucose (mgg/dl) |
179.8 (32.3) |
180.2 (31.8) |
186.3 (42.1) |
161.9 (45.3) |
P-Value |
0.197 |
0.016* |
2Hr postprandial
Glucose (mg/dl) |
189.7 (42.8) |
183 (38.7) |
192.2 (41.7) |
167.9 (37.5) |
P-Value |
0.312 |
0.010* |
HbA1c (%) |
8.79 (0.55) |
8.82.9 (0.73) |
8.70 (0.67) |
8.52 (0.68) |
P-Value |
0.730 |
0.003* |
BMI (kg/m2) |
30.92 (3.67) |
30.81 (3.55) |
31.12 (3.73) |
29.52 (3.5) |
P-Value |
0.373 |
0.028* |
|
Consenting diabetics subjects (N=70) were randomized to treatment to either N. sativa oil (2.5 ml) or its matching placebo. The test drugs were administered twice daily doses in conjunction with their oral hypoglycemic drugs (metformin 500 bid, glyburide 5 mg bid). Blood withdrawal and analysis were determined after 12 hours fast.
(*) Statistically significant difference. |
The findings in these controlled clinical studies are consistent with the pre-clinical studies in which NS had shown hypoglycemic effects in animal models with experimental diabetes [2,11-13]. However, unlike the animal studies, NS was used as an adjuvant rather than a principal therapy for the treatment of uncontrolled type-2 diabetes mellitus.
Hyperlipidemia: Three studies investigated the hypolipidemic effects of NS [14-16]. The Badar, et al. [14] investigated NS hypolipidemic effects in 114 consenting subjects with uncontrolled type-2 DM and who were also receiving standard oral hypoglycemic drugs (glibenclamide, metformin or rosiglitazone). The study was a single-blind, non-randomized investigation in comparison with placebo. The study protocol was reviewed and approved by the Research and Ethical Committee of the University of Dammam in Saudi Arabia; the trial was registered in the Clinical Trial Registry of India. The subjects were recruited from the Diabetes Clinic of King Fahd Hospital of the University of Dammam. The inclusion criteria were poorly controlled type-2 DM (determined by two readings of HbA1C in excess of 7% taken 3 months apart), aged 18-60 years and receiving stable oral hypoglycemic drugs. The exclusion criteria were: HbA1C in excess of 9%, insulin therapy, BMI in excess of 40 kg/m2, triglycerides of more than 400 mg/dL, major cardiovascular, renal and hepatic disease, pregnancy and lactation. Lipid levels, heart rates, systolic blood pressure, diastolic blood pressure and mean arterial pressure were determined at baseline, 3 months, 6 months, 9 months and 12 months following the treatment with 2 g/day of NS crushed seeds or its matching placebo administered at the frequency of twice daily. Serum triglycerides, total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C) were determined
As is evident from Table 5, NS treatment significantly reduced total cholesterol and LDL-C but did not affect the other lipid parameters. Although data not shown in this table, the investigators noted small, but statistically significant decreases in heart rates, diastolic blood pressure and mean arterial blood pressure.
Table 5.Hypolipidemic Effects of Adjuvant Nigella Sativa in Type-2 Diabetic Subjects Receiving Oral Hypoglycemics for 12 Months. Values Represent Means and Standard Deviation (SD) [14]
Parameter |
Treatment Duration |
Placebo Means (SD) |
N. sativa |
P Value |
Total Cholesterol (mg/dL) |
Baseline |
195.63 (46.30) |
194.19 (40.58) |
0.86 |
3 months |
199.30 (45.84) |
185.56 (40.94) |
0.11 |
6 months |
200.94 (43.31) |
177.61(41.24) |
0.007 |
9 months |
195.64 (34.54) |
180.18 (42.99) |
0.05 |
12 months |
199.27 (39.29) |
180.66 (41.91) |
0.02 |
Triglycerides (mg/dL) |
Baseline |
180.82 (124.14) |
170.87 (102.19) |
0.64 |
3 months |
185.82 (111.09) |
168.35 (92.38) |
0.38 |
6 months |
193.49 (110.28) |
167.06 (108.72) |
0.22 |
9 months |
184.02 (115.19) |
162.34 (85.08) |
0.29 |
12 months |
189.72 (114.99) |
169.62 (102.60) |
0.36 |
LDL-C (mg/dL) |
Baseline |
122.98 (33.12) |
126.49 (33.91) |
0.57 |
3 months |
127.98 (30.02) |
114.23 (35.29) |
0.03 |
6 months |
128.62 (35.93) |
107.14 (36.48) |
0.004 |
9 months |
120.58 (27.73) |
115.34 (38.50) |
0.44 |
12 months |
120.72 (26.96) |
114.25 (34.62) |
0.30 |
HDL-C(mg/dL) |
Baseline |
42.45 (10.05) |
42.54 (43.01) |
0.96 |
3 months |
42.21 (9.83) |
43.01 (10.36) |
0.68 |
6 months |
41.70 (8.41) |
42.68 (10.66) |
0.61 |
9 months |
43.25 (11.13) |
45.02 (10.67) |
0.42 |
12 months |
43.79 (9.81) |
44.02 (10.45) |
0.91 |
Consenting subjects (N=114) with total cholesterol concentration of >200 mg/dL were randomized to either NS or its matching placebo capsules given twice daily for 4 weeks. Powdered NS capsules (2 g/day given twice daily) were administered for 12 months with oral hypoglycemic drugs. Although NS significantly reduced total cholesterol and low-density lipoprotein cholesterol (LDL-C), it did not affect the concentration of the triglycerides nor the high-density lipoprotein cholesterol (HDL-C). |
Sabzghabaee, et al. [16] investigated the hypolipidemic effects of NS using a randomized, placebo-control clinical trial in consenting subjects with hyperlipidemia. The study was conducted in Isfahan University, Iran. Subjects (N=88) were included if they had a total cholesterol value of >200 mg/dl. Capsules of NS crushed seeds containing 500 mg or its matching placebo were administered in two divided daily doses (total daily dose of 2.0 g/day) for 4 weeks. Fasting baseline blood glucose, total cholesterol, low density lipoprotein, high density lipoprotein and triglycerides were measured at baseline and after 4 weeks of treatment. Statistically significant decreases in total cholesterol, low density lipoprotein and triglycerides were shown with NS treatment (Table 6). However, there were no statistically significant changes in either the high-density lipoprotein or fasting blood glucose.
Table 6.Hypolipidemic Effects Powdered Nigella Sativa in Human Subjects Receiving a Total Daily Dose of 2.0 g/day for Four Weeks [16]
Parameter |
Percent Reductions from controls at 4 weeks |
P values NS vs. Placebo |
Total Cholesterol (mg/dL) |
4.78% |
P < 0.05 |
Low Density Lipoprotein C (mg/dL) |
7.6 % |
P < 0.05 |
Triglycerides (mg/dL) |
16.65 % |
P < 0.05 |
| Eighty-eight consenting subjects with total cholesterol concentration of >200 mg/dL were randomized to either NS or its matching placebo capsules given twice daily for 4 weeks. NS dose was 2 g/day. Although NS significantly reduced total cholesterol, LDL-C cholesterol and triglycerides, it did not affect the concentration of fasting blood glucose nor the high-density lipoprotein cholesterol (HDL-C). |
As will be subsequently discussed in the Hashimoto’s thyroiditis section, the adjuvant treatment with NS (2 g/day for 8 weeks) given with levothyroxine significantly improved thyroid functions, insulin concentration, triglycerides, LDL and HDL but it did not affect the fasting blood glucose concentration (Farhangi, et al. [15]; Table 7).
Table 7.Effect of Nigella Sativa on Metabolic Parameters in Patients with Hashimoto’s Thyroiditis [26]
Laboratory tests |
Nigella sativa (N=20) |
Placebo (N=20) |
Statistics (a) P* |
|
Fasting blood glucose (mg/dL) |
Before |
86.6 ± 4.46 |
88.10 ± 8.56 |
0.58 |
After |
84.90± 7.01 |
87.80 ± 6.03 |
0.16 |
P** |
0.31 |
0.85 |
|
|
Insulin (ulU/ml) |
Before |
10.62 ± 7.51 |
7.71 ± 4.12 |
0.14 |
After |
29.18 ± 19.93 |
17.30 ± 9.16 |
0.023 |
P** |
<0.001 |
<0.001 |
|
|
HDL (mg/dL) |
Before |
41.55 ± 4.67 |
41.70 ± 6.50 |
0.93 |
After |
43.75 ± 3.72 |
40.57 ± 4.87 |
0.027 |
P** |
0.046 |
0.26 |
|
|
LDL (mg/dL) |
Before |
130.65 ± 30.68 |
105.00 ± 34.48 |
0.018 |
After |
107.85 ± 36.99 |
108.90 ± 32.88 |
0.92 |
P** |
0.002 |
0.06 |
|
|
Triglycerides (mg/dL) |
Before |
177.10 ± 34.50 |
186.00 ± 66.63 |
0.59 |
After |
156.00 ± 5.91 |
185.55 ± 74.98 |
0.11 |
P** |
0.02 |
0.93 |
|
|
Total Cholesterol (mg/dL) |
Before |
183.70 ± 45.72 |
179.10 ± 43.66 |
0.74 |
After |
175.10 ± 29.06 |
180.17 ± 44.95 |
0.64 |
P** |
0.22 |
0.55 |
|
|
Subjects with Hashimoto’s thyroiditis receiving stable dosage of thyroxine were randomized to either NS (2 g/day given twice daily before lunch and dinner) or its matching placebo given for 8 weeks duration. Blood was withdrawn before and after the treatment and assayed for various metabolic parameters. Data are presented as mean ± SD.
(a) P* values for ANOCOVA after adjustment for age, gender and baseline concentration of parameters. P** Values for paired t test. |
The results of the three NS trials are consistent with the results of the systematic review and meta-analysis regarding glucose homeostasis and serum lipids in type-2 diabetic patients conducted by Daraybeygi-Khotbehsara, et al. [17] In their review, the supplementation of various pharmaceutical preparations of NS improved fasting blood glucose, HbA1C, total cholesterol, and LDL-C. However, there were no statistically significant effects on triglycerides and HDL-C but subgroup analyses disclosed that the NS oil, but not the powdered seeds, showed significant reduction of serum triglycerides [17].
Hashimoto’s Thyroiditis: Hashimoto’s thyroiditis is the most common cause of hypothyroidism. The illness is a T-cell mediated autoimmune disease which is characterized by the presence of thyroid autoantibodies such as anti-thyroid peroxidase (TPO-Ab) and anti-thyroglobulin (TG-Ab) in the serum. Patients usually exhibit gradual thyroid failure and occasional goiter development which could lead to papillary thyroid carcinoma in the untreated patients [18,19]. Levothyroxine sodium is an effective treatment of Hashimoto’s thyroiditis; however, its chronic use is associated with cardiac dysfunction, left ventricular hypertrophy and bone loss [15,20-22]. Several growth and vasoactive factors may potentially be responsible for the pathological changes associated with Hashimoto’s thyroiditis blood flow: these include the vascular endothelium growth factor (VEGF) and nesfatin-1, a peptide which is also involved in regulation of energy homeostasis related to food consumption [23].
A pre-clinical study had shown a protective role of NS in reversing hypothyroid status, ameliorating oxidative stress and decreasing thyroid damage in propylthiouracil-induced hypothyroidism in rats [24]. Given the pre-clinical evidence of the efficacy of NS in hypothyroidism, one major controlled human study investigated the effects of NS on thyroid function and several important parameters which included serum vascular endothelium growth factor (VEGF)-1, nesfatin-1, transforming growth factor B (TGF-b), interleukin-23 (IL-23) and anthropometric features in patients with Hashimoto’s thyroiditis. The three publications discussed below were conducted at the same institution and by the same investigators [15,25,26].
Farhangi, et al. [15] conducted a double-blind, placebo-controlled trial in 47 patients with Hashimoto’s thyroiditis who were attending the endocrinology and metabolism clinics of Isfahan University of Medical Sciences in Iran. The study examined the adjuvant use of NS in patients who were receiving stable doses of levothyroxine from 6 weeks prior to enrollment to the end of the trial. The study protocol was reviewed and approved by the Institutional Review Board of Tabriz University Medical Sciences in Iran. All study subjects provided written informed consent and the study was registered in the Iranian Registry of Clinical Trials. Subjects, aged 20 to 50 years, had a clinically established diagnosis of Hashimoto’s thyroiditis. Subjects were excluded if they were: taking any nutritional supplements for at least 3 months prior to the enrollment or during the trial, history of autoimmune disease, cardiovascular event, Grave’s disease, thyroid surgeries, pregnancy or lactation. The average replacement maintenance dose of levothyroxine sodium was 1.7 mcg/kg/day (about 100 mcg/day for a 60 kg adult). Subjects were randomized to either NS treatment group (N=23) who received capsules filled with 1.0 g of NS powder taken twice daily at a total dose of 2.0 g/day before lunch and dinner for 8 weeks or its control (placebo; N=24) group who received capsules filled with starch that had similar appearance to the active treatment. Three subjects assigned to NS developed itching and nausea and four subjects in the placebo group refused to continue the trial and thus were excluded from the analysis. Thus, 20 subjects in each arm were allotted to the active and to the placebo group. In each test arm, there were 17 women and 3 men. The results were analyzed on the basis of the evaluable patients who had completed this study. The high preponderance of women enrolled in the study was consistent with fact that women are ten times more likely to develop Hashimoto’s thyroiditis than men [27].
NS capsules and its matching placebo were prepared by a contract pharmaceutical manufacturer who had adhered to Good Manufacturing Practices (GMP). However, the selection of the NS dose employed in this study was based on the authors’ review of previous clinical studies indicating the effectiveness of 2 g/day in several metabolic disorders including immune disturbances and lipid abnormalities [15].
The adjuvant employment of NS supplementation with levothyroxine for 8 weeks significantly (P<0.05) improved thyroid functions and anthropometric variables including body weight (kg), body mass index (BMI in kg/m2), waist and hip circumference when compared with the control group. The serum concentrations of thyroid stimulating hormone (TSH) and anti-thyroid peroxidase (anti-TPO) were significantly decreased while serum T3 concentrations increased in the NS treated group (Table 8). Serum VEGF, but not nesfatin-1, concentrations were reduced with NS treatment.
Table 8.Effect of Nigella Sativa on Various Metabolic Parameters in Patients with Hashimoto’s Thyroiditis [15]
Laboratory test |
Nigella sativa (N=20) |
Placebo (N=20) |
Statistics P* |
|
TSH (mlU/l) |
Before |
6.42 ± 3.86 |
8.14 ± 7.28 |
0.35 |
After |
4.13 ± 2.35 |
8.27 ± 7.21 |
0.02 |
P** |
0.03 |
0.40 |
|
|
T3 (mm/l) |
Before |
0.92 ± 0.27 |
1.18 ± 0.36 |
0.017 |
After |
1.06 ± 0.34 |
1.16 ± 0.35 |
0.39 |
P** |
0.008 |
0.15 |
|
|
T4 (mmol/l) |
Before |
8.07 ± 2.56 |
7.97 ± 3.11 |
0.91 |
After |
8.89 ± 1.43 |
7.63 ± 2.23 |
0.04 |
P** |
0.21 |
0.32 |
|
|
Anti-TPO (IU/ml) |
Before |
294.55 ± 210.05 |
278.10 ±170.77 |
0.78 |
After |
147.99 ± 158.33 |
274.30 ±167.20 |
0.01 |
P** |
0.019 |
0.28 |
|
|
Nesfatin-1 (ng/ml) |
Before |
41.80 ± 28.33 |
25.86 ± 20.91 |
0.049 |
After |
37.63 ± 5.91 |
26.75 ± 23.95 |
|
P** |
0.34 |
0.69 |
|
|
VEGF (ng/L) |
Before |
3521.13 ± 395.95 |
2101.73 ± 339.29 |
0.17 |
After |
2100.17 ± 36.082 |
2100.17 ± 360.82 |
0.25 |
P** |
0.02 |
0.99 |
|
|
Subjects with Hashimoto thyroiditis receiving stable dosage of thyroxine were randomized to either NS (2 g/day given twice daily before lunch and dinner) or its matching placebo for 8 weeks duration. Blood was withdrawn before and after the treatment and assayed for various metabolic parameters. Data are presented as mean ± SD.
(a) P* values for ANOCOVA after adjustment for age, gender and baseline concentration of parameters. P** Values for paired t-test. TSH: thyroid stimulating hormone, T3: triiodothyronine, T4: thyroxine, VEGF: vascular endothelial growth factor. The bolded P values are statistically significant.
|
In a separate analysis performed on the same set of Hashimoto’s thyroiditis patients, the treatment of NS significantly reduced the concentration of interleukin-23 (IL-23), but not the concentration of the transforming growth factor beta (TGF-b) indicating a protective anti-inflammatory role of NS in this disease [25]. In addition, the treatment with NS improved the lipid profile of the Hashimoto’s thyroiditis patients as previously discussed under the hyperlipidemia section of this review (Table 7; Farhangi, et al. [25]). All these observations suggest that NS has a potentially important therapeutic value in the management of patients with Hashimoto’s thyroiditis.
Asthma: Pre-clinical studies established that NS exhibits bronchodilation and anti-allergic actions. Several pharmacological mechanisms mediate the effects of NS on tracheal chain which include a functional antagonistic effect of the muscarinic receptor, inhibitory effects on histamine (H1) receptor, inhibitory effect on calcium channels, opening effects of potassium channels, stimulatory effects on beta-adrenergic receptors and antitussive actions [28-31]. A total of four controlled clinical trials investigated the anti-asthmatic effect of NS [32-35]. Three of the four trials were performed in a single clinical center (Asthma Clinic of the Mashhad University of Medical Sciences, Iran by the same principal investigator, Professor Boskabady). The protocols for the three Boskabady studies were approved by the Institutional Ethics Committee and all patients provided written informed consent. Since all Boskabady, et al. studies had employed similar experimental design and similar pulmonary functions tests, they will be discussed together. Listed below is a summary of the Boskabady studies:
The first investigation was a placebo-controlled study which examined the adjuvant use of NS in 29 asthmatic patients, studied over a period of 3 month [32]. Subjects meeting the following criteria were included: (a) They had asthma diagnosed by a physician and had two or more of the following symptoms: recurrent wheeze, recurrent cough or tightness at rest, cough or tightness during the night or early morning, wheeze or cough during the exercise; (b) They had forced expiratory volume in 1 sec (FEV1) and peak expiratory flow (PEF) less than 80% of predicted value; (c) They had no history or symptoms of cardiovascular or other respiratory diseases that required treatment (excluding the common cold). All study subjects had moderate to severe asthma according to the United States National Institutes of Health Global Strategy and Asthma Management-The Gina Guidelines [36]. The treatment regimen for all subjects included inhaled corticosteroids, mostly beclomethasone diproprionate (400-1400 mcg) inhaler, beta-agonists, oral corticosteroids and oral theophylline. Subjects in the NS study group (N=15) were instructed to take 15 ml/kg/day of 0.1 g% of boiled NS extract (a tea consisting of NS extract and 10 % roasted glucose solution) daily and those in the control (placebo) group (N=14) were given roasted glucose solution (10 % glucose in a saline solution). The actual daily dose of the NS tea corresponded to about 100 gm of the unextracted seeds given in a total daily volume of about one liter of the solution for an average 70 kg human subject.
Asthma symptoms and pulmonary function tests (PFTs) were performed using acceptable standards as outlined by the American Thoracic Society with subjects in the standing position and wearing nose clips [37]. All study subjects were assessed at enrollment, after 45 days and after 90 days of the treatment.
Some of the asthmatic symptoms improved statistically after 45 days of treatment with the NS tea but no symptoms improvement had occurred in the control (placebo) group. However, at the 90 days treatment period, asthmatic symptoms significantly improved when compared with the control group (Table 9). Likewise, the results of pulmonary function tests showed statistically significant improvement in the NS group when compared with the control group (Table 10). Furthermore, the concomitant use of beta agonists, oral corticosteroids, oral theophylline and inhaled corticosteroids in the NS group was significantly (P<0.001) decreased at the end of the 90-day treatment period when compared with the control group. Thus, subjective symptoms improvement correlated with the objective PFTs improvement. This study clearly suggests that adjuvant use of NS has significant prophylactic effect on asthma symptoms.
Table 9.Asthma Symptoms and Severity in the Nigella sativa and Control Groups at the Beginning and After 90 days of Treatment. Data are presented as percentage decrease ± SEM [32]
Symptoms |
% Decrease at the Beginning |
% Decrease After 90 Days of treatment |
|
Control |
N. sativa |
Control |
N. Sativa |
Night wheezing |
2.36 ± 0.25 |
2.20 ±0.20 NS |
5.99 ± 8.50 |
65.56 ± 0.12*** |
Night coughing |
2.00 ± 0.30 |
1.87 ± 0.27 NS |
33.33 ± 11.59 |
74.45 ± 7.78*** |
Exercising W & C |
2.43 ± 0.17 |
2.47 ± 0.16 NS |
15.46 ± 8.97 |
53.32 ± 7.49*** |
Morning W & C |
1.79 ± 0.28 |
2.27 ± 0.23 NS |
14.29 ± 8.35 |
51.11 ± 7.54*** |
Daily W & C |
2.00 ± 0.3 |
2.13 ± 0.19 NS |
7.14 ± 9.48 |
60.00 ± 10.26*** |
Weekly W & C |
6.07 ± 0.74 |
4.47 ± 0.45 NS |
10.48 ± 9.97 |
58.45 ± 5.25*** |
Chest wheezing |
2.57 ± 0.17 |
2.6 ± 0.13 NS |
22.61 ± 7.74 |
44.43 ± 3.12*** |
Asthma Severity |
3.2 ± 0.19 |
3.27 ± 0.18 NS |
5.36 ± 3.87 |
49.45 ± 3.50*** |
|
N. sativa was administered in a form of a hot water extract containing roasted (boiled) 10 % glucose. The total daily dose of the extract corresponded to 100 g of NS seeds. The control group received only 10% roasted glucose in a saline solution. Each subject received a total daily dose contained in one liter of the fluid extract which was taken several times per day.
W: wheezing; C: coughing.
Statistical difference between placebo and study group. NS: non-significant difference;***P=0.001. |
Table 10.Pulmonary Function Tests (PFTs) for Nigella sativa and Control Groups in Asthmatic Patients at the Beginning and After 90 Days of Treatment [32]
PFTs |
At the beginning |
After 90 days of treatment |
|
Control |
N. sativa |
Control |
N. sativa |
FVC |
54.21 ± 3.30 |
62.27 ± 4.50 NS |
9.26 ± 5.47 |
27.71 ± 5.15* |
FEV1 (L) |
52.1 ± 4.50 |
58.8 ± 5.00 NS |
3.30 ± 6.50 |
29.47 ± 5.04** |
PEF (L/s) |
38.8 ± 3.70 |
53 ± 5.21* |
-0.66 ± 7.20 |
31.18 ± 3.80*** |
MEF75 (L/s) |
33 ± 4.85 |
50 ± 7.40 NS |
7.71 ± 8.52 |
31.74 ± 11.00*** |
MEF50 (L/s) |
37.71 ± 5.18 |
41.67 ± 5.45 NS |
1.26 ± 9.93 |
42.00 ± 7.06*** |
MEF25 (L/s) |
45.9 ± 7.45 |
49.87 ± 5.70 NS |
3.64 ± 11.30 |
20.95 ±5.40 NS |
|
N. sativa was administered in a form of a hot water extract containing roasted (boiled) 10 % glucose. The total NS daily dose corresponded to the content of 100 g of NS seeds. The control group received only roasted 10% glucose in a saline solution. Each subject received a total daily dose contained in approximately one liter of the fluid extract which was taken several times per day.
FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; PEF: Peak expiratory flow; MEF75, MEF50, MEF25 maximal expiratory flow at 75%, 50% and 25% of the FVC, respectively. All PFTs values were quoted as means ± SEM of percentage predicted.
Statistical difference between control and study group. NS: non-significant difference; *P<0.01; **P<0.005; ***P;<0.001. |
The second study was a randomized, double-blind, placebo-controlled investigation conducted in 40 chemical (sulfur mustard gas) war victims in which a boiled extract of NS tea was given for two months; the study showed statistically significant therapeutic efficacy on respiratory symptoms and PFTs when compared with placebo [34]. The symptoms and PFTs assessments were similar to the previously discussed study [32].
A third comparative study by the same investigators was conducted in 15 asthmatic patients showed that the NS boiled extract was more effective than placebo but NS was quantitatively less effective when compared with the reference drugs salbutamol and theophylline [33].
More recently, Koshak, et al. [35] conducted a randomized, double-blind, placebo-controlled trial in which NS oil (500 mg twice daily) was used as a supplementary treatment in 80 asthmatic patients. After 4 weeks, the NS treated group (N=40) showed statistically significant improvement in asthma test scores and a trend of improvement in PFTs when compared with the placebo group (N=40). Of interest, the improvement was associated with a remarkable normalization of blood eosinophilia in these patients.
No untoward toxicity or intolerance was reported with any of the four asthma trials. Furthermore, the clinical studies support the efficacy of NS, administered either as a water extract or in the form of its oil, for the improvement of asthmatic symptoms.
Since these four studies employed different pharmaceutical preparations, we need to establish a preferred pharmaceutical dosage form. However, the fact that poor compliance with conventional asthma medications remains a major problem in achieving asthma control, there is a need to investigate NS supplemental therapy compares with the standard of care therapies.
Intractable Pediatric Seizures: Pre-clinical studies had established that NS had anti-epileptic action in diverse animal models [2]. Three small, single centers, pilot clinical studies investigated the adjuvant therapeutic value of NS, administered in three types of pharmaceutical preparations, for the control of intractable seizures in children receiving stable anti-epileptic drugs (AEDs) [38-40]. All of the three studies had similar study design but different types of pharmaceutical dosage preparations and therefore will be discussed together in a chronological order of their publication. Briefly, Akhondian, et al. [38] conducted a double-blind, crossover clinical trial on children with refractory epilepsy. The study subjects were aged 13 months to 13 years and consisted of 10 boys and 10 girls. The subjects were receiving constant and stable AEDs treatment for at least one month prior to the enrollment. The subjects received either aqueous extract of NS (40 mg/kg every 8 hour) or its placebo for a period of 4-weeks. A two-week washout period was allowed before crossing of one treatment to the second treatment. The mean frequency of seizures between the NS treatments and placebo were compared. The treatment with the NS extracts significantly (P<0.05) decreased the frequency of seizures when compared with the placebo treatment.
A follow-up pilot study, which was conducted by the same investigators, examined the efficacy of thymoquinone, an active component of NS, for the treatment of refractory epilepsy [39]. The study was a double-blind, placebo-controlled, crossover clinical trial conducted on 22 children in which either thymoquinone (1.0 mg/kg every 8 hours) or its matching placebo were orally administered. The children were maintained on stable dosages of AEDs and the frequency of seizure and parenteral satisfaction were compared between the two treatment groups at the end of four weeks of treatment period. The reduction of the frequency of seizures at the end of the first period was compared with the second period. Thymoquinone treatment resulted in a statistically significant (P=0.02) suppression of seizure frequency. Furthermore, the parental satisfaction was significantly increased (P=0.03) when the children were treated with thymoquinone but not with the placebo.
A third pilot study examined the adjuvant role of the NS oil in the treatment of intractable pediatric seizures and oxidative status marker [40]. The study was a randomized, single blinded, crossover study conducted in 30 children with intractable seizures who had a seizure frequency of greater or equal to 2 seizures/month and who were also maintained on stable dosage of AEDs. Subjects with a history of psychiatric, renal, hepatic, thyroid, cardiac, or any systemic chronic illness or metabolic disease other than epilepsy were excluded. Eligible subjects were randomly assigned to either Group-I who received placebo for the first 4-weeks followed by a washout period of two weeks and then another 4-weeks treatment with the black seed oil at a dosage of 40-80 mg/day or Group-II who received the same treatment but in a reverse order. Blood samples were withdrawn at baseline and after several periods with placebo and the NS treatment in order to evaluate the oxidative stress markers (the total anti-oxidant capacity and malondialdehyde). Blood derived from five healthy children was obtained as controls for the investigation of oxidative status. The subjects were assessed at weeks 4 and 10 for oxidative stress markers and seizure frequency and severity. Eight subjects did not complete the study for the following reasons: Noncompliance (N=3), changing AEDs during the study (N=2), exacerbation of seizures when receiving black seeds oil (N=2), nausea and vomiting after receiving black seeds oil (N=1). Only 22 subjects completed the study, comprising 12 females and 10 males.
Comparing the seizure frequency and severity between the placebo and the black seed oil period, a significant difference was found in group I, while no significant difference was found in Group II between the two periods (Table 11). Furthermore, there were no significant difference found between the two groups over time, indicating the non-significance of the treatment sequence on seizure frequency and severity. However, 8 of the 20 treated subjects assigned to the black seeds oil had >50% reduction in seizure frequency/severity. In addition, there were no statistically significant differences between the black seed oil and the placebo groups with respect to the stress oxidative markers. The authors showed that children with seizures had significantly lower total antioxidant capacity relative to the healthy controls (P=0.007), but the malondialdehyde level was not different between the healthy children and children with seizures (Table 12). Malondialdehyde is one of the by-products of lipid peroxidase [41].
Table 11.Effect of Nigella sativa Black Seed Oil on Seizure Frequency and Severity in Children with Intractable Seizures Treated with Standard Anti-epileptic Drugs (AEDs) [40]
|
During 4 Weeks of Placebo |
During 4 Weeks of Black Seed Oil |
P value |
|
Seizure Frequency |
Group I; Median (Range) |
33 (2-608) |
12 (0-461) |
0.034* |
Group II; Median (Range) |
28 (4-516) |
17 (5-200) |
0.44 |
|
Seizure Severity |
Group I; Median (Range) |
28 (6-123) |
24 (0-93) |
0.038* |
Group II, Median (Range) |
35 (8-127) |
31 (7-84) |
0.225 |
|
The investigation was a crossover study design conducted in 22 children with intractable seizures and maintained on standard anti-epileptic drugs (AEDs). Group I or Group II was assigned to 4-weeks of treatment with either Nigella sativa oil (40-80 mg/day) or its matching placebo for four weeks. A two-week washout period was allowed before crossing of one treatment to another treatment.
(*) Wilcoxon Rank Signed test. |
Table 12.Baseline Oxidative Stress Markers in Children with Intractable Seizures Who were Maintained on Anti-epileptic Drugs (AEDs) in Comparison with Healthy Control Subjects [40]
Serum Tests (Mean ± SD) |
Group I Epilepsy Patients |
Group II Epilepsy Patients |
Healthy Subjects (Controls) |
P-Value |
Serum TAC
(mmol/L) |
1.19 ± 0.49 |
1.26 ± 0.41 |
1.36 ± 0.49 |
0.007 |
Serum MDA (nMol/ml) |
6.3 ± 2.37 |
6.23 ± 1.94 |
6.34 +/2.04 |
0.776 |
|
The study was a crossover study conducted in 22 children with intractable seizures and maintained on standard anti-epileptic drugs (AEDs). Group I or Group 2 was assigned to 4-weeks of treatment with either Nigella sativa or its matching placebo for 4-weeks. A two-week washout period was allowed before crossing of one treatment to another treatment. Prior to the treatment, epileptic children had significantly reduced serum level of Total Anti-oxidant Capacity (TAC) when compared with healthy children. However, there were no significant differences in malondialdehyde (MDA) serum concentration between the epileptic patients and the healthy control subjects.
TAC represented total anti-oxidant capacity; MDA represented malondialdehyde. |
Although the three pilot studies used similar study design, the studies differed from each other with respect to the pharmaceutical dosage forms and the amount of the active components contained in these pharmaceutical preparations (Table 13). Despite the fact that these studies were not optimum, the findings clearly suggest a potential efficacy of NS for the treatment intractable pediatric seizures. Clearly, the optimization of the pharmaceutical dosages forms and the optimization of the effective components of NS should be investigated in future studies.
Table 13.Summary of the Adjuvant Anti-epileptic Studies of Nigella sativa, Administered in various Pharmaceutical dosage Forms, in Children with Intractable Seizures Treated with Standard Antiepileptic Drugs (AEDs).
Reference |
No of subjects |
Drug Treatment |
Treatment Outcome |
Akhondian, et al. [38] |
20 |
Aqueous extract 40 mg/kg Q 8 hr |
Significant (P<0.05) reduction in the frequency of seizures. |
Akhondian, et al. [39] |
22 |
Thymoquinone 1 mg/kg Q 8 hr |
Significant (P<0.02) reduction in seizure frequency and significant (P<0.05) parenteral satisfaction. |
Shawki, et al. [40] |
22 |
Black seed oil |
No statistically significant improvement between the 40-80 mg/Day test groups. However, 8 of 22 of NS subjects showed >50% reduction in the seizure frequency and severity. NS treatment did not significantly change the levels of the serum oxidative stress markers. |
All three investigations were crossover studies conducted in children with intractable seizures and maintained on standard anti-epileptic drugs (AEDs). The subjects received either Nigella sativa, in various pharmaceutical preparations and dosages, or its matching placebo for a period of 4-weeks. A two-week washout period was allowed before crossing of one treatment to the other treatment for another 4-week period. |
Memory, Attention, Cognition and Anxiety: Pre-clinical studies showed that NS improved the memory of aged rats and prevented the loss of hippocampal pyramidal cell [42]. Nigella sativa also enhanced the consolidation and recall capability of stored information and spatial memory in rats [43].
Given these preclinical pharmacological actions, two prospective placebo-controlled studies investigated the action of NS on memory, attention, cognition, mood and anxiety in consenting healthy human subjects. Both trials were conducted by the same principal investigator but with a different age of the subject cohorts [44,45]. The ethics of the trials complied with the Helsinki Declaration for the Protection of Human Subjects and its Subsequent Revisions. Written informed consent was obtained from each subject prior to the enrollment. A summary of the two studies is provided below:
The first trial recruited 40 healthy men (55 years or older), who were randomized to two treatment groups each consisting of 20 subjects/group [44]. Subjects were included if they had no previous neuropathological history or hospitalizations for psychiatric illness, nor had a history of drug or alcohol abuse and had normal psychomotor development. Subjects were excluded if they had diabetes, hypothyroidism, renal disease, malignancy, ischemic heart disease, peripheral arterial disease, aneurysm, or were receiving thiazides diuretics, or beta blockers or corticosteroids.
Capsules containing 500 mg of crushed seeds of NS were manufactured by a contract pharmaceutical company (Incepta Pharmaceuticals, Dhaka, Bangladesh) and the manufacturer was compliant with the general requirements of Good Manufacturing Practices (GMP). Psyllium seed husks were used for the preparation of the matching placebo capsules. One group (Group A) received two 500 mg capsules of crushed NS once daily after dinner for nine weeks. The second group (Group B) received matching placebo capsules.
Baseline data concerning memory, attention and cognition were obtained before and after the administration of the NS or its matching placebo. Routine interim history and clinical laboratory tests were conducted at the start and end of the investigation. Standard neuropsychological tests were conducted at baseline and after nine weeks of treatment for Group A and Group B including: logical memory, digit span, Rey-Osterrieth Complex Figure test, attention and cognitive tests. Blood pressure and standard clinical laboratory tests (Cardiac, liver and kidney function) were performed before and after the treatment as part of the safety investigation of Nigella sativa.
After nine weeks of treatment, NS showed a statistically significant (P<0.05) improvement on memory, attention and cognitive functions (Table 14). The subjects tolerated the treatment very well and did not show any changes in any of the standard clinical laboratory tests. Furthermore, NS treatment did not exhibit any effect on body weight, systolic and diastolic blood pressure when compared with the placebo.
Table 14.Effect ofNigella sativaCrushed Seeds (500 mg Taken Twice Daily) or Placebo on Neuropsychological Tests (Memory, Attention, Cognition, Mood and Anxiety) in Healthy Adult Men
Reference |
Age (Yr) |
N |
Treatment Duration in weeks |
Memory Tests |
Attention Tests |
Cognition Tests |
Bin Sayed, et al. [44] |
55 |
40 |
9 |
Enhanced P<0.05 in 3 of 4 tests |
Enhanced P< 0.05 in 3 of 4 tests |
Enhanced P<0.05 in 3 of 4 tests |
Bin Sayed, et al. [45] |
14-17 |
48 |
4 |
Enhanced |
Enhanced |
Enhanced cognition; stabilized mood & decreased anxiety |
|
N. sativa was administered in a form of gelatin capsules containing crushed seeds and prepared by a GMP-compliant contract manufacturer (Incepta Pharmaceuticals LTD., Dhaka, Bangladesh). Psyllium seeds husks were used as placebo. The details of the neuropsychological tests are fully described in these two publications. |
The second study investigated the effect of NS crushed seeds on mood, anxiety and cognition [45]. Nigella sativa was administered at the dosage of 500 mg twice daily or its matching placebo for four weeks in 48 healthy adolescent male subjects, aged 14 to 17 years. The treatment with NS, but not with placebo was shown to stabilize mood, to decrease the anxiety and to modulate cognition (Table 14). No observable adverse effects were noted in any of the study subjects.
Even though the total dose of NS crushed seeds was 1000 mg/day for both studies, it was not clear why the investigators elected a twice daily dosage regimen for the adolescent subjects and only studied the once daily dosage regimen for the older subjects. Future neuropsychological studies with memory and cognition in healthy adult subjects should address NS dose-response and the pharmacokinetics basis for the selection of the dose frequency. Furthermore, these neuropsychological studies should also be conducted in patients with impaired memory and/or impaired cognition.
Rheumatoid Arthritis: Several animal studies provided evidence that NS elicits broad anti-inflammatory and anti-oxidant activities which would provide a basis for its human investigation [2]. Three published studies, conducted in patients with rheumatoid arthritis (RA), investigated the safety and efficacy of NS in this disease [46-48]. Although not stated in the publications, the Hadi, et al. and Kheirouri, et al. studies [47,48] appeared to be conducted on the same RA patient population and in the same institution (Tabriz University of Medical Sciences, Tabriz, Iran), these two studies will be briefly described together. Briefly, Hadi, et al. [47] investigated NS oil for its anti-inflammatory action, inflammatory cytokine response and oxidative stress status in patients with rheumatoid arthritis. The study was a randomized, double-blinded, placebo-controlled trial conducted in 40 consenting subjects. The protocol of the trial reviewed and approved by the Tabriz University Review Board for Human Investigations. The trial was also registered on the Iranian Registry of Clinical Trials. Subjects were included in the study if they were: (a) Aged between 20 to 50 years with mild to moderate RA, according to 2010 criteria of the American College of Rheumatology-European League Against Rheumatism (ACR-EULAR) criteria; (b) Subjects were receiving concomitant therapy with disease-modifying anti-rheumatic drugs (DMARDs) which included: methotrexate, hydroxychloroquine and prednisolone less than 10 mg/day; (c) Subjects were not taking any non-steroidal anti-inflammatory drugs (NSAIDs) or cytokine inhibitors and had stable medications for at least two months; and (d) Subjects with a body mass index less than 40. Subjects were excluded: (a) If they were pregnant or lactating; (b) Subjects taking hormones or oral contraceptives; (c) Subjects with any metabolic disorders, kidney or liver diseases, chronic inflammatory diseases including inflammatory bowel disease; and (d) Subjects consuming anti-oxidant or anti-inflammatory supplements four weeks prior to the intervention. The active group received two 500 mg capsules containing NS oil daily for 8 weeks. The placebo group received capsules filled with paraffin oil and the capsules had identical appearance to the NS capsules to insure the double blindness of the study. The disease activity scores (DAS28) were determined at baseline and at the end of the study. In addition, at the baseline and at the endpoint of the study, venous blood was assayed for cytokine levels Tumor Necrosis Factor-alpha (TNF-alpha), Interleukine-10 (IL-10), total anti-oxidant capacity, superoxide dismutase, catalase, malondialdehyde and nitric oxide.
In this study, the disease activity scores at baseline were not significantly different between the NS and placebo groups. However, at the end of the 8 weeks, the DAS28 score was significantly (P<0.05) decreased in the NS group when compared with the placebo group (Table 15). The NS treatment was well tolerated and led to improvement of the number of the swollen joints when compared with the baseline and the placebo treatment. Furthermore, the serum level of the anti-inflammatory cytokine IL-10 was significantly (P < 0.01) increased in the NS group when compared with placebo (Table 16). However, the level of the inflammatory cytokine TNF-alpha was not significantly changed with the NS treatment when compared with the placebo arm. Moreover, the treatment with NS led to significant reduction of the serum of malondialdehyde and nitric oxide but did not change the level of superoxide dismutase, catalase and total anti-oxidant capacity.
Table 15.Controlled Clinical Trials Examining the Efficacy of Nigella sativa Oil (500 mg Twice Daily) in the Management of Rheumatoid Arthritis in Patients Receiving DMARDs Therapy
Reference |
Number of subjects (N) |
Treatment Duration (Weeks) |
Study Outcome |
Gheita and Kenawy [46] |
40 |
4 |
Significant (P=0.017) improvement in the disease activity score (DAS-28) with NS treatment.
ACR20 and EULAR response criteria for symptoms improvement was observed in 42.5% and 30% of the patients, respectively. |
Hadi, et al. [47] |
42 |
8 |
DAS-28 score was significantly (P<0.05) improved in the NS group but not in the placebo. NS treatment significantly (P=0.009) increased serum IL-10, but had no effect on serum TNF-alpha. NS treatment significantly reduced serum malondialdehyde and nitric oxide but had no effect on superoxide dismutase, catalase and total anti-oxidant capacity |
|
DMARDs: Disease-modifying antirheumatic drugs used in the studies included methotrexate, hydroxychloroquine and glucocorticoids; DAS-28: Disease Activity Score; EULAR: European League against Rheumatism; ACR: American College of Rheumatology; IL-10: Interleukine-10; TNF-alpha: Tumor Necrosis Factor-alpha; NS: Nigella sativa oil.
The Gheita and Kenawy [46] trial was placebo-controlled, parallel groups study. Patients received capsules filled NS oil for the active treatment or starch filled capsules for the placebo group.
The Hadi, et al. [47] trial was placebo controlled double-blind, randomized study. |
Table 16.Effect of Nigella sativa vs. placebo on inflammatory, antioxidant, and stress oxidative biomarkers in female patients with rheumatoid arthritis. Adapted from Hadi,et al.[47]
Parameter Value |
Placebo group (N=16) |
Nigella sativa |
P |
|
TNF-alpha |
Baseline |
12.20 (7.82, 15.06) |
13.29 (8.19, 17.30) |
0.65(a) |
End of the study |
12.20 (9.04, 18.69) |
9.42 (5.72, 14.83) |
0.27(b) |
Mean difference (95% CI) |
0.00 (-3.47, 6.79) |
0.15 (-7.87, 4.48) |
|
P-value(c) |
0.72 |
0.74 |
|
|
IL-10 |
Baseline |
5.55 (1.90, 20.55) |
7.8 (2.40, 12.2) |
0.84(a) |
End of the study |
7.8 (0.60, 43.50) |
11.30 (3.50, 53.70) |
0.34(b) |
Mean difference (95% CI) |
0.4 (-1.62, 22.95) |
8.90 (1.50, 45.90) |
|
P-value(c) |
0.21 |
0.009 |
|
|
The trial was placebo controlled double-blind, randomized study which examined the efficacy of Nigella sativa Oil (500 mg Twice Daily) in the management of rheumatoid arthritis in patients receiving DMARDs Therapy.
(a)Mann-Whitney U test.
(b)Based on ANCOVA adjusted for baseline measures and confounding factors |
The serum high-sensitivity C-reactive protein (CRP) and the immunomodulatory effects of NS oil on T-lymphocytes were also investigated in the RA patients, especially with respect to the involvement of CD4(+), CD8(+) and CD4(+) CD25(+) T-cells using flow cytometry [48]. The treatment with NS induced a significant reduction of the serum high-sensitivity CRP. In addition, the NS treatment reduced CD8 (+), and increased CD4 (+) CD25 (+) T-cell percentage and the CD4 (+)/CD8 (+) ratio as compared with placebo and baseline. Such results suggest that the anti-inflammatory mechanisms of action of NS in the RA patients are multi-factorial and could be mediated through increased levels of the anti-inflammatory cytokine IL-10, a selective anti-oxidant actions and possible modulation of the T- lymphocytes.
Gheita and Kenawy [46] (Faculty of Medicine, Cairo University, Egypt) conducted a placebo-controlled clinical study in forty consenting female RA patients diagnosed according to 2010 ACR/EULAR criteria and concurrently receiving DMARDs therapy. Capsules of NS oil (500 mg) and placebo (starch filled) were prepared and administered twice daily. All subjects received a lead-in treatment period of one month with placebo capsules taken twice daily. At the end of the lead-in period, the subjects were allocated to treatment with either NS or the placebo capsules given twice daily. The disease activity scores (means +/- standard error of the means), determined at 28 days, was significantly (P=0.017) decreased in the group receiving the NS capsules (4.55 +/- 0.82) when compared with before the treatment and with the placebo scores of (4.98 +/- 0.79 and 4.99 +/-0.72, respectively). Similarly, the number of swollen joints and the duration of morning stiffness improved with NS treatment. A marked improvement in the disease activity was shown by both ACR20 and EULAR response criteria in 42.5% and 30% of the NS patients, respectively. However, the Gheita and Kenawy study was limited due to the fact that it was not a double-blind, randomized study [46]. Despite the methodology limitation, the authors concluded that “the supplementation of Nigella sativa during DMARDs therapy in RA may be considered an affordable potential adjuvant biological therapy”.
Helicobacter pylori and Hepatitis-C: Pre-clinical studies have disclosed that Nigella sativa has several important gastrointestinal pharmacological actions relevant to human therapeutics [2,49]. One of such potential use concerns its anti-microbiological actions. Nigella sativa has a broad antimicrobial spectrum including Gram-negative, Gram positive bacteria, viruses, parasites, schistosoma and fungi [50,51]. In particular, NS inhibits the growth of bacteria associated with significant gastrointestinal morbidity such as Salmonella, Escherichia coli and Helicobacter pylori [52,53]. We will briefly discuss the human clinical studies, which examined the anti-bacterial and anti-viral actions of Nigella sativa.
Nigella sativa therapy eradicated Helicobacter pylori (H. pylori) and provided relief the dyspeptic pain in patients with functional dyspepsia [53-55]. Briefly, Salem, et al. [54] investigated the dose-response effects of powdered NS seeds in 88 dyspeptic patients positive for H. pylori infection by histopathology and urease test. Patients were randomly assigned to four treatment groups receiving: Group 1 subjects receiving triple standard therapy of clarithromycin, amoxicillin and omeprazole (N=23); Group 2 subjects receiving 1 g NS (taken as capsule, each containing 500 mg of powdered NS seed and administered twice daily after meals) + 40 mg omeprazole (N=21); Group 3 subjects receiving 2 g NS + omeprazole (N=21); and Group 4 subjects receiving 3 g NS + omeprazole (N=23). The eradication was confirmed by a negative H. pylori stool antigen test which was conducted four weeks after the end of the active treatment period. The percent H. pylori eradication rates for the four groups were 82.6, 47.6, 66.7 and 47.8 with triple therapy, 1g NS, 2 g NS and 3 g NS, respectively. Eradication rate with 2 g NS was statistically not different from the standard triple therapy, whereas the eradication rates with other NS doses were significantly less than that with the triple therapy (P<0.05). Dyspeptic symptoms were significantly improved in all groups to the same extent. However, the lack of dose-response for NS for H. pylori eradication is not understood and should be further investigated.
Hashem-Dabaghian, et al. [55] studied the combined effect of NS and honey (6 g/day of NS ground seeds and 12 g/day of honey administered three times daily after meals for two weeks) in 14 dyspeptic patients who were positive H. pylori infection confirmed by urea breath test. The second urea breath test was made four weeks after the completion of the two weeks of active treatment. Negative urea breath test was observed in 57 % (8/14) of the subjects treated with combined treatment with NS and honey. Furthermore, the dyspeptic symptoms were significantly (P=0.005) improved when compared with the pretreatment evaluation. No serious adverse events were reported. Since the study did not have a control group for NS alone and honey alone, it would be difficult to ascertain whether the beneficial effect of the combined treatment was entirely due to NS alone.
Mohtashami, et al. [56] studied the effect of NS oil (5 ml orally daily) or placebo in dyspeptic patients with H. pylori infection confirmed by the urea breath test. The study was a double-blind, randomized placebo controlled clinical study conducted in 70 dyspeptic patients. Nigella sativa treatment significantly (P<0.001) reduced the number of patients with H. pylori infection and significantly improved the dyspeptic symptoms when compared with the placebo group. However, the absence of positive comparator (gold standard) in this double-blind randomized study was an obvious deficiency of the study.
Clearly, these three clinical studies showed that NS, administered in various pharmaceutical dosage forms, has efficacy in inducing partial eradication of H. pylori and resolution of the symptoms in patients with functional dyspepsia. Unfortunately, only one study [54] had a reference standard (triple therapy) but the other two studies did not. Until we have additional information with comparative standard treatments, it is difficult to ascertain the advantages and disadvantages of NS vs. reference standard antibiotics for the treatments of H. pylori infection. Also, it would be interesting to test whether NS co-treatment with antibiotics would augment their anti-bacterial efficacy as is now common with the currently used combinations of several antibiotics for H. pylori eradication [57]. Clearly; additional prospective studies are needed to clarify the role of NS for the eradication of H. pylori infections.
Nigella sativa was also shown to possess anti-viral activity against hepatitis-C (Hep-C) infection in patients with type-2 diabetes [58]. In this single-arm pilot study, conducted in type-2 diabetic patients who were not eligible for interferon/ribavirin therapy at the time of the conduct of the study, the administration of NS (450 mg of N. sativa oil capsules three times daily for three months) markedly decreased the viral load, improved the oxidative stress and the glycemic control. However, this study was not a controlled investigation and the NS treatment did not reduce the viral load to an extent achieved with the currently available direct acting antiviral drugs effective for the treatment of Hep-C infections.
Miscellaneous Controlled Studies: Unlike the previously discussed studies in which there were more than one clinical study which examined a given potential therapeutic action of NS, our literature search uncovered three interesting controlled studies which investigated the potential therapeutic actions of NS in eczema, cyclic mastalgia and male fertility. These studies will be briefly discussed to highlight NS broad therapeutic values.
- Treatment of Eczema
Yousefi, et al. [59] (The Skin Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran), conducted a randomized, controlled clinical trial in sixty subjects with hand eczema. Consenting subjects, aged 18-60 years, were randomized to three treatment groups: Nigella sativa ointment, betamethasone ointment and Eucerin, a commercial emollient. Subjects were instructed to apply the topical medications twice daily to the affected areas and were followed for four weeks. The primary outcome of the study was changes in the eczema severity and life quality which were assessed at the beginning, 14th and 28th day of the study by the Hand Eczema Severity Index (HECSI) and the Dermatology Life Quality Index (DLQI), respectively [59].
Nigella sativa and betamethasone ointments showed significantly more rapid improvement in hand eczema when compared with Eucerin (P=0.003 and P=0.012, respectively). Nigella sativa and betamethasone induced significant decreases in DLQI scores compared with Eucerin (P<0.0001 and P=0.007, respectively). However, no significant difference was detected between the mean DLQI and HECSI of the NS and betamethasone over time (P=0.38 and P=0.99, respectively). This controlled study indicated that topical application of NS ointment had comparable clinical efficacy with betamethasone in decreasing severity of hand eczema and in improving the quality of life. However, the authors did not provide sufficient details regarding the pharmaceutical preparation of all the ointments and their active concentrations nor provided any information regarding their chemical stability to ensure lack of degradation of the NS active components during the treatment. However, the fact that the work was conducted in a major academic skin research center, we believe that the authors had probably addressed these requirements but did not include such details in their publication.
- Treatment of Male Infertility
In traditional (complementary) medicine in Iran, NS is used for the treatment of male infertility. A randomized, double-blind, placebo-controlled study was conducted in 68 infertile men, aged 20-45 years and who had abnormal semen quality [7]. The protocol was reviewed and approved by the Ethics Committee of the Infertility Center of Mahjdieh Hospital. A written informed consent was obtained from each participant. Subjects were included if the abnormal sperm morphology was less than 30% or sperm counts 20X10 (6)/ml or type A and type B motility were less than 25% and 50%, respectively. The subjects were randomized to oral treatment with either NS oil (2.5 ml given twice daily for two months) or to its matching placebo (2.5 ml of liquid paraffin taken twice daily for two months). At baseline and after 2 months, the sperm count, sperm motility, sperm morphology, semen volume, semen pH and round cells were used as primary outcomes of the treatment and were determined in both groups.
This clinical study is scientifically very important since the authors conducted an extensive chemical analysis of the NS oil used in their study. The chemical analyses of the Iranian sourced NS oil assessed the fixed oil (non-volatile) and the volatile oil components. The authors had identified 11 fatty acids in the fixed oil; three of these fatty acids were present with the highest concentration: linoleic (58%), oleic acid (22.6%) and palmitic acid (12.8 %). In addition, NS oil contained 1.1% volatile constituents consisting of some 20 different compounds; however, only three components were present in the high concentrations: p-cymene (51.6%), thymoquinone (14.5%) and alpha-thujene (13.95%).
In this study, the sperm count, sperm motility, semen volume, semen pH and round cells determined at 2 months were significantly improved with NS oral treatment in comparison with the concurrent placebo and baseline values7. In addition, there were no adverse effects reported by any of the study subjects allocated to either the active treatment or to placebo treatment. However, the authors did not provide sufficient details with respect to the stability of the active components during the treatment period. Furthermore, it would be important to know whether the improvement of the human sperms quality would also be associated with improvement of conception and pregnancy rates. These fertility issues should be investigated in future prospective studies.
- Treatment of Cyclic Mastalgia
Nigella sativa has been reported to possess analgesic activities in several animal models of pain [2]. Given this inherent analgesic activity, it will not be surprising that a clinical investigation would be undertaken in women with cyclic mastalgia because it is a common disorder that has no optimal therapy. Cyclic Mastalgia usually starts within two weeks before menses and resolves or diminishes with the onset of menses. After ruling out significant disease, most patients respond to a combination of assurance and non-pharmacological measures [60]. However, cyclic mastalgia may sometimes be very severe and long lasting that would require pharmacological interventions. Topical nonsteroidal anti-inflammatory drugs (NSAIDs) and the endocrine drugs danazol, bromocriptine and tamoxifen have significant efficacy for the management of cyclic mastalgia [60]. However, NSAIDs are the first line drug for the treatment, but if the NSAIDs are not effective, then endocrine drugs should be used [60].
Huseini, et al. [61] conducted a 3-arm, randomized, triple-blind, placebo-controlled study in 189 consenting women with cyclic mastalgia. The study protocol was reviewed and approved by the Medical Ethics Committee of the Yazd University Medical Sciences in Iran. The trial was registered in the Iranian Registry of Clinical Trials. Outpatient women were included in the study if they were aged 25-45 years, with regular menstrual cycles and diagnosed with a history of cyclic mastalgia for at least during the past three consecutive menstrual cycles (with pain duration for at least 7 days per month) and a mastalgia severity score greater than 4 on a 10 cm on a visual analog scale (VAS) of pain. Exclusion criteria included subjects receiving NSAIDs, hormone therapy or hormonal contraceptives, irregular menstrual cycles, history of breast or endometrial cancers, hysterectomy, pregnancy, lactation, planning a pregnancy, or serious health problems. After three non-medicated baseline menstrual cycles, the subjects were randomized to three treatment groups: topical Nigella sativa oil gel (600 mg) or topical diclofenac (20 mg) gel or its placebo gel. The treatment was applied to the site of pain twice daily for two cycles. The pharmaceutical formulation of all three treatment groups used the same gel vehicle, which was prepared by the same contract manufacturer in Iran. However, the composition of the gel vehicle was not disclosed to the investigators due to manufacturer’s proprietary trade secret. Each patient self-assessed the intensity of mastalgia with the VAS in their late luteal phase of the three baseline cycles and at each treatment cycle, five times in total. Primary and secondary outcomes were pain relief and potential adverse reactions, respectively. The proportion of subjects with more than 50% pain relief at 1 and 2 treatment cycles were determined.
The endpoint reductions of the pain score by NS oil, diclofenac and placebo from baseline were 82%, 83% and 18%, respectively. The pain scores for the two active treatments at the first and the second cycles did not differ significantly (P>0.05). The active treatments decreased the pain score significantly at the end point compared with baseline (both P<0.001). No adverse events were observed during the trial.
This interesting study confirmed the topical analgesic action of the NS oil which was comparable to the reference diclofenac gel. However, the basis of the selection of the NS dose used in this study was not disclosed. Clearly, pharmacokinetics and dose-response analgesic studies should be prospectively investigated.
Discussion
Complementary and alternative medicines (CAM) are commonly used world-wide. In the United States, 1 in 4 older adult diabetic patients use CAM, especially herbal products [62]. Many botanical drugs were initially used as folk remedies and eventually evolved to become accepted main stream drugs for human therapeutics. Nigella sativa is a complementary drug commonly used for the treatment of several diseases based on cultural experience.
During the last 30 years, pre-clinical pharmacological characterizations of Nigella sativa disclosed diverse and interesting properties which are relevant to human therapeutics [2]. Therefore, it was our objective to provide, for the first time, a comprehensive review of the evidence-based clinical investigations which explored NS human therapeutic properties.
The conduct and analyses of the clinical studies reviewed in this communication were generally similar to American and European drug development guidelines for new drugs. Since the clinical trials were relatively small and only conducted in a single clinical research center, there is a need for the conduct of prospective multi-clinic studies to ensure reliable clinical outcomes in diverse patient populations and clinical settings. However, it is essential that a comprehensive Chemistry and Manufacturing Controls for either the black seeds or its components is established prior to the conduct of any future multi-clinic studies. Hopefully, the outcome of these trials should influence the clinical practice regarding the uses of this complementary drug.
The studies disclosed that NS administered in several pharmaceutical dosage forms possesses therapeutic effects in diabetes and hypothyroidism. In uncontrolled type-2 DM patients who were receiving standard oral hypoglycemic drugs, the adjuvant treatment with NS improved the glycemic control and hyperlipidemia. More importantly, NS improved the management of Hashimoto’s thyroiditis by decreasing TSH and the inflammatory markers (anti-TPO) and vascular endothelial growth factor (VEGF). In addition, NS exhibits anti-asthmatic, analgesic and anti-seizure actions. The adjuvant anti-seizure action of NS was demonstrated in children with intractable seizures who were also receiving standard anti-epileptic drugs. Limited but promising studies disclosed that NS has an important role in the management of rheumatoid arthritis in patients receiving DMARDs therapy. This anti-inflammatory action may be mediated by an increased interleukine-10, an anti-inflammatory cytokine and modulating the T-cell response. This novel immunomodulating action of NS is not shared by biological RA drugs acting principally through the inhibition of TNF-alpha, an inflammatory cytokine. Furthermore, the inherent anti-inflammatory activity of NS was demonstrated, when applied topically to patients with hand eczema. The topical efficacy of NS in eczema was similar to the topical efficacy of the reference standard steroid betamethasone. An intriguing observation indicates that NS may also aid in the treatment of male infertility by providing favorable effects on sperm counts, sperm motility, semen volume and semen pH. Nigella sativa was also shown to possess analgesic action when used for the treatment of cyclic mastalgia. Finally, in healthy adolescent and healthy elderly human subjects, NS showed promising effects on memory and cognition; however, additional prospective studies should also examine potential uses in patients with such impairments.
The preclinical characterization of Nigella sativa showed promising profile for the treatment of various types of cancer and for the prevention of the chemotherapy-induced toxicity in the gastrointestinal tract, heart and kidneys [2,63]. In fact, about two third of the NS preclinical pharmacology abstracts listed in the PubMed search were devoted to its anti-proliferative actions and its adjuvant use with anti-cancer drugs. Surprisingly, we did not uncover any meaningful human investigations for its use of NS in oncology therapeutics.
The dosages for NS selected for most of the reviewed studies were not based on the conventional escalating multi-dose tolerance, dose-response or safety considerations. Therefore, the selected dosage used in the clinical studies may not have been optimum for all NS clinical investigations. However, only one study examined a dose-response relationship of powdered NS for its adjuvant use in diabetes mellitus. With respect to its chronic safety, one study conducted in type-2 DM patients showed that the administration of the powdered NS at a total dosage of one gram per day given twice daily for one year did not show any major adverse events or serious toxicity.
There are three limitations to our review that needs to be pointed out: Firstly, we only examined published literature dating from 1960 and did not examine older literature. Secondly, we had rejected some older studies due to their preliminary nature or poor conduct. Thirdly, we only examined studies written in the English language.
Nigella sativa clearly warrants formal drug development in order to define uniform methods for its pharmaceutical preparations, to standardize the dosage forms either for the powdered seeds or seed extract, black seed oil, or a specific active component of the seeds (e.g. thymoquinone or other components) and to insure the stability of the active components to storage. The pharmacokinetics characteristic of the active components needs to be determined. In addition, the maximally tolerated chronic human doses and the safety profile for a specific medical use needs to be investigated. However, the priority of development for a specific use will require close cooperation between the clinical investigators, pharmaceutical scientists and the government(s) to fund this research.
It is hoped that this review would provide a stimulus for the pharmaceutical and clinical scientists to further investigate the potential drug development of black seeds for human therapeutics. Despite the reservations expressed about the pharmaceutical dosage forms and the stability of its components, there are several potential and promising clinical uses, especially for unmet therapeutic indications in the fields of oncology, endocrinology, neurology, rheumatology, pulmonary medicine and infectious diseases.
Conflict of Interests and Acknowledgements
The authors declare that there is no conflict of interests regarding the contents of this manuscript. The authors also wish to thank the many authors who had kindly provided their complete published manuscripts to us when we had requested them. ; Without such co-operation, the undertaking of this overview would not have been possible.
References
- Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, et al. (2013) A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trop Biomed 3: 337-352. [Crossref]
- Dajani EZ, Shahwan TG, Dajani NE (2016) Overview of the pre-clinical pharmacological properties of Nigella sativa (Black Seeds): A complementary drug with historical and clinical significance. J Physiol Pharmacol 67: 801-817.
- Banerjee S, Padhye A, Azmi A, Wang Z, Phillip PA, et al. (2010) Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr Cancer 62: 938-946. [Crossref]
- Sharma, NK, Ahirwar D, Jhade D (2009) Medicinal and pharmacological potential of Nigella sativa: A review. Ethnobotanical Rev 13: 946-55.
- Khan MA, Afzal M (2016) Chemical composition of Nigella sativa Linn: Part 2 Recent advances. Inflammopharmacology 24: 67-79. [Crossref]
- Agbaria R, Gabarin A, Dahan A, Ben-Shabat S (2015) Anti-cancer activity of Nigella sativa (black seed) and its relationship with the thermal processing and quinine composition of the seeds. Drug Des Develop Therap 9: 3119-3124. [Crossref]
- Kolahdooz M, Nasri S, Modarres SZ, Kianbakht S, Huseini HF (2014) Effects of Nigella sativa L. seed oil on abnormal semen quality in infertile men: a randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 21: 901-915. [Crossref]
- Bamosa AO, Kaatabi H, Lebdaa FM, Elq AM, Al-Sultanb A (2010) Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J Physiol Pharmacol 54: 344-354.
- Kaatabi H, Bamosa AO, Badar A, Al-Elq A, Abou-Hozaifa B, et al (2015) Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: Placebo controlled participant blinded clinical trial 2015. PLoS One 10: e0113486.
- Hosseini MS, Mirkarimi SA, Amini M, Mohtashami R, Kianbakht S, et al. (2013) Effects of Nigella sativa L. seed oil in type II diabetic patients: a randomized, double-blind, placebo-controlled clinical trial. J Med Plants 12: 93-99.
- Kanter M, Coskun O, Korkmaz A, Oter S (2004) Effects of Nigella sativa on oxidative stress and beta cell damage in streptozotocin-induced diabetic rats. Anat Rec A Discov Mol Cell Evol Biol 279: 685-691. [Crossref]
- Kaleem M, Kirman D, Asif M, Ahmad Q, Bano B (2006) Biochemical effects of Nigella sativa L seeds in diabetic rats. Indian J Exp Biol 44: 745-748.
- Sultan MT, Butt MS, Karim R, Iqbal SZ, Ahmad S, et al. (2014) Effect of Nigella sativa fixed and essential oils on antioxidant status, hepatic enzymes, and immunity in streptozotocin induced diabetes mellitus. BMC Complement Altern Med 14: 193.
- Badar A, Kaatabi H, Bamosa A, Al-Elq A, Abou-Hozaifa B, et al. (2017) Effect of Nigella sativa supplementation over a one-year period on lipid levels, blood pressure and heart rate in type-2 diabetic patients receiving oral hypoglycemic agents: nonrandomized clinical trial. Ann Saudi Med 37: 56-63. [Crossref]
- Farhangi MA, Dehghan P, Tajmiri S, Abbasi NM (2016) The effects of Nigella sativa on thyroid function, serum endothelia growth factor (VEGF)-1, Nesfatin-1 and anthropometric features in patients with Hashimoto’s thyroiditis: a randomized controlled trial. BMC Complement Altern Med 16: 471. [Crossref]
- Sabzghabaee AM, Dianatkhah M, Sarrafzadegan N, Asgary S, Ghannadi A (2012) Clinical evaluation of Nigella sativa seeds for the treatment of hyperlipidemia: A randomized, placebo controlled clinical trial. Med Arch 66: 198-200.
- Daryabeygi-Khotbehsara R, Golzarand M, Ghaffari MP, Djafarian K (2017) Nigella sativa improves glucose homeostasis and serum lipids in type 2 diabetes: A systematic review and meta-analysis. Complement Ther Med 35: 6-13. [Crossref]
- Ott RA, McCall AR, McHenry C, Jarosz H, Armin A, et al. (1987) The incidence of thyroid carcinoma in Hashimoto’s thyroiditis. Am Surg 53: 442-445.
- Repplinger D, Bargren A, Zhang YW, Adler JT, Haymart M, et al. (2008) Hashimoto’s thyroiditis a risk factor for papillary thyroid cancer. J Surg Res 150: 49-52. [Crossref]
- Lida M, Yamamoto M, Ishiguro Y, Yamazaki M, Honjo H, et al. (2012) Thyroid hormone within the normal range is associated with left ventricular mass in patients with hypertension. J Am Soc Hyper 64: 261-269. [Crossref]
- Nguyen KD, Bagheri B, Bagheri H (2018) Drug-induced bone loss: A major safety concern in Europe. Expert Opin Drug Safety 17: 1-10. [Crossref]
- Ko YJ, Kim JY, Lee J, Song HJ, Kim JY, et al. (2014) Levothyroxine dose and fracture risk according to the osteoporosis status in elderly women. J Prev Med Public Health 47: 36-46. [Crossref]
- Ayada C, Toru U, Korkut Y (2015) Nesfatin-1 and its effects on different organs. Hippokratia 19: 4-10.
- Khalawi AA, Al-Robai AA, Khoja SM, Shaker AS (2013) Can Nigella sativa oil (NSO) reverse hypothyroid status induced by PTU in rats? Biochemical and histological studies. Life Sci J 10: 1-5.
- Tajmiri S, Farhangi MA, Dehghan P (2016) Nigella sativa treatment and serum concentrations of thyroid hormones, transforming growth factor B (TGF-B) and interleukin 23 (IL-23) in patients with Hashimoto’s Thyroiditis. Eur J Int Med 8: 576-580. [Crossref]
- Farhangi MA, Dehghan P, Tajmiri S (2018) Powdered black cumin seeds strongly improve serum lipids, atherogenic index of plasma and modulate anthropometric features in patients with Hashimoto’s thyroiditis. Lipids Health Dis 17: 59. [Crossref]
- Ajjan RA, Weetman AP (2015) The pathogenesis of Hashimoto’s thyroiditis: Further development in our understanding. Horm Metab Res 47: 702-710. [Crossref]
- Boskabady MH, Shahabi M (1997) Brochodilatory and anti-cholinergic effects of Nigella sativa on isolated guinea pig tracheal chain. Iran J Med Sci 22: 127-133.
- Boskabady MH, Shiravi N (2000) Inhibitory effect of Nigella sativa on histamine H1 receptors of the isolated guinea pigs tracheal chains. Eur Respir J 16: 461S. [Crossref]
- Boskabady MH, Shirmohammadi B (2002) Effect of Nigella sativa on isolated guinea pig tracheal chains. Arch Iran Med 5: 103-107.
- Boskabady MH, Kiani S, Jandaghi P (2004) Antitussive of Nigella sativa. Pakistan J Med Sci 20: 224-228.
- Boskabady MH, Javan H, Sajady M (2007) The possible prophylactic effect of Nigella sativa seed extract in asthmatic patients. Fundam Clin Pharmacol 21: 559-66. [Crossref]
- Boskabady MH, Mohsenpoor N, Takaloo L (2010) Antiasthmatic effect of Nigella sativa in airways of asthmatic patients. Phytomedicine 17: 707-713. [Crossref]
- Boskabady MH, Farhadi J (2008) The possible prophylactic effect of Nigella sativa seed aqueous extract on respiratory symptoms and pulmonary function tests on chemical war victims: A randomized, double blind, placebo-controlled trial. J Altern Complement Med14: 1137-1144. [Crossref]
- Koshak A, Wei L, Koshak E, Wali S, Alamoudi O, et al. (2017) Nigella sativa supplementation improves asthma control and biomarkers: A randomized, double-blind, placebo-controlled trial. Phytotherap Res 31: 403-409. [Crossref]
- National Institutes of Health (2002) Global strategy for asthma management and prevention: NHBLI Workshop report, NIH, Bethesda, MD, Publication No. 02-3659.
- American Thoracic Society (1995) Standardization of spirometry: 1994 Update. Official Statement of American Thoracic Society. Am J Respir Crit Care Med 152: 1107-1136.
- Akhondian J, Parsa A, Rakhshande H (2007) The effect of Nigella sativa L. (Black cumin seed) on intractable pediatric seizures. Med Sci Mon 13: 555-559.
- Akhondian J, Kianifar H, Raoofziaee M, Moayedpour A, Toosi MB, et al. (2011) The effect of thymoquinone on intractable pediatric seizures (pilot study). Epilepsy Res 93: 39-43. [Crossref]
- Shawki M, Wakeel EL, Shatla R, El-Saeed G, Ibrahim S, et al. (2013) The clinical outcome of adjuvant therapy with black seed oil on intractable pediatric seizures: a pilot study. Epileptic Disord 15: 295-301. [Crossref]
- Hendre AS, Shariff AK, Patil SR, Durgawale PP, Sontakke AV, et al. (2013) Evaluation of oxidative stress and anti-oxidant status in essential hypertension. J Indian Med Assoc 111: 377-378.
- Azzubaidi MS, Saxena AK, Talib NA, Ahmad QU (2011) Mnemonic effects of fixed oil of black cumin seeds (Nigella sativa) on aged rats with memory impairment. In: Malaysia Australia Research Colloquium on Exercise, Nutrition, Health and Wellness.
- Jalali MR, Roghani M (2009) The effect of Nigella sativa on learning and memory in male diabetic rats. Basic and Clinical Neurosciences 1: 32-34.
- Bin Sayed MS, Asaduzzaman MD, Morshed H, Hossain MM, Kadir MF, et al. (2013) The effect of Nigella sativa Linn seed on memory, attention and cognition in healthy human volunteers. J Ethnopharmacol 148: 780-786. [Crossref]
- Bin Sayeed MS, Shams T, Fahim Hossain S, Rahman MR, Mostofa A, et al. (2014) Nigella sativa L. seeds modulate mood, anxiety and cognition in healthy adolescent males. J Ethnopharmacol 152: 156-162.
- Gheita TA, Kenawy SA (2012) Effectiveness of Nigella sativa oil in the management of rheumatoid arthritis patients: A placebo-controlled study. Phytotherap Res 26: 1246-1248. [Crossref]
- Hadi V, Kheirouri S, Alizadeh M, Khabbazi A, Hosseini H (2016) Effects of Nigella sativa oil extract on inflammatory cytokine response and oxidative stress status in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled clinical trial. Avicenna J Phytomed 6: 34-43.
- Kheirouri S, Hadi V, Alizadeh M (2016) Immunomodulatory effect of Nigella sativa oil on T lymphocytes in patients with rheumatoid arthritis. Immunol Invest 45: 271-283. [Crossref]
- Kapoor S (2009) Emerging clinical and therapeutic applications of Nigella sativa in gastroenterology. World J Gastroenterology 15: 2170-2171.
- Hosseinzadeh H, Fazly Bazzaz BS, Motevaly-Haghi M (2007) Antibacterial activity of total extracts and essential oil of Nigella sativa L. seeds in mice. Pharmacol Online 2: 429-435.
- Forouzanfar F, Bazzaz BSF, Hosseinzadeh H (2014) Black cumin (Nigella sativa) and its constituent (thymoquinone): a review on antimicrobial effects. Iran J Basic Med Sci 201417: 929-938.
- O’Mahony R, Al-Khtheeri H, Weerasekera D, Fernando N, Vaira D, et al. (2005) Bactericidal and anti-adhesive properties of culinary and medicinal plants against Helicobacter pylori. World J Gastroenterol 11: 7499-7507.
- Bakal SN, Bereswill S, Heimesaat MM (2017) Finding Novel Antibiotic Substances from Medicinal Plants - Antimicrobial Properties of Nigella Sativa Directed against Multidrug-resistant Bacteria. Eur J Microbiol Immunol (Bp) 7: 92-98. [Crossref]
- Salem EM, Yar T, Bamosa AO, Al-Quorain A, Yasaway MI, et al. (2010) Comparative study of Nigella sativa and triple therapy in eradication of Helicobacter pylori in patients with non-ulcer dyspepsia. Saudi J Gastroenterol 16: 207-214. [Crossref]
- Hashem-Dabaghian F, Agha S, Taghavi-Shirazi M, Ghobado A (2016) Combination of Nigella sativa and honey in eradication of gastric Helicobacter pylori infection. Iran Red Crescent Med J 18: e23771. [Crossref]
- Mohtashami R, Huseini HF, Heydari M, Amini M, Sadeghi Z, et al. (2015) Efficacy and safety of honey-based formulation of Nigella sativa seed oil in functional dyspepsia: A double blind randomized controlled clinical trial. J Ethnopharmacol 175: 147-152. [Crossref]
- Johnson DA (2018) With Three Guidelines on H. Pylori, What Should Clinicians Do Differently? Medscape.
- Barakat EMF, El-Wakeel LM, Hagag RS (2013) Effects of Nigella sativa on outcome of hepatitis C in Egypt. World J of Gastroenterol 19: 2529-2536. [Crossref]
- Yousefi M, Barikbin B, Kamalinejad M, Abolhasani E, Ebadi A, et al. (2013) Comparison of therapeutic effect of topical Nigella sativa with betamethasone and Eucerin in hand eczema. J Europ Acad Dermatol Venereol 27: 1498-1504. [Crossref]
- Smith RI, Pruthi S, Fitzpatrick LA (2004) Evaluation and management of breast pain. Mayo Clinic Proc 79: 353-372.
- Huseini HF, Kianbakht S, Mirshamsi MH, Zarch AB (2016) Effectiveness of topical Nigella sativa seed oil in the treatment of cyclic mastalgia: A randomized, triple-blind, active, and placebo-controlled clinical trial. Planta Med 82: 285-288. [Crossref]
- Rhee TG, Westberg SM, Harris IM (20118) Use of complementary and alternative medicine in older adult in diabetes care. Diabetes Care 41: 95-96. [Crossref]
- Mostafa AGM, Hossain MK, Basak D, Bin Sayeed MS (2017) Thymoquinone as a potential therapy for cancer treatment: Evidence from preclinical studies. Frontiers Pharmacol 8: 295. [Crossref]
- Rahmani AH, Alzohairy MA, Khan MA, Salah M, Aly SM (2014) Therapeutic implications of black seed and its constituent thymoquinone in the prevention of cancer through inactivation and activation of molecular pathways. Evid Based Complement Alternat Med 1: 724658. [Crossref]


