Abstract
Objectives: Suprasellar meningiomas are generally a difficult tumors to treat with a mortality rate as high as 20% to 67% [1], and the lack of postoperative improvement in vision has often been disappointing. The early diagnosis and treatment of suprasellar meningiomas remain a challenge to the neurosurgeon due to the size of these tumors, their proximity to the optic apparatus and major vessels and their vascularity. The onset of visual disturbance is very common and might be very severe. The goal of this study is to assess the outcome and prognostic factors of suprasellar meningiomas
Patients and methods: Data was collected from all patients presented at Hôpital de spécialité Rabat, with suprasellar meningiomas over a 13 years period (between Jan. 2003, and Jan 2016). Age, gender, medical history, Glasgow Coma Scale (GCS) score , symptoms and visual field defects upon initial presentation, neurological examination, CT scan findings, MRI findings, hospital admission and length of stay, blood test, preoperative medication , surgical intervention and type of surgery, grade resection, functional dependency at discharge as per Karnofsky Performance Scale Index (functionally able to carry on normal activity > 70; functionally independent = 100%), Histopathology, morbidity, mortality and follow up results were all collected from medical files.
Results: 42 patients having suprasellar meningiomas were included. The most affected age group was between 40 and 60 with a female predominance of 71.4%.
The most common complaint at the presentation was visual impairment, which was seen in 36 patients (85.71%). Neuroradiological assessment showed Calcifications in 14 cases (33.33%). Bone changes in 7 cases (16.66%), and perilesional edema in 22 cases (52.38%).
The rate of morbidity was 44.73 %, and the postoperative mortality was 9.52%. The poor prognostic factors found in this study was tumor size>4.5cm with p value 0.006, as duration of symptoms more than 1 year, as well as perilesional edema with p value 0.0053. Postoperative vision improvement was noticed in 13 cases (44.82%), no change was found in 11 cases (37.93%), and deterioration was seen in 4 cases (13,79%).
Conclusion: Visual impairment is frequently the most common complaint because of proximity of suprasellar meningiomas to the optic apparatus. Tumor size has been found to play a role as a prognostic factor in term of post-operative visual impairment. Tumor larger than 4.5cm, duration of symptoms more than one-year, perilesional edema has been a significant influential factor on the post-operative mortality.
Introduction
As a result of the advent of cranial imaging with computed tomography (CT) and magnetic resonance imaging (MRI), detection and diagnosis of intracranial tumors increased upon the years. However, the early diagnosis and treatment of some kind of tumors, especially suprasellar meningiomas remain always a challenge.
Suprasellar meningiomas are tumors arising from the base of the skull near the pituitary gland and the optic nerve. One reason these tumors are difficult to diagnose at an early stage, is the paucity of symptoms associated with small tumors. Thus, early diagnosis of suprasellar meningiomas continues to be a problem. As these tumors are near the optic apparatus and major neurovascular vessels, this has led many to investigate the prognostic factors as well as the best surgical approach, however, results have been always diverging.
Material and methods
The present study is a retrospective analysis of surgical outcomes in 42 patients having suprasellar meningiomas admitted at the department of Neurosurgery, Mohamed Vth University, School of Medicine, Hôpital des Spécialités, ONO CHU Ibn Sina, Rabat, over a period of 13 years (between Jan. 2003, and Jan 2016). This study received full approval by the institutional research Ethics of the faculty of medicine and pharmacy Rabat.
The criteria used for suprasellar meningioma diagnosis was based on MRI and especially on intraoperative findings. Only cases with tumor arising from the tuberculum sellae, planum sphenoidale and diaphragma sellae were included, Tumors arising lateral to the anterior clinoid processes or from the olfactory grooves with secondary extension to the suprasellar area were excluded. The following data were collected from file records: age, gender, medical history, Glasgow Coma Scale (GCS) score , symptoms and visual field defects upon initial presentation, neurological examination, CT scan findings, MRI findings, hospital admission and length of stay, blood test, preoperative medication , surgical intervention and type of surgery, grade resection, functional dependency at discharge as per Karnofsky Performance Scale Index (functionally dependent = > 70. ; functionally independent = 100 , see annex), Histopathology, morbidity, mortality and follow up.
Follow up results were gathered by finding the information on the data files, or reaching out to patients, either by phone calls or meet them in person.
Continuous variables were analyzed using the T-test. Significance was defined as a p value of less than 0.05.
Results
Epidemiology
Of the 42 patients, 30 (71.40%) were female and 12 (28.60 %) were male (Figure 1). The youngest patient was a 6-years old female and the oldest was a 65-year-old woman. Most patients were between 40 and 60 years of age, and females predominated in all age groups.
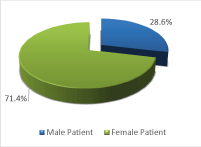
Figure 1: Sex distribution in 42 patients
Symptoms
The most common complaint upon presentation was visual impairment, which was present in 36 patients (85.71%) (Table 1).
Table 1: Suprasellar meningiomas symptoms upon presentation
Symptoms |
cases |
|
N |
Percent % |
visual loss |
36 |
85.71 |
Headaches |
34 |
80.95 |
Vomiting |
14 |
33.33 |
Memory disorder |
7 |
16.66 |
Concentration disorder |
4 |
9.52 |
Behavior Disorder |
5 |
11.9 |
Motor Deficit |
4 |
9.52 |
seizure |
4 |
9.52 |
Endocrinological signs |
1 |
2.38 |
Proptosis |
2 |
4.76 |
Aphasia or disarthia |
1 |
2.38 |
endocrine dysfunction |
1 |
2.38 |
Smell loss |
10 |
23.8 |
Headache was the next most common symptom in 34 cases (80.95%). Frontal headache was most common and was seen in 31 patients, temporal headache was present in 2 patients, and one patient had generalized headaches.
Mental changes were present in 16 cases (38.09%). 7 patients complained of memory impairment, 4 patients were having concentration impairment, and 5 patients had behavior changes. Motor deficits were noted in 4 patients (9.52%). 14 cases had vomiting (33.33%), 10 patients complained of hyposmia and anosmia (23.8%), and 4 suffered from epilepsy (9.52%). Endocrine dysfunction was recorded in only 1 patient having diaphragma sellae which presented as a galactorrhea. 2 patients had exophthalmos and 1 patient presented with dysarthria. Foster-Kenedy syndrome was found in 2 patients having planum sphenoidale meningiomas.
Physical examination
Visual acuity was decreased in 36 cases:
- 20 cases were found to have binocular visual loss (55.55%), 4 cases between 3-9/10 on examination (11.11%) and 12 cases had a preblindness (33.33%).
- 16 cases had monocular impairment.
Other signs are mentioned in (table 2), including optic atrophy presented in 6 of 36 patients whose funduscopic findings were recorded, papillary edema in 9 patients and papillary pallor in 9 patients as well, 2 patients had unilateral papillary edema and contralateral optic atrophy, 2 patients had unilateral papillary palor and contralateral optic atrophy as well. while 8 patients had normal optic discs.
Table 2: CT scan and MRI features of suprasellar meningiomas in our series
|
cases |
|
N |
Percent % |
CT scan |
hyperdense |
30 |
71.42 |
hypodense |
1 |
2.85 |
isodense |
11 |
26.19 |
Calcification |
14 |
33.33 |
Perilesional edema |
22 |
52.38 |
|
Bone reaction |
7 |
16.66 |
MRI |
T1 |
Iso |
24 |
68.57 |
Hypo |
10 |
28.57 |
Hyper |
1 |
2.8 |
T2 |
Iso |
7 |
2 |
Hypo |
4 |
11.42 |
Hyper |
24 |
68.57 |
Mean size |
3.58cm |
In neurological examination of cranial nerves, 10 patients were found to have a smell disorder, 3 patients had 3rd cranial nerve impairment, one patient with 4th cranial nerve deficit and one patient with 6th cranial nerve impairment.
Diagnostic investigations
The neuroradiological assessment included a CT head scan, an MRI as well as Cerebral angiography.
Computerized tomography (CT) scanning, both with and without contrast material was performed in all cases, In 30 cases (71.42%), hyperdensity mass was detected in the suprasellar area which strongly enhanced on administration of contrast. 11 tumors were isointense. Calcifications were seen in 14 cases (33.33%). Bone changes was detected in 7 cases (16.66%), while perilesional edema was seen in 22 cases (52.38%) (Figure 2).
Figure 2: MRI of planum sphenoidale meningioma in a patient of 45 age years old admitted at Hôpital Des Spécialités Rabat
MRI examination was performed in all patients; however, the details of the MRI results were found in only 35 patients data. The tumor was mainly isointense in 24 cases (68.57%) in T1 and mainly hyperintense in 23 cases (68.57%) in T2. The mean size was 3.58cm.
The use of routine laboratory studies, including complete blood count, coagulation studies, electrolytes, endocrinology evaluation was performed in all the patients. Laboratory testing for endocrine disorders were performed to assess for hormonal abnormalities related to pituitary stalk effect or hypothalamic compression. Workup included evaluation of thyroid function (thyroid stimulating hormone [TSH] and free T4), growth hormone function (somatomedin-c), serum prolactin, random or morning cortisol level.
MR Angiography was done in 16 cases and abnormalities were noted in all the cases. Arterial displacement was the most common findings, this was present in nearly all the cases and the anterior cerebral complex was the most commonly involved (Table 2).
Treatment
Several days prior to surgery, all our patients were given dexamethasone in order to reduce and prevent cerebral swelling, it was used at a loading dose of 2 mg/kg per day divided into 3 doses, it was maintained for at least 5 days with gradual regression and stopped on the 7th day. Antibiotic prophylaxis was administered systematically before incision in all patients. Surgery was carried out under general endotracheal anesthesia with controlled respiration
A supine position with slight rotation and light extension of the head was the usual position. The head placed on the Mayfield head holder.
Surgical management and approach were depended on location and extension of the meningioma.
A subfrontal osteoplastic bone flap was used in 19 cases: unilateral in 17 cases and bilateral in 2 cases; a frontotemporal approach was used in 22 cases and an interhemispheric approach was used in only one case.
These tumors were generally firm in consistency. Small tumors were removed with sharp dissection using bipolar coagulation. For large tumors we have used, first, suction, small loops, or fragmentation with a hook or small rongeurs, and once the tumor was adequately debulked and the capsule sufficiently thinned, we dissect the tumor away from the optic nerves, chiasm, and regional blood vessels.
The dural attachment was coagulated in 35 cases (83.33%) (Table 3).
Table 3: Surgical approaches used in our series
|
PS |
TS |
DS |
subfrontal |
Unilateral |
15 (44.11) |
2 (40) |
0 |
Bilateral |
2 (5.88%) |
0 |
0 |
Frontotemporal |
16 (47.05) |
3 (60) |
3 (100) |
interhemispheric |
1 (2.94) |
0 |
0 |
The tumor was attached to the tuberculum sellae in 5 cases, 34 patients had tumor attachment to the planum sphenoidale. The diaphragma sellae was involved in 3 cases.
After surgery, the patients were closely monitored, Antiepileptic medication was continued during the perioperative period in patients with a known history of seizures (Table 4).
Table 4: Grade resection during suprasellar meningiomas surgery
|
PS
Cases (%) |
TS
Cases (%) |
DS
Cases (%) |
Grade I |
- |
- |
- |
Grade II |
28 (82.35) |
4 (80) |
3 (100) |
Grade III |
1 (2.9) |
1 (25) |
- |
Grade IV |
5 (14.70) |
- |
- |
Preventive anticoagulant therapy was postoperatively given in patients with or without a risk of developing thromboembolic disease, using calcium heparin or low molecular weight heparin.
patients were carefully observed for signs of diabetes insipidus or inappropriate secretion of antidiuretic hormone (SIADH). Methylprednisolone was usually tapered over several days.
Histopathological results were obtained in 40 cases, in which transitional meningiomas were the most common result found, and 3 cases had a Grade II meningioma, including 2 cases of invasive meningothelial meningiomas and one case of clear cell meningioma.
Short term results
Mortality
Among the 42 operated patients, 4 patients died within one month, for a mortality rate of 9.52%, all of them had a planum sphenoidale meningiomas. One of these patients had a pneumonia after surgery, another patient had pneumonia and meningitis, with an extradural hematoma, the third patient had meningitis and the remaining patient had bradycardia and cardiac arrest.
Of the patients followed up after the discharge, one patient died one year after the surgery, the reason was a suicide post-depression after a remaining blindness in both eyes according to his father.
Morbidity
Morbidity following surgery was common, patients who died after surgery were excluded. Diabetes insipidus was seen in 4 cases, complete anosmia was present in 4 cases, Cerebrospinal fluid leak developed in 2 cases, which stopped spontaneously.
Electrolyte disturbance occurred in one case, including hyponatremia and hypokalemia. And one case of significant papiloedema occurred after surgery. Postoperative meningitis occurred in 4 cases and empyema in one case requiring another surgery for drainage (Table 5).
Table 5: Postsurgery morbidity in our series
Morbidity |
cases |
Percent% |
Diabetes insipidus |
4 |
10.52 |
anosmia |
4 |
10.52 |
Cerebrospinal fluid leak |
2 |
3.26 |
meningitis |
4 |
10.52 |
empyema |
1 |
2.63 |
Electrolyte disturbance |
1 |
2.63 |
Significant papiloedema |
1 |
2.63 |
Neurological outcome
Visual outcome: 3 months post-operative
Visual function outcome was found in 29 files, improvement was noticed in 13 cases (44,82%), no change was found in 11 cases (37.93%), and deterioration was seen in 4 cases (13,79%). All patients with unchanged or worsened visual outcome have had visual symptoms of duration > 1year.
Long term results
Of the 38 cases who survived after the surgery, 7 were lost to the follow-up review within 6 months of operation, 6 other patients were lost to follow up visit in more after 5 years. The ranging extremes follow-up period was from one year and a half to 13 years, with a mean follow-up of 5 years.
The good outcome after surgery is defined by a normal state of patient or the presence of minor sequelae allowing him/her to carry on normal activities. The Karnofsky Performance Scale Index allows patients to be classified as to their functional impairment. This can be used to compare effectiveness of different therapies and to assess the prognosis in individual patients. The lower the Karnofsky score, the worse the survival for most serious illnesses.
In 6months, 11 patients had KS<70% (35.48%) while 20 patients had a KS>70% (64.51%).
Recurrence is defined as clinical or neuroradiological evidence of disease progression. It had occurred in 4 patients, all with planum sphenoidale meningiomas, one of these patients had a partial resection (Simpson grade IV) and 3 recurrence occurred even with a Simpson grade II resection. One of them had a radiosurgery and one of them required another surgery which was a complete resection and had as a post-operative incident a diabetes insipidus.
Case in our series
45 YO, Female. complained from headaches and visual acuity near blind at left eye which pushed her into investigation (Figure 3).

Figure 3: Sagittal view of preoperative suprasellar meningioma in 45 YO female patient admitted in hospital de specialité Rabat
Surgery excision was Simpson grade II of a tuberculum sella meningioma with extension int the left optic canal (Figures 4,5).
Figure 4: Coronal view of suprasellar meningioma in 45 year old female patient.

Figure 5:Postoperative MRI of suprasellar meningioma
Prognosis:
To have a better understanding of the factors that could play a role on the mortality rate, we calculated the p Value of preoperative karnovsky scale, the tumor size, perilesional edema, histopathology as well as the location (Table 6).
Table 6: Different features analyzed in our series
Features analyzed |
N total patients
(%) |
P value |
Karnovsky scale<70
|
2
(4.76) |
0,86 |
Tumor size>4.5cm
|
9
(21.42) |
0,006 |
perilesional edema
|
22
(52.23) |
0.0053 |
Histopathology
( Grade II ) |
3
(71.42) |
0.16 |
Location |
- |
0,70 |
Duration of symptoms > 1 year |
10
(23,80) |
0.005 |
Discussion
Classification
Suprasellar meningiomas usually arise in the midline from the region of the tuberculum sellae and planum sphenoidale, but they can arise from the diaphragm sellae, or be located primarily to one side, arising from the anterior clinoid region. (Table 7) shows some classifications of the suprasellar meningiomas.
Table 7: Suprasellar classifications
Authors |
Classification |
LINDSAY SYMON, F.R.C.S., AND JACOB ROSENSTEIN, M.D.
1984 [1]
|
tuberculum sellae, planum sphenoidale diaphragma sellae, and/or anterior clinoid processes. |
BRIAN T. ANDREWS, M.D., AND CHARLES B. WILSON, M.D.
1988 [2] |
tuberculum sellae, planum sphenoidale, diaphragma sellae, and anterior clinoid processes |
ABDULRAZAG M. AJLAN
2015 [3] |
tuberculum sellae, planum sphenoidale, diaphragma sellae, |
OUR CLASSIFICATION |
tuberculum sellae, planum sphenoidale, diaphragma sellae, |
Age and gender
Several studies have demonstrated that among patients who sustained a suprasellar meningiomas, female gender was predominant up to 80% with a mean age of 50 years old [1,3,4] (Table 8).
Table 8 : Age and gender distribution of suprasellar meningiomas
Authors |
Male |
Female |
Range |
LINDSAY SYMON, F.R.C.S., AND JACOB ROSENSTEIN
1984 [1] |
32.7 % |
67.3% |
Most patients between 40-60yrs |
BRIAN T. ANDREWS, M.D., AND CHARLES B. WILSON,
1988 [2] |
26% |
74% |
56 years (range 21 to 84 years)
Most patients between 40-60yrs |
Chuan-Wei Wang1 Ying-Yu Li and associates
2011 [4] |
20% |
80% |
51 years (ranging from 23 to 75 years) |
Our series
|
28.6%
|
71.4%
|
Most patients between 40-60yrs
|
Clinical presentation
Tuberculum sellae
TSM comprise approximately 3%–10% of all intracranial meningiomas [5], with a rate of 5% of skull base meningiomas, anterior and middle fossa in our study. These tumors raise generally in a suprasellar subchiasmal midline position, and they characteristically shift the optic chiasm posteriorly and slightly superiorly, and the optic nerves laterally [5].
More than 95% of patients suffer visual acuity and approximately 75 to 90% have optic atrophy [6, 7, 8, 9]. The same finding was obtained in our study from the fundus examination, that has identified the optic atrophy as the most common result. The pattern of vision loss can vary, and although a bitemporal hemianopsia has been described as the most frequent field cut observed [6, 8].
Planum sphenoidale
Planum sphenoidale meningiomas are closely related to tumors of the tuberculum sellae, but their clinical presentations and surgical outcomes are very different. Given their proximity to the optic chiasm, tuberculum sellae tumors can manifest early, and visual deficits can be present even when the lesions are small. However, this is not always applied for planum sphenoidal meningiomas, which symptoms occur in most of the cases once the tumors reach significant size. In series reported by Hentschel and DeMonte [5] more than 50% of patients had tumors larger than 6 cm.
Diaphragmae sellae
Preoperative differentiation between tuberculum and diaphragma sellae meningiomas may be difficult to make based on clinical features or neuroimaging findings, and diagnosis is made intraoperatively when dural attachment to the diaphragma is visualized. Diaphragma sellae meningiomas are generaly less common than TS meningiomas [10-12], in a series of 67 suprasellar meningiomas, 25 were classified as TS meningiomas and only one as a DS meningioma [11]. In our sudy of 42 suprasellar menigiomas, 5 were TS meningiomas (11.90%) and only 3 were having diaphragma sellae meningioma (7.14%) based on the intraoperative findings.
Several studies have included diaphragma sellae meningiomas with TS meningiomas in their series, Even though they have different sites of origin that can be distinguished anatomically []10,11,13]. The reason behind this, it the differentiation difficulty encountered because of the similarity in clinical presentation and anatomical adjacency [14, 12].
Surgery
For several studies, skull base navigation, using CT/MRI fusion was found to provide a valuable intraoperative information and it was considered to be important to use especially for suprasellar meningiomas
During surgery, neuronavigation can be used before and after dural opening to frontal sinus identification, in order to determine tumor relationship with midline, optic nerves and internal carotid arteries. Thus, the relevant anatomy may be marked in surgical planning.
Three different approaches have been described for the suprasellar meningioma, subfrontal, bifrontal and pterional approach.
For most patients, a subfronal exposure elevating the frontal lobe just in front of the sphenoid wing was the preferred exposure in many studies [15, 16]; a left subfrontal exposure was used when the tumor bulk is greater on that side. For large tumors, a bifrontal exposure was indicated Occasionally, Mac carty [17] and co-workers and Andrews and Wilson have also used the right subfrontal approach unless the visual loss is greater on the left side. Kempe and gristoli and associates [8] have also described this approach. logue and simon use a unilateral right subfrontal exposure but approach the tumor along the midline. In large tumors, macCarty and colleagues and al-Mefty and associates [10] have used bifrontal craniotomy.
Diaphragma sellae meningiomas have been grouped into 3 types by Al-Mefty, Type A, originating from the upper leaf of the diaphragma sellae anterior to the pituitary stalk; Type B, originating from the upper leaf of the diaphragma sellae posterior to the pituitary stalk; and Type C, originating from the inferior leaf of the diaphragma sellae [18].
Surgical approaches for these tumors included the cranio-orbital approach for Types A and B and the transcranial-transsphenoidal approach for Type C. Surgery was described as more difficult than for tuberculum sellae meningiomas because of the deep location and the difficulty of dissecting Types A and B from the pituitary stalk. Repair of the sphenoid sinus to prevent cerebrospinal fluid leakage is mandatory for Type C tumors
For tuberculum sellae meningiomas, the superior interhemispheric approach t has been found to decrease surgical injury to the olfactory tractus and to be more effective in treating the visual deterioration without inducing injury on the brain surface in contact with the medial surface of the frontal lobe [18] [19], this was explained by the minimal unilateral retraction of the frontal lobe and avoidance of elevation from the orbital roof in comparison with the subfrontal approaches. The upward retraction of the frontal lobe determines the risk of sectioning, avulsion, or forming an ischemic lesion on the olfactory nerves, and must be limited to 1.5 cm based on Cardali and Al [20, 21, 22]. Moreover, the trajectory of the superior interhemispheric approach is located far away from the olfactory tractus because the trajectory line is located under the genu of the corpus callosum which decreased injury risk of olfactory tractus as well as surgical risk to the microvasculature [18].
Some disadvantages of the interhemispheric approach were reported and were essentially related to the initial dissection of the interhemispheric fissure. The injury to the venous drainage system such as a thrombosis of the superior sagittal sinus or the bridging veins may occur, especially at the beginning of the intervention. [19].
Several studies have demonstrated the benefits of EET for meningiomas arising from diaphragma and tuberculum sellae regions. However, many recent studies failed to show the advantages of EET versus transcranial approaches, especially regarding the postoperative vision outcome. What was described about EET, is the possibility to have lower morbidity rate with incomplete resection tumor compared to transcranial approaches [3].
According to a recent studies , a patient with a tumor <2 cm, without or with minimal extension into the optic canals as well as minimal vascular encasement in the absence of luminal narrowing, can have an endoscopic transsphenoidal resection, whereas a patient with brain invasion, >180 vascular encasement, size more than 4 cm, and severe optic canal invasion should undergo a transcranial approach as a better approach [23]. This is also what De Divitiis, Cappabianca et al reported [13]
Morbidity
Preservation of vision is the most important goal of surgery and improvement in vision has been reported in 40–80% of patients [23, 24, 25, 26]. Nakamura et al. reported 72 cases of tuberculum sellae meningiomas where total resection was achieved in 91.7% of the patients; Postoperative visual improvement was seen in 65% of the patients [27]. Visual improvement was found to be dependent on the duration of preoperative visual symptoms, but not on the preoperative visual acuity or tumor size. Patients with visual symptoms of a duration <6 months tended to recover more often than those in which the duration of symptoms was longer than 7 months Zevgaridis et al. [27].
Our findings were consistent with the other studies; all patients with unchanged or worsened visual outcome have had visual symptoms of duration > 1year with a p=0.005. The possible reason already described is the compressive mechanical injury which can lead to small vessel compromise and demyelination, especially in patients with a long duration of visual decrease before surgery
In Pamir et al. study [28], peritumoral edema had a significantly worse visual outcome. It was correlated with a higher proliferative index and a higher angiogenetic activity [28]. Thus, it has been suggested that the visual impairement might be related to a higher growth rate and accelerated compression nerve process. We in our study too found similar results with a p value 0.0053
In the cohort series by Pamir et al. [28] and Zevgaridis et al. [26] have demonstrated that vision outcome was significantly better with an intact arachnoid plane than those, in whom no plane was identified. Early identification and preservation of the arachnoid anatomy facilitates easy dissection of the tumor from its surroundings and optic and chiasmatic vessels. Another factor found in the literature was the arterial encasement by the tumor that was having a direct impact on the complete resectability of the tumor, which in turn has significant influence on the visual outcome [31].
Another important factor is the optic canal decompression which was discussed in a new study, and described as a key factor in determining visual outcome. In most studies, especially those that reported postoperative visual deterioration, intradural optic canal decompression was performed selectively at a late stage of surgery, not preceding tumor resection, and in some cases, the optic canal was not decompressed at all. Some authors have suggested that early extradural optic canal decompression might be associated with better visual outcome, by reducing tension on the optic nerve during tumor resection [32,22].
Larger tumors appeared to be associated more frequently with postoperative visual deterioration [2]. Symon and Rosenstein reported that 68 % of their patients had postoperative complications. This may have been related to the deep and prolonged frontal lobe retraction often required for this type of operation [2].
In our study, vision deterioration was found in 6% (n=2/34) of planum sphenoidal meningiomas, 20% (n=1/5) of TSMs, and 33% (n=1/3) of diaphragm sellae meningiomas. The overall rate of worsening vision was found in 4 cases (9,52%). the prognostic factors of vision deterioration have not been included in this study.
Mortality
The mortality rate with these tumors has varied widely in the various series that have been published.
Jane and McKissock reported a mortality rate of 42% in 32 cases with tumors larger than 3 cm in size, although there were no deaths among 17 patients having tumors less than 3 cm. Complete tumor removal was achieved in 59% of cases with small tumors as compared to 50% of cases with large tumors [34].
Kadis, et al. reported 13 deaths among 95 patients with large tumors (> 3 cm), but no deaths were noted in 10 cases with small tumors (< 3 cm). In all cases the mortality was related to the large size of the tumor and encasement of major blood vessels [35]. Solero et al. 19 reporting on 55 cases of suprasellar meningioma, noted a mortality rate of 14.8% in 27 cases with small tumors (< 4 cm) as compared to a mortality rate of 32.1% in 28 cases with large tumors (> 4 cm) [1]. Also, in a recent study done in 2011, One of the 47 patients died after surgery (overall mortality was 2.13%) [3].
In our series, mortality rate was significant in patients having tumors more than 4.5cm (p=0.006) (Table 6).
When duration of symptoms was examined, patients with symptoms for less than 1 year fared better than those with a duration of greater than that, despite equivalent average tumor size and average preoperative visual loss in the two groups. Operative mortality was lower, total removal rate higher, and overall outcome generally better in the short-duration group. There was no difference, however, in the recurrence rate [1].
the effect of microsurgery on surgical outcome was analyzed in literature. Although the fact that Mortality rate was lower, general outcome better, and tumor recurrence rate lower in the group with microsurgery than in the group with classical operative techniques, the beneficial effects on visual outcome couldn’t demonstrate no significant effect. This might be related to irreversible changes induced in the optic nerves and chiasm by long-term standing tumors. Thus, early diagnosis and treatment remains the important factor in the successful management of these difficult cases [10].
The overall morbidity and mortality rates associated with the resection of these tumors have been observed to have decreased substantially in modern series. Total resection can be achieved in 76.4– 93% of cases with an incidence mortality of 0–8.7% [7,23,28-31]. In our series, as mentioned above, mortality rate was 9.52%.
Tumor recurrence in TSMs is still common. One study demonstrated a 39% recurrence rate in a mean follow-up duration of 10.7 years [1]. Patients with tumor recurrence are likely to lose vision in at least one eye and are unlikely to experience visual improvement with subsequent surgery or radiation therapy (Table 9).
Unlike other series reported in literature, no recurrence was found in our patients with TSMs at a mean follow up period of 5years, excluding from these findings the patient who committed suicide at 6 months as he suffered a major depression, due to preblindness status not improved after surgery.
The recurrence rate for planum sphenoidale meningiomas in our series is 7%, even when gross total resection grade II was achieved. Therefore, postoperative patients should undergo long-term, serial clinical and radiological examination to allow early detection and management of recurrence.
Limitations
Some limitations in the current study should be noted. As this study is retrospective, some results as the vision outcome are based on self-reporting and completeness of file medical records.
Conclusion
Suprasellar meningiomas are very challenging tumors due to their proximity to the optic apparatus and major vessels and their vascularity as well as their size, they mostly present with decreased vision. An early consultation would be needed as the postoperative vision outcome depends mainly on the duration of vision deficit and choosing the most appropriate way for the management of these region is very crucial. Continued efforts to refine operative procedures are still required to obtain further satisfactory outcomes. Many factors have been questionable to play a role on the post-operative mortality, which include duration of visual decrease, preoperative condition of optic disc, peritumoral edema, age, severity of visual disturbance, tumor size, location, involvement with optic canal, arachnoid membrane interface and extent of tumor removal. Our series showed that tumor larger than 4.5cm, duration of symptoms more than one-year, perilesional edema have been a significant influential factor, however clinical significances of all these items still need to be testified with more researches.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- Symon L, Rosenstein J (1984) The influence of tumor size, duration of symptoms, and microsurgery on surgical outcome in 101 consecutive cases. J Neurosurg 61: 633-641. [Crossref]
- Andrews BT, Wilson CB (1988) Suprasellar meningiomas: the effect of tumor location on postoperative visual outcome. J Neurosurg 69: 523-528. [Crossref]
- Ajlan AM, Choudhri O, Hwang P, Harsh G (2015) Meningiomas of the Tuberculum and Diaphragma Sellae. J Neurol Surg B Skull Base 76: 74-79. [Crossref]
- Wang CW, Li YY, Zhu SG, Yang Y, Wang HW, et al. (2010) Surgical Management and Evaluation of Prognostic Factors Influencing Postoperative. World Neurosurg 75: 294-302. [Crossref]
- D. F. Hentschel SJ, Olfactory groove meningiomas. Neurosurg Focus, 2003. [Crossref]
- Andrews BT, Wilson CB (1988) the effect of tumor location on postoperative visual outcome. J Neurosurg 69: 523-528 [Crossref]
- Goel A, Muzumdar D, Desai KI (2002) Tuberculum sellae meningioma: a report on management on the basis of a surgical experience with 70 patients. Neurosurgery 51: 1358-1363. [Crossref]
- Grisoli F, Diaz-Vasquez P, Riss M, Vincentelli F, Leclercq TA, et al. (1986) Microsurgical management of tuberculum sellae meningiomas. Re sults in 28 consecutive cases. Surg Neurol 26: 37-44. [Crossref]
- Sklar EM, Schatz NJ, Glaser JS, Sternau L, Seffo F (2000) Optic tract edema in a meningioma of the tuberculum sellae. AJNR Am J Neuroradiol 21: 1661-1663. [Crossref]
- Al-Mefty O, Holoubi A, Rifai A, Fox JL (1985) Microsurgical removal of suprasellar meningiomas. Neurosurgery 16: 364-372. [Crossref]
- Rubin G, Ben David U, Gornish M, Rappaport ZH (1994) Meningiomas of the anterior cranial fossa floor. Review of 67 cases. Acta Neurochir (Wien) 129: 26-30. [Crossref]
- Kinjo T, al-Mefty O, Ciric I (1995) Diaphragma sellae meningiomas. Neurosurgery 36: 1082-1092. [Crossref]
- de Divitiis E, Esposito F, Cappabianca P, Cavallo LM, de Divitiis O, et al. (2008) Endoscopic transnasal resection of anterior cranial fossa meningiomas. Neurosurg Focus 25: E8. [Crossref]
- Kwancharoen R, Blitz AM, Tavares F, Caturegli P, Gallia GL, et al. (2014) Clinical features of sellar and suprasellar meningiomas. Pituitary 17: 342-348. [Crossref]
- Ojemann R (1980) meningiomas of the basal parapituitary region: technical considergations. clin Neurosurg [Crossref]
- R. a. s. K. Ojemann (1988) Surgical management of olfactory groove, suprasellar and medial sphenoid wing meningiomas. [Crossref]
- C. D. E. M. MacCartey (1982) meningeal tumors of the brain. in Youmans, J.R. (ed): Neurosurgical surgery, ed. 2. Philadelphia, W.B. Saundres [Crossref]
- Kinjo T, al-Mefty O, Ciric I (1995) Diaphragma Sellae Meningiomas Clinical Study. Neurosurgery 36: 1082-1092. [Crossref]
- Curey S, Derrey S, Hannequin P, Hannequin D, Fréger P, et al. (2012) Validation of the superior interhemispheric approach for tuberculum sellae meningioma. J Neurosurg 117: 1013-1021. [Crossref]
- Curey S, Derrey S, Hannequin P, Hannequin D, Fréger P, et al. (2012) Validation of the superior interhemispheric approach for tuberculum sellae meningioma. J Neurosurg 117: 1013-1021. [Crossref]
- Cardali S, Romano A, Angileri FF, Conti A, La Torre D, et al. (2005) Microsurgical anatomic features of the olfactory nerve: relevance to olfaction preservation in the pterional approach. Neurosurgery 57: 17-21. [Crossref]
- Cömert A, Uğur HC, Kahiloğullar G, Cömert E, Elhan A, et al. (2011) Microsurgical anatomy for intraoperative preservation of the olfactory bulb and tract. J Craniofac Surg 22: 1080-1082. [Crossref]
- Dare AO, Balos LL, Grand W (2001) Olfaction preservation in anterior cranial base approaches: an anatomic study. Neurosurgery 48: 1142-1145. [Crossref]
- Dhandapani S, Negm HM, Cohen S, Anand VK, Schwartz TH (2015) Endonasal Endoscopic Transsphenoidal Resection of Tuberculum Sella Meningioma with Anterior Cerebral Artery Encasement. Cureus 7: e311. [Crossref]
- Fahlbusch R, Schott W (2002) Pterional surgery of meningiomas of the tuberculum sellae and planum sphenoidale: surgical results with special consideration of ophthalmological and endocrinological outcomes. J Neurosurg 96: 235-243. [Crossref]
- Nozaki K, Kikuta K, Takagi Y, Mineharu Y, Takahashi JA, et al. (2008) Effect of early optic canal unroofing on the outcome of visual functions in surgery for meningiomas of the tuberculum sellae and planum sphenoidale. Neurosurgery 62: 839-844. [Crossref]
- Schick U, Hassler W (2005) Surgical management of tuberculum sellae meningiomas: involvement of the optic canal and visual outcome. J Neurol Neurosurg Psychiatry 76: 977-983. [Crossref]
- Zevgaridis D, Medele RJ, Müller A, Hischa AC, Steiger HJ, et al. (2001) Meningiomas of the sellar region presenting with visual impairment: impact of various prognostic factors on surgical outcome in 62 patients. Acta Neurochir (Wien) 143: 471-476. [Crossref]
- Nakamura M, Roser F, Struck M, Vorkapic P, Samii M (2006) Tuberculum sellae meningiomas: clinical outcome considering different surgical approaches. Neurosurgery 59: 1019-1028. [Crossref]
- Pamir MN, Ozduman K, Belirgen M, Kilic T, Ozek MM (2005) Outcome determinants of pterional surgery for tuberculum sellae meningiomas. Acta Neurochir (Wien) 147: 1121-1130. [Crossref]
- Palani A, Panigrahi MK, Purohit AK (2012) Tuberculum sellae meningiomas: A series of 41 cases; surgical and ophthalmological outcomes with proposal of a new prognostic scoring system. J Neurosci Rural Pract
3: 286-293. [Crossref]
- Lehmberg J, Krieg SM, Mueller B, Meyer B (2013) Impact of anterior clinoidectomy on visual function after resection of meningiomas in and around the optic canal. Acta Neurochir (Wien) 155: 1293-1299. [Crossref]
- Unteroberdörster M, Müller O, Özkan N, Pierscianek D, Hadamitzky M, et al. (2017) Impact of optic canal decompression on visual outcome in subtotal resected skull base meningiomas. J Neurosurg Sci 64: 440-445. [Crossref]
- JANE JA, McKISSOCK W (1962) Importance of failing vision in early diagnosis of suprasellar meningiomas. Br J Med 2: 5-7. [Crossref]
- Chan RC, Thompson GB (1984) Morbidity, mortality, and quality of life following surgery for intracranial meningiomas. J Neurosurg 60: 52-60. [Crossref]
- Arai H, Sato K, Okuda, Miyajima M, Hishii M, et al. (2000) Transcranial transsphenoidal approach for tuberculum sellae meningiomas. Acta Neurochir (Wien) 142: 751-756. [Crossref]
- Jallo GI, Benjamin V (2002) Tuberculum sellae meningiomas microsurgical anatomy and surgical technique. Neurosurgery 51: 1432-1439. [Crossref]
- Schick U, Hassler W (2005) Surgical management of tuberculum sellae meningiomas: involvement of the optic canal and visual outcome. J Neurol Neurosurg Psychiatry 76: 977-983. [Crossref]
- Bonnal J, Thibaut A, Brotchi J, Born J (1980) Invading meningiomas of the sphenoid ridge. J Neurosurg 53: 587-599. [Crossref]
- F. A. J. R. M. LINDSAY SYMON (1984) J Neurosurg 61: 633-641. [Crossref]
- Turel MK, Tsermoulas G, Reddy D, Andrade-Barazarte H, Zadeh G, et al. (2016) Endonasal endoscopic transsphenoidal excision of tuberculum sellae meningiomas: a systematic review. J Neurosurg Sci 60: 463-475. [Crossref]
- Douglas Fox et . V. G. K. a. R. F. S (2009) Olfactory Groove/Planum Sphenoidale. Meningiomas. [Crossref]
- Rangel-Castilla L, Russin JJ, Spetzler RF (2014) Surgical management of skull base tumors. Rep Pract Oncol Radiother 21: 325-335. [Crossref]





