Palliative anticancer drug therapy is often performed for unresectable, advanced-stage oesophageal cancer. Patients with poor general condition may not be able to undergo high-dose cisplatin plus fluorouracil (5-FU) therapy due to a high-risk of adverse events. Herein, we report a patient with advanced oesophageal carcinoma and poor general condition, accompanied by severe liver injury due to metastasis, who received palliative chemoradiotherapy. The administration of high-dose cisplatin was not found to be beneficial, and the treatment regimen was switched to low-dose nedaplatin and 5-FU therapy. Subsequently, there was a significant reduction in the liver metastasis that resulted in an improvement of the hepatic function; this allowed the patient to receive outpatient anticancer treatment. Even after the patient became resistant to the primary treatment, changing anticancer drugs allowed treatment to continue, and as a result we were able to prolong the patient’s life by approximately 600 days. This therapy with low-dose nedaplatin resulted in an improvement in the general condition and long-term survival of the patient; additionally, the drug has low nephrotoxicity. Hence, it may be considered a significant advancement in the treatment of unresectable, advanced-stage oesophageal cancer.
chemoradiotherapy, liver metastasis, low-dose nedaplatin, oesophageal cancer
Approximately 90% of oesophageal cancers reported in Japan are squamous cell carcinomas, and chemotherapy using cisplatin and fluorouracil (5-FU) has been established as the standard treatment regimen [1]. Although multidisciplinary treatment combining surgery, radiotherapy, and anticancer drug therapy is performed for up to stage III oesophageal cancer, and since curative surgery and chemoradiotherapy is not possible for patients with distant metastases to other organs, anticancer drug therapy is generally recommended. The Guideline for Diagnosis and Treatment of Carcinoma of the Esophagus (2017) in Japan [1] proposes palliative treatments such as chemoradiotherapy, or bypass surgery, including oesophageal stenting and enterostomy, or gastrostomy.
The NCCN guidelines (2018) [2] also recommend anticancer drug therapy if the patient has a good overall condition with a performance status (PS) of less than 2, and palliative treatment for patients with PS of 3 or higher and a poor general condition. With respect to anticancer drug therapy, the NCCN guidelines recommend FP therapy (cisplatin 75–100 mg/m2 d1; 5-FU 750–1,000 mg/m2 CIV(continuous intravenous infusion) d1–4; repeating every 3–4 weeks; category 1) [3]; in Japan, the usefulness of FP therapy (cisplatin 70 mg/m2 d1; 5-FU 700 mg/m2 CIV d1–5; repeating every 4 weeks) in cases with distant metastasis has been proven [4-6]. Nedaplatin is a third-generation platinum drug developed in Japan for the purpose of alleviating nephrotoxicity caused by cisplatin [7-9], and its dose-limiting toxicity is myelotoxicity. It is characterized by needing almost no moisture loading, but due to a lack of adequate clinical trial results, a standard treatment regimen has not been established.
Herein, we report the case of an oesophageal carcinoma patient with poor general condition, multiple liver metastases, and severe liver dysfunction, who showed a complete response to chemoradiotherapy using low-dose nedaplatin and 5-FU.
A 64-year-old male with a complaint of epigastralgia and difficulty swallowing for about 3 months presented at a local clinic. He had a history of ureteral stones and percutaneous coronary vasodilation for coronary stenosis approximately 10 years and 5 years ago, respectively. An upper gastrointestinal endoscopy was performed on the day of presentation to the clinic, and the patient was diagnosed with oesophageal cancer. In addition, an abdominal ultrasonography revealed multiple liver tumours, and the patient was referred to our hospital for examination and treatment.
Upon examination at our hospital the patient had the following clinical features: height, 167.5 cm, body weight, 73.0 kg, blood pressure, 124/80 mmHg, body temperature, 36.2℃, heart rate, 80 bpm, no abnormalities of the chest, and tenderness when the enlarged liver in the upper midline of the abdomen was palpated with 4-finger breadths.
The upper gastrointestinal endoscopy identified a type 2 tumour in the lower thoracic oesophagus (Figure 1). The biopsy-based diagnosis according to the patient’s previous physician was low to moderately differentiated squamous cell carcinoma (Figure 2). An oesophageal ultrasonic endoscopy did not find any evidence of infiltration in the aorta. A contrast-enhanced, chest computed tomography (CT) scan found no evidence of regional lymph node metastasis (Figure 3a). However, both large and small nodular shadows were observed in both lobes of the liver. The S1 mass excluded the inferior vena cava.
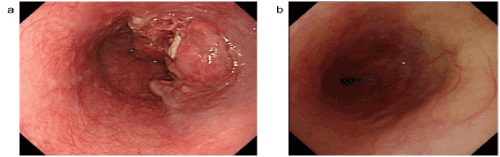
Figure 1. Upper gastrointestinal endoscopy examination
a) Before treatment: type 2 tumour identified in the lower oesophagus
b) Day 405 of disease after treatment: the primary lesion has scaring, with no relapse identified
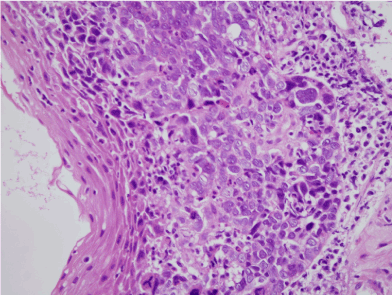
Figure 2. Almost no keratinization was identified, exhibiting a clinical presentation of lowly to moderately differentiated squamous cell carcinoma (Haematoxylin and eosin staining, x400 magnification)
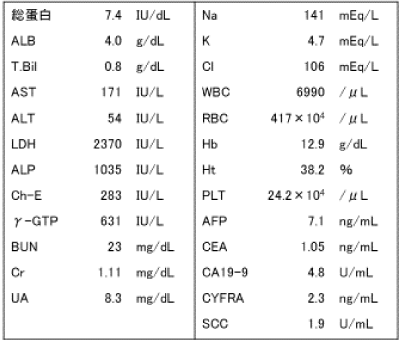
A haematological examination found no rise in total bilirubin, but both lactate dehydrogenase and alkaline phosphatase levels were abnormally high, at 2,370 IU/L and 1,035 IU/L, respectively. Serum creatinine was moderately high at 1.11 mg/dL (Table 1).
Table 1. Haematological findings at the time of hospitalization
After obtaining informed consent from the patient, low-dose nedaplatin plus 5-FU therapy (nedaplatin at 10 mg/body/day IV(intravenous drip) d1–5, 8–2, and 15–19; 5-FU at 500 mg/body/day CIV d1–5, 8–12, and 15–19; total regimen repeated every 4 weeks) [9,10], as well as simultaneous palliative radiation therapy at 40 Gy of total dose, delivered in 20 fractions, was initiated. After the start of treatment, the blood biochemistry examination on day 15 of the disease tended to improve, as shown in figure 4, and the CT scan taken on day 27 (Figure 3b) revealed a partial response (PR) to treatment. Even though the patient was scheduled to start the second course of treatment on day 29, this was interrupted due to the development of a grade 2 decline in platelet counts (56,000/μL). Upon resuming treatment, the patient requested a shortened hospitalization period and refused the administration of anticancer drugs on consecutive days. For this reason, the patient was switched to a nedaplatin plus 5-FU therapy regimen (nedaplatin at 50 mg/m2 IV d1; 5-FU at 500 mg/m2 CIV d1–5, repeated every 2 to 3 weeks) [10], beginning on day 49, and the patient was discharged on day 56. Using an infuser pump during outpatient visits, the patient underwent a total of 7 courses of the new chemotherapy regimen by day 245. The CT scan taken on day 179 showed the largest treatment effect (Figure 3c), with the original liver enlargement almost completely gone with normal biochemical test findings (Figure 4). Despite maintaining a PR thereafter, we identified the re-enlargement of the metastatic liver tumours according to a CT scan on day 255. Therefore, the patient was switched to 2 courses of docetaxel plus cisplatin therapy (docetaxel at 60 mg/m2 d1; cisplatin at 60 mg/m2 d1; repeated every 3 to 4 weeks) [11], as the tertiary treatment after the determination of progressive disease (PD). A CT scan on day 308 revealed tumour shrinkage and resulted in a determination of PR, but due to general malaise, loss of appetite, and a refusal to undergo in-patient treatment, the patient was switched to 4 courses of docetaxel monotherapy (docetaxel at 70 mg/m2 d1; repeated every 3 weeks) [12] as a quaternary treatment. However, due to the determination of PD by a CT scan performed on day 394, the patient was switched to 5 courses of outpatient vindesine plus nedaplatin chemotherapy (vindesine at 3 mg/m2 d1 and d8; nedaplatin at 90 mg/m2 d1; repeated every 4 weeks) [13] beginning on day 399 as a quinary treatment. However, the patient died on day 611, following rapid enlargement of the liver tumours and cancerous peritonitis. With respect to the primary lesion, the patient had shown a complete response (CR), according to an upper gastrointestinal endoscopy performed on day 76 and had maintained CR, even on an endoscopy examination performed on day 405 (Figure 1b). In addition, the patient was able to consume food orally until right before death.
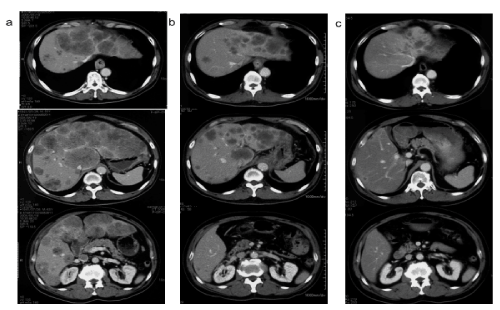
Figure 3. Abdominal contrast-enhanced CT scan
a) Before the start of treatment, multiple metastatic lesions on both sides of the liver are present. Note that there is significant enlargement of the liver and thickening of the oesophageal wall, which is where the primary lesion was located
b) Day 27 of the disease: the tumour shrunk and the liver enlargement was reduced
c) Day 179 of disease: further shrinkage of the tumour
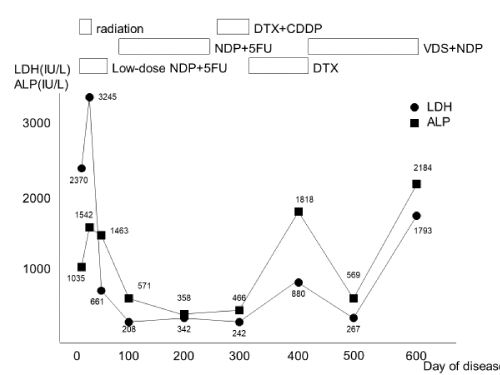
Figure 4. Clinical course treatment was initiated with low-dose nedaplatin plus 5-FU, and the liver function normalized
We reported a case of oesophageal carcinoma having a poor general condition, multiple liver metastases, and severe liver dysfunction, which showed a complete response to chemoradiotherapy using low-dose nedaplatin and 5-FU.
Although this patient had a PS of 1 at the time of hospitalization, he rapidly worsened to a PS of 3 thereafter. The liver tumour size was palpated by a 10-finger breadth at the midline of the abdomen. In addition, the blood biochemistry examination revealed an elevated serum creatinine level of 1.29 mg/dL. Furthermore, because the S1 section of the liver tumour excluded the inferior vena cava and could have caused blood-flow dysfunction through rapid tumour enlargement, we decided that FP therapy with normal doses [3,4] was not possible. It was considered that the exacerbation of PS was very likely due to pain associated with the enlargement of the liver tumour, and we decided to treat this patient with a nedaplatin-concomitant regimen because the initial anticancer treatment, which combined two agents, was expected to provide tumour shrinkage, in addition to the relatively young age of the patient and the fact that he was proactively seeking treatment. Moreover, since obstruction of the oesophagus was the biggest cause of our patient’s discomfort, we decided to provide radiation concurrently.
In general, advanced oesophageal cancer has a poor prognosis. However, with regards to stage IV patients with a good general condition (PS of 0–2), who are able to undergo curative chemoradiotherapy, it has been reported that chemoradiotherapy regimens that combine cisplatin and 5-FU with radiation provide a high response rate of 87%, as well as a cure rate of approximately 20%, which has resulted in this approach being considered the standard treatment for this clinical scenario [6]. However, for cases such as the one reported here with distant metastases and a poor general condition (PS of 3–4), no standard treatment exists.
The Guidelines for the Diagnosis and Treatment of Carcinoma of the Esophagus (2014) [1], NCCN Guidelines (2018) [2], and the European Society of Medical Oncology Guidelines (2016) [14] recommend best supportive care (BSC) or chemotherapy alone for cases with distant metastasis, and radiation and oesophageal stenting for cases with malignant stenosis. However, in recent years, treatments have begun to be selected according to individual cases, and several reviews and reports have indicated the efficacy of chemoradiotherapy [15-18]. In particular, there have been reports of patients with long-term survival who, after receiving palliative radiotherapy, were able to consume food orally over a long-term and maintained their quality-of-life (QOL). Taking the risk of side effects into consideration, palliative radiotherapy is provided at doses between 40 and 50 Gy, which are lower than curative doses since the clinical aim is to preserve QOL [18]. On the other hand, chemotherapy for distant metastasis usually include either single-agent treatments or a combination of 2–3 agents, with the treatment being selected according to the general condition of each individual patient. In general, elderly patients are given single-agent regimens, while patients with a good general condition tend to be treated with combined-agent regimens [19]. With regards to the nephrotoxicity of nedaplatin, there are reports that it is mild, including non-haematological toxicities, according to animal experiments and phase I clinical trials [20,21]. A Grade 2–4 elevation in serum creatinine levels for each organ during phase II trials (100 of mg/m2 d1; repeated every 4 weeks) was 1.5% in lung cancer patients [22], 0.0% in head and neck cancer patients [23], and 3.3% in gastrointestinal cancer patients [24]. However, Grade 3 or higher increases in serum creatinine levels for oesophageal cancer in phase 2 trials (nedaplatin at 90 mg/m2 d1; 5-FU 800 mg/m2 CIV d1-5; repeated every 4 weeks) was 0.0% [25], indicating a low incidence of nephrotoxicity. Although there are no reports that compare cisplatin and nedaplatin directly, nedaplatin is thought to be a drug that suitable in situations where large moisture loading is not possible, such as cases with decreased kidney function, elderly patients, and when cardiovascular complications are present.
In conclusion, we experienced a case of oesophageal carcinoma with multiple liver metastases, where the primary tumour size decreased using a chemotherapy regimen combining low-dose nedaplatin. The patient was able to continue onto another regimen and had the disease progression controlled over a long period. We believe that chemotherapy regimens combining low-dose nedaplatin should be studied in the future, as a safe and effective treatment that can be optimal for patients with a poor general condition.
- Guideline for Diagnosis and Treatment of Carcinoma of the Esophagus 2017, The Japan Esophageal Society, ISBN 978-4-307-20369-2
- NCCN Clinical Practice Guidelines in Oncology. https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf [Accessed on July 1 2018]
- Lorenzen S, Schuster T, Porschen R, Al-Batran SE, Hofheinz R, et al. (2009) Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 20: 1667-1673. [Crossref]
- Iizuka T, Kakegawa T, Ide H, Ando N, Watanabe H, et al. (1992) Phase II evaluation of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japanese Esophageal Oncology Group Trial. Jpn J Clin Oncol 22: 172-176. [Crossref]
- Hayashi K, Ando N, Watanabe H, Ide H, Nagai K, et al. (2001) Phase II evaluation of protracted infusion of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG) Trial (JCOG9407). Jpn J Clin Oncol 31: 419-423. [Crossref]
- Ohtsu A, Boku N, Muro K, Chin K, Muto M, et al. (1999) Definitive Chemoradiotherapy for T4 and/or M1 Lymph Node Squamous Cell Carcinoma of the Esophagus. J Clin Oncol 17: 2915-2921. [Crossref]
- Fukuda M, Shinkai T, Eguchi K, Sasaki Y, Tamura T, et al. (1990) Phase II study of (glycolate-O,O') diammineplatinum(II), a novel platinum complex, in the treatment of non-small-cell lung cancer. Cancer Chemother Pharmacol 26: 393-396. [Crossref]
- Akaza H, Togashi M, Nishio Y, Miki T, Kotake T, et al. (1992) Phase II study of cis-diammine(glycolato)platinum, 254-S, in patients with advanced germ-cell testicular cancer, prostatic cancer, and transitional-cell carcinoma of the urinary tract. 254-S Urological Cancer Study Group. Cancer Chemother Pharmacol 31: 187-192. [Crossref]
- Muro K (2003) A phase II study of nedaplatin and 5-fluorouracil in metastatic squamous cell carcinoma of the esophagus: The Japan Clinical Oncology Group (JCOG) trial (JCOG 9905). The 39 Annual Meeting of the American Society of Clinical Oncology.
- Kaneko K, Ito H, Ito T, Konishi K, Kurahashi T, et al. (2001) A case of esophageal carcinoma with multiple liver metastasis successfully treated with nedaplatine (NDP) and 5-fluorouracil (5-FU) combined with radiotherapy. Gan To Kagaku Ryoho 28: 831-834. [Crossref]
- Park SH, Kang WK, Lee HR, Park J, Lee KE, et al. (2004) Docetaxel plus cisplatin as second-line therapy in metastatic or recurrent advanced gastric cancer progressing on 5-fluorouracil-based regimen. Am J Clin Oncol 27: 477-480. [Crossref]
- Muro K, Hamaguchi T, Ohtsu A, Boku N, Chin K, et al. (2004) phase II study of single-agent docetaxel in patients with metastatic esophageal cancer. Ann Oncol 15: 955-959. [Crossref]
- Yamazaki K, Hironaka S, Boku N, Yasui H, Fukutomi A, et al. (2008) A retrospective study of second-line chemotherapy for unresectable or recurrent squamous cell carcinoma of the esophagus refractory to chemotherapy with 5-fluorouracil plus platinum. Int J Clin Oncol 13: 150-155. [Crossref]
- Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D, et al. (2016) Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 27: v50-v57. [Crossref]
- Harvey JA, Bessell JR, Beller E, Thomas J, Gotley DC, et al. (2004) Chemoradiation therapy is effective for the palliative treatment of malignant dysphagia. Dis Esophagus 17: 260-265. [Crossref]
- Coia LR, Soffen EM, Schultheiss TE, Martin EE, Hanks GE (1993) Swallowing function in patients with esophageal cancer treated with concurrent radiation and chemotherapy. Cancer 15: 281-286. [Crossref]
- Javle M, Ailawadhi S, Yang GY, Nwogu CE, Schiff MD, et al. (2006) Palliation of malignant dysphagia in esophageal cancer: a literature-based review. J Support Oncol 4: 365-373. [Crossref]
- Ahmad NR, Goosenberg EB, Frucht H, Coia LR (1994) Palliative Treatment of Esophageal Cancer. Semin Radiat Oncol 4: 202-214. [Crossref]
- Koshy M, Esiashvilli N, Landry JC, Thomas CR Jr, Matthews RH (2004) Multiple management modalities in esophageal cancer: combined modality management approaches. Oncologist 9: 147-159. [Crossref]
- Sasaki Y, Tamura T, Eguchi K, Shinkai T, Fujiwara Y, et al. (1989) Pharmacokinetics of (glycolate-0,0')-diammine platinum (II), a new platinum derivative, in comparison with cisplatin and carboplatin. Cancer Chemother Pharmacol 23: 243-246. [Crossref]
- Ota K, Wakui A, Majima H, Niitani H, Inuyama Y, et al. (1992) Phase I study of a new platinum complex 254-S, cis-diammine (glycolato)-platinum (II). Gan To Kagaku Ryoho 19: 855-861. [Crossref]
- Furuse K, Fukuoka M, Kurita Y, Ariyoshi Y, Niitani H, et al. (1992) A phase II clinical study of cis-diammine glycolato platinum, 254-S, for primary lung cancer. Gan To Kagaku Ryoho 19: 879-884. [Crossref]
- Inuyama Y, Miyake H, Horiuchi M, Hayasaki K, Komiyama S, et al. (1992) A late phase II clinical study of cis-diammine glycolato platinum, 254-S, for head and neck cancers. Gan To Kagaku Ryoho 19: 871-877. [Crossref]
- Taguchi T, Wakui A, Nabeya K, Kurihara M, Isono K, et al. (1992) A phase II clinical study of cis-diammine glycolato platinum, 254-S, for gastrointestinal cancers. 254-S Gastrointestinal Cancer Study Group. Gan To Kagaku Ryoho 19: 483-488. [Crossref]
- Kato K, Muro K, Ando N, Nishimaki T, Ohtsu A, et al. (2014) A phase II study of nedaplatin and 5-fluorouracil in metastatic squamous cell carcinoma of the esophagus: The Japan Clinical Oncology Group (JCOG) Trial (JCOG 9905-DI). Esophagus. 11: 183-188.





