Abstract
Obesity cardiomyopathy (OCM) is a recently described form of heart muscle disorder. However, with the prevalence of obesity rapidly rising, research interest on its deleterious effect on cardiac morphology and function has been on the increase. However, with morphological and functional features mimicking those of dilated cardiomyopathy, research on OCM has been reduced to a sub-type or a secondary cause of dilated cardiomyopathy (DCM). Considerable debate also persists as to whether isolated obesity can directly cause cardiomyopathy in humans since its effect on cardiac function and structure cannot be isolated from those of comorbidities such as hypertension, dyslipidemia, glucose intolerance and coronary artery disease. With such extraneous factors, research on IOC has been fragmented and at most inconsistent. The purpose of the present review and meta-analysis is to synthesize current scholarly and practitioner understanding of IOC with a focus on broadening the knowledge on diagnosis and clinical management.
Key words
heart failure, morbid obesity, obesity cardiomyopathy.
Introduction
Obesity is fast becoming a major public health burden worldwide. It is a significant risk factor for the development of cardiovascular diseases (CVD) and an important cause of premature death [1]. It has an independent association with the development of heart failure (HF) [2,3] and involved in the development of several comorbid conditions principally atherosclerosis, hypertension and diabetes mellitus (DM) that predispose patients to the development of HF [4]. An extreme consequence of obesity is its involvement in the development of a range of pathological alterations in ventricular morphology and function, a condition clinically termed as obesity cardiomyopathy (OCM). Over the past three decades, the global prevalence of obesity has nearly doubled and the mean body mass index (BMI) has risen by 0.4 kg/m2 [5]. Current projections predict by 2025, the prevalence of obesity will reach epidemic proportions [6]. The deleterious effect of obesity on cardiac performance vis-à-vis its rapidly rising prevalence warrants a precise understanding of its relationship with ventricular structure and function to guide diagnosis and clinical management. Thus, this review article aggregates current research findings and practitioner knowledge on the clinical status of obesity cardiomyopathy including two meta-analyses of its diagnosis and clinical management methods.
Description
Historical context: Despite the recency of increased research interest in the relationship between obesity and cardiac function, description of obesity-associated heart failure in medical literature dates as far back as the late 1700s [7]. The initial mention of excess deposition of fat involving the heart of obese individuals was in 1783. About two decades later (in 1806), adipose tissue surrounding the heart in obese individuals was suspected to oppress the heart leading to sudden death [8]. In the late 19th Century [9,10] and in the early 20th Century [11] research evidence of the deleterious effect of obesity on cardiac function began to accumulate. However, the initial clinical descriptions of a pathologic obesity-associated cardiac morphology and dysfunction appeared in 1933. Saphir and Corrigan [12], and Smith and Willius [13] described the deleterious effect of fatty infiltration or adiposity of the heart on cardiac function and structure.
Since then, several subsequent studies have demonstrated cardiomyopathic processes caused by obesity may involve both the left and right sides of the heart, which occur in the absence of other cardiac or extra cardiac conditions associated with morbid obesity [14-18]. These conditions mainly include systemic hypertension, diabetes mellitus and coronary artery disease (CAD) but which on their own could contribute to cardiac decompensation, and as such, they remain significant risk factors accelerating the development of OCM [18-20]. More recently, the Framingham Heart Study (FHS) further reported obesity is an independent risk factor for the development of HF, demonstrating its deleterious effect on the ventricular function [21,22]. Despite the decades-long accumulated evidence of obesity associated heart failure, the recent research interest on OCM is attributable to the rapidly increasing global prevalence of obesity in the general population. Since the 1933 initial clinical description of OCM, more than 57,000 articles whose titles include the term obesity and CVD have been published in PubMed, with the bulk of the studies published towards the end of the 1990s.
Research on the prevalence of obesity in the general population has been extensive. However, a longitudinal systematic epidemiological study by Ng and associates [23] remains the biggest and most powerful. Spanning over three decades (1980-2013) and recruiting individuals aged between two (2) years and over 80 years old from 188 countries, the study reported the highest prevalence of obesity (defined as BMI ≥ 30 kg/m2) in Oceania, North Africa and the Middle East, which exceeded 50% of the general population. The prevalence was lower but still very high on other parts of the world. In North America, a third of the population was obese while in Western Europe, a fifth of the population was obese [23]. These extremely alarming global levels and trends of obesity in the general population and its harmful effect on many different physical and mental conditions together with its involvement in cardiac diseases explains the renewed research interest in obesity
Classification: Obesity cardiomyopathy has been long recognized as a clinical entity but current morphological-and functional-based classification systems have excluded it as a distinct form of cardiomyopathy. The European Society of Cardiology (ESC) Working Group on Myocardial and Pericardial Diseases [24] does not classify OCM as a type of cardiomyopathy. On the other hand, the 2016 Scientific Statement of the American Heart Association (AHA) classifies OCM as a sub-type of dilated cardiomyopathy (DM) under endocrine or metabolic etiologies of DCM [25]. In support of AHA’s classification, Kasper et al. [26] found a higher incidence of idiopathic DCM (77% versus 36%) among obese patients (mean body weight 130 kg) compared with lean patients (mean body weight 71kg). Several other studies have also demonstrated an association between obesity and idiopathic DCM [27-30] with some reporting a direct toxic effect of obesity on cardiac morphology and function [27,29,30].
Classification of OCM as a sub-type or an etiology of DCM adds to the current considerable debate to the existence of a true obesity-induced cardiomyopathy. Although OCM has been documented in rodent models, it is unclear whether isolated obesity can directly lead to cardiomyopathy in humans. Moreover, majorities of obese individuals have concomitant and likely synergistic risk factors for developing cardiac dysfunction, DCM and heart failure [27] [29,31]. In addition, pure obesity in the absence of hypertension, dyslipidemia, glucose intolerance and CAD is very rare, and therefore, difficult to isolate the deleterious cardiac effects of these comorbid disease entities from that caused exclusively by obesity.
Definition
Morbid/Extreme Obesity: The term obesity defines individuals with a body mass index (BMI) of 30 kg/m2 or higher while overweight defines individuals with BMI of between 25 to 29 kg/m2. Morbid or extreme obesity defines individuals with BMI of 35 kg/m2 or higher [32]. Morbid obese individuals are of particular interest in the study of OCM because of a significantly elevated risk (50 to 100 times) of death from any cause compared to normal-weight individuals as well as are at the greatest risk of developing OCM [20,32].
Obesity Cardiomyopathy: Current definitions of obesity cardiomyopathy (OCM) lack a standardized definition. Contributing to the lack of a definitional uniformity is the ongoing controversy surrounding its recognition as a true cardiomyopathy and its varied terminologies, which include fatty heart, fatty infiltration, lipotoxic cardiomyopathy, cardiac steatosis or adiposity of the heart [12,13,33]. One category of definitions emphasize on the cause of cardiomyopathy, defining OCM as a potentially reversible cardiac condition in which an individual develops a form of non-ischemic DCM secondary to direct toxic effects of lipid accumulation in the myocardial cells [34,35]. The other category of definitions emphasize on pathological structural and functional alterations occurring in the myocardium. In this category, the AHA defines OCM as myocardial disorder characterized by alterations in ventricular morphology and function including left ventricular (LV) dilatation, eccentric or concentric LV hypertrophy, LV systolic and diastolic dysfunction, and right ventricular (RV) dysfunction occurring in the setting of morbid obesity [25]. The 1985 National Institutes of Health Consensus Development Conference [36] and the World Health Organization (WHO) Expert Committee Anthropometric reference data for international use defined OCM as a cardiac condition characterized with clinically demonstrable LV dysfunction not explained by structural heart disease or systemic hypertension [37]. Although the current definitions agree that morbid obesity is the culprit etiology of OCM, the condition lacks pothognomic features to distinguish it from DCM, hence supporting the 2016 AHA guidelines classifying it as a sub-type of DCM.
Effect on cardiac performance: Morbid obesity (defined as BMI > 35 kg/m2) potentially causes a wide range of alterations in cardiac performance including changes in cardiac hemodynamics, and LV morphology and function (Table 1).
Table 1. Impact of Obesity on Hemodynamics and LV Morphology and Function.
Cardiac Function/Structure |
- Description of the Effect of Obesity
|
Hemodynamics |
Increased blood volume |
Increased stroke volume/Work |
Increased arterial pressure |
Increased LV wall stress |
Pulmonary artery hypertension |
Cardiac Structure |
LV concentric remodeling |
LV hypertrophy (eccentric/concentric) |
Left atrial enlargement |
RV hypertrophy |
Cardiac Function |
LV diastolic dysfunction |
LV systolic dysfunction |
RV failure |
Inflammation |
Increased C-reactive protein |
Over-expression of tumor necrosis factors (TNF) |
Neurohumoral |
Insulin resistance and hyperinsulinemia |
Leptin resistance and hyperleptinemia |
Reduced adiponectin |
Sympathetic nervous system over-activation |
Activation of renin-angiotensin-aldosterone system |
Cellular |
Hypertrophy |
Apoptosis |
Fibrosis |
Research interest to clarify the relationship between obesity, and cardiac structure and function began in the 1950s. The initial studies centered on the effect of obesity on cardiac hemodynamics, chamber size, LV wall thickness and mass and ventricular diastolic or systolic function [7]. Recent studies involving animal models expanded the focus to include the effect of obesity-associated neurohumoral activation and metabolic abnormalities on cardiac structure and function [38]. Today, the scientific consensus is that excessive adipose accumulation in myocardial tissues is the primary etiologic agent in the development of OCM through the synergistic effect of five key pathophysiologic mechanisms. (a) Alterations in cardiac hemodynamic, structure and function; (b) metabolic disturbances; (c) myocardial lipotoxicity; (d) neurohumoral derangement (over activation of the renin-angiotensin-aldosterone and sympathetic nervous systems); and (e) small-vessel disease (microangiopathy and endothelial dysfunction) [38-41] (Figure 1).
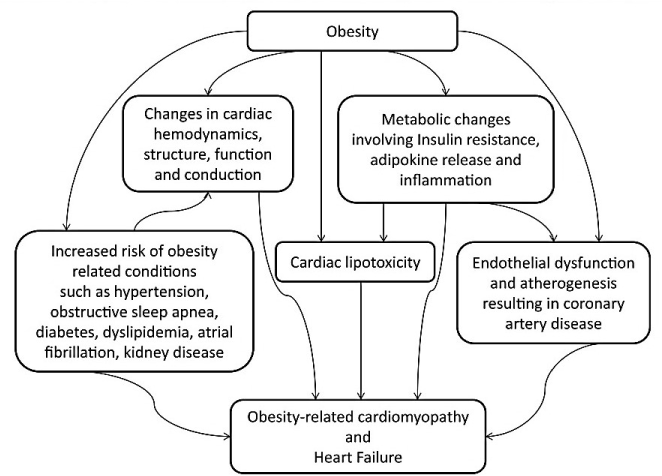
Figure 1. Pathophysiologic Mechanisms of Obesity Cardiomyopathy [39]. In morbid obese patients, cardiomyopathy may result from obesity, which may be potentiated with increased predisposition to other risk factors such as coronary artery disease, diabetes mellitus, hypertension, dyslipidemia, insulin resistance, metabolic syndrome, kidney disease, obstructive sleep apnea and cardiac conduction abnormalities.
Changes in Cardiac Performance: Extensive research evidence has clearly demonstrated an injurious association between adiposity (obesity), and changes cardiac performance (hemodynamics, structure and function) [7,26,43-47]. Systematic involvement of excess adipose accumulation causes alterations in ventricular hemodynamics, structure and function contributing in the pathophysiology of obesity (Figure 2).
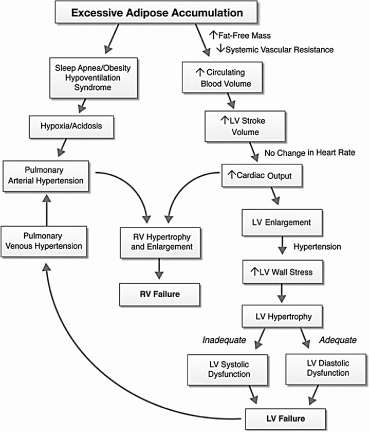
Figure 2. Hemodynamics, Structural and Functional Alterations in Obesity Cardiomyopathy [42]. This diagram schematically represents hemodynamic changes resulting from excessive adipose accumulation in morbid obese patients and their subsequent effect on cardiac morphology and ventricular function and eventually heart failure. LV hypertrophy maybe eccentric in normotensive morbid obese patients or concentric in morbid obese patients with chronic systemic hypertension. Neurohormonal and metabolic abnormalities may contribute to the development of LV failure, and pulmonary arterial hypertension due to sleep apnea/obesity may lead to RV failure.
Hemodynamic Changes: Excessive adipose tissue and increased fat-free mass in obese individuals increases metabolic demands leading to hyperdynamic circulation: increased circulating blood volume (CBV) and increased cardiac output (CO) [48]. The increase in circulating blood volume causes an increase in venous return to the right and left ventricles and a subsequent increase in wall tension and chamber dilatation [49]. In obese individuals, heart rate remains unchanged or is mildly increased but LV stroke volume (volume of blood pumped from the left ventricle per beat) increases relative to the excess body weight leading to increased LV stroke work above that predicted for the ideal body weight [38,50,51]. Obesity widens arteriovenous oxygen difference due to elevated LV pressure and volume, which increases oxygen consumption causing a leftward shift in the Frank-Starling curve [43,44,52]. These changes cause hemodynamic overload, increased LV stroke workload and eventually causes LV failure [50,53]. Obesity increases LV afterload because of increased peripheral vascular resistance and greater aortic stiffness mainly in obese patients with hypertension [48,54]. RV afterload may be increased because of the LV changes, or obstructive sleep apnea (OSA) and/or obesity hypoventilation syndrome (OHS), which may cause hypoxia-induced vasoconstriction and pulmonary arterial hypertension (PAH) [38,54,55].
Myocardial Remodeling: Obesity causes myocardial remodeling (structural changes to the heart), which is a key mechanism in the subsequent development of heart failure [38,56,57]. Cardiac weight and body weight have a positive correlation with morbid obese patients. In chronic obesity, especially occurring concomitant with systemic hypertension, LV hypertrophy and dilatation are common, and to a lesser extent, RV hypertrophy and dilatation may occur [39,58]. Both eccentric and concentric LV hypertrophy occur in obese individuals. The degree of cardiac remodeling correlates with the severity and duration of obesity [53,54]. Obesity (higher BMI) or waist circumference also correlate with higher LV mass compared to age-matched lean individuals [50]. Increased CBV, LV hypertrophy, LV stiffness and LV-end diastolic pressure may cause left atrial (LA) enlargement [39]. Obesity individuals also have excessive epicardial fat, which correlates with visceral adiposity but not with overall adiposity [58]. Obesity-associated epicardial fat extension into the ventricular and atrial myocardium may result in fatty infiltration in the RV and perivascular regions. Myocardial fibrosis occurring concomitant with tissue regeneration and inflammation is common in obese individuals and corresponds with the severity of obesity. It represents an important change in cardiac morphology preceding the development of LV hypertrophy and a key contributor to cardiac dysfunction [7,26].
Ventricular Dysfunction: Excess adipose accumulation in the myocardium has been shown to produce a deleterious effect on diastolic cardiac function. Obesity-associated alterations in LV filling occur in the setting of increased LV mass and abnormal loading conditions, which reduce ventricular compliance [54,59]. Decreased E/A ratio, prolonged isovolumetric relaxation times, decreased mitral annular velocity and myocardial early diastolic velocity are common in obesity [59,60]. The presence of myocardial fat infiltration does not always cause LV systolic dysfunction because it is not always present in obesity. The initial compensatory LV hypertrophy may normalize LV wall stress and preserves systolic function [21,61,62]. However, increased blood volume may lead to LV dilatation, which in turn may reduce systolic contraction because of reduced myofibril shortening [21]. Moreover, chronic obesity and continued increase in LV blood volume may lead to systolic dysfunction accompanied by LV hypertrophy and fibrosis, cardiomyocytes injury and progressive collagen deposition and subsequent diastolic dysfunction. Persisting LV dysfunction may cause pulmonary edema and pulmonary hypertension, eventually leading into RV dysfunction [32] [39]. However, the assessment of RV dysfunction in obesity is challenging because of a high comorbid rate of obstructive sleep apnea and pulmonary hypertension [32].
Metabolic Disturbances
Insulin Resistance: Insulin resistance is an independent predictor of the incidence of heart failure [63]. Since insulin resistance states correlate highly with obesity, it may potentiate the association between obesity and heart failure [64]. Insulin resistance changes myocardial substrate metabolism through an imbalance between free fatty acid (FFA) oxidation (uptake) and use leading to increased myocardial oxygen demand [65,66]. Decreased myocardial oxidative capacity reduces cardiac contractility and efficiency by altering sarcoplasmic reticular calcium stores and promoting mitochondrial dysfunction ultimately leading to cardiac dysfunction and increased susceptibility to pressure overload and ischemic injury [63,66]. Increased LV stroke work due to changes in hemodynamics further stimulates myocardial metabolism setting up a vicious cycle [66]. Stimulated global insulin resistance in obesity could result in hyperinsulinemia and chronic systemic hyperglycemia and hyperglycemia-induced cardiomyocytes injury or glucotoxicity-induced cardiomyocytes apoptosis [64]. Hyperglycemia is also involved in alterations of cardiac structure and function by producing advanced glycated end-products and modifying extracellular matrix (ECM), which alters the expression and function of intra-myocellular calcium channels. Collectively, insulin resistance mediated changes lead to systolic and diastolic dysfunction [64].
Adipokines Release: Adipokines are cytokines (cell signaling proteins) secreted by adipose tissue involved in the regulation of myocardial function. They participate in myocardial metabolism, cardiomyocyte hypertrophy, cardiomyocyte apoptosis, and changes in the structure and composition of the ECM [67]. In vitro and in vivo studies have demonstrated that adipokines directly regulates myocardial remodeling components (matrix metalloproteinase, tissue inhibitor of metalloproteinases and collagens) [67]. Two key adipokines (adiponectin and leptin) are involved in cardiac disease and myocardial remodeling. Adiponectin has cardioprotective effect of decreasing the action of insulin-induced adrenergic receptor-mediated cardiac myocyte hypertrophy. In obese individuals, reduced adiponectin levels and over-expression of tumor necrosis factor (TNF), which suppresses adiponectin, mitigate the cardioprotective effect of adiponectin [68,69]. Leptin on the other hand appear to have an opposite effect to adiponectin. It stimulates cardiac hypertrophy directly through cell signaling mechanisms and indirectly through its effect on hypertension and sympathetic nervous system (SNS) [70,71]. Leptin conveys a cardioprotective effect against ischemic reperfusion injury and limiting cardiac lipotoxicity [72]. Although Shibata et al. [69] reports that obese individuals are insensitive to leptin, it is postulated that, since leptin has an opposite effect to adiponectin, leptin/adiponectin ratio may play a role in myocardial remodeling and heart failure development [72]. The effect of other adipokines such as resistin, apelin, visfatin, vastin, omentin and chemerin on cardiac function and structure is not well-established [72].
Inflammation: Inflammation is involved in the pathophysiology of obesity cardiomyopathy. Obese individuals usually exhibit chronic inflammation with elevated circulating levels of inflammatory markers and increased expression and/or release of inflammation-associated adipokines except adiponectin whose expression is decreased [73]. Interleukin-6, a pro-inflammatory cytokine, modulates hepatic secretion of C-reactive peptide, an inflammatory marker [49]. Interleukin-6, C-reactive peptide and TNF are associated with heart failure and subclinical LV dysfunction. Inflammatory pathways and Interleukin-6 in particular has the strongest prediction for heart failure in obese patients [74]. In addition to adipokines-associated inflammation, FFA in the epicardial adipose tissue may potentiate obesity-associated inflammation by activating inflammatory responses in epicardial macrophages [75]. Chronic inflammation promotes insulin resistance and fibrotic changes in the myocardium, leading to cardiac dysfunction [21,40,75].
Myocardial Lipotoxicity: Myocardial lipotoxicity is the deposition of excess FFA and triglycerides (TG) in parenchymal cardiomyocytes through a process called cardiac steatosis leading to cellular dysfunction and apoptosis and ultimately myocardial dysfunction [40,76,77]. In obese rat models, defects in lipid oxidation (mismatch between FFA uptake and use) causes the accumulation of FFA and TG in cardiomyocytes causing ventricular hypertrophy and dysfunction. In humans, cardiac steatosis correlates positively with BMI and the severity of adiposity due to elevated LV mass and suppressed septal wall thickening [68,78]. Although high myocardial TG content is non-toxic, and may initially provide a buffer against FFA toxic pathways [79], cardiomyocytes have limited storage and shunt excess FFA into non-oxidative pathways resulting into lipotoxicity and apoptosis of lipid-filled cardiomyocytes [49,79,80]. Fat in intervening cells may also produce pressure-induced atrophy of adjacent cardiomyocytes leading to restrictive pattern of cardiomyopathy [49].
Neurohumoral Derangement: Certain obesity-associated neurohumoral derangements (over-activation of the renin-angiotensin-aldosterone system [RAAS] and increased sympathetic nervous system [SNS] tone) have been linked to the pathogenesis of OCM. The two systems (RAAS and SNS) serve as baseline regulatory signaling cascade controlled by negative feedback to preserve normal hemodynamics and facilitate adequate myocardial perfusion [8,32]. Insulin resistance and hyperinsulinemia (excess levels of insulin circulating in the blood relative to the level of glucose) lead to an increase in angiotensinogen, a component of the renin-angiotensin system [21,81]. Elevated levels of angiotensinogen causes an increase in angiotensin II and aldosterone, which potentiate cardiomyocyte growth factors and promote myocardial and perivascular changes leading to ventricular hypertrophy, fibrosis and ultimately cardiac dysfunction [21,38,57]. Continuous sympathetic activation due to cardiac dysfunction increases and impaired signal transduction due to down-regulation of beta-adrenergic receptors and decreased sarcoplasmic reticular calcium ATPase depresses cardiac contractility and leads to further myocardial dysfunction [21,26,50,57].
Small-Vessel Disease: Small vessel disease also contributes to the pathophysiology of OCM. Endothelial dysfunction and inflammation of the small vessel wall are important events in the development of atherosclerosis. Obesity is an independent pathobiological determinant for atherosclerosis and directly contributes to atherogenesis by creating a prothrombotic and proinflammatory state [49,52,82]. Obesity in young adults accelerates the progression of atherosclerosis decades before the appearance of clinical manifestations [52]. Excessive circulating FFA, TG, and low-density lipoprotein cholesterol are risk factors for the development of lipotoxicity, which may cause injury to vascular tissues or alter their functions [83]. Lipotoxicity causes endothelial dysfunction independently or in concert with hyperglycemia, oxidative stress, over-activated RAAS and elevated levels of proinflammatory cytokines [84].
Comorbidities: Besides direct involvement of obesity in the pathophysiology of OCM, it also conveys an indirect involvement. Obesity predisposes to the risk of comorbidities that may potentiate or increase the risk of developing OCM. Hypertension, diabetes mellitus, dyslipidemia, insulin resistance, metabolic syndrome, obstructive sleep apnea and kidney diseases are frequently encountered comorbidities in obese patients [32,39,54]. In the setting of obesity, these comorbidities increase the risk of atherogenesis and heart failure [54]. In addition, obesity hypertension and obstructive sleep apnea are predictors of LV hypertrophy (a risk factor for heart failure) [85], while CAD is involved in the development of LV hypertrophy [86].
Risk factors: Several factors have been identified that increase the risk of developing heart failure in OCM patients. In a study of cardiac structure and LV function in normotensive morbidly obese patients with or without congestive heart failure, Alpert and associates [15] identified five factors that increase the risk of heart failure in morbidly obese patients. (a) The duration of morbid obesity; (b) LV internal dimension in diastole; (c) LV end-systolic wall stress; (d) Left atrial (LA) dimension; and (e) RV internal dimension (Table 2).
Table 2. Risk Factors for Obesity-Related Heart Failure.
Risk Factor |
Significance (p value) |
Duration of morbid obese |
< 0.00000002 |
LV internal dimension in diastole |
< 0.00003 |
LV end systolic wall stress |
< 0.00004 |
Left Atrial dimension |
< 0.000001 |
RV internal dimension |
< 0.00004 |
In subsequent studies, Alpert and associates [15,38] isolated the duration of morbid obesity (BMI > 25 kg/m2) as the strongest risk factor for HF in obese patients. They reported that twenty (20) years of obesity conveyed a 66% risk for developing obesity-related HF, which rose to 95% for 25 years duration of morbid obesity. Moreover, obesity cardiomyopathy is now well recognized in the HF community, accounting for about 11% of HF in men and 14% in women [87]. Duration and severity of adverse loading conditions particularly hypertension increases the risk of LV dysfunction and HF in obese patients [39]. The severity of obesity is another risk factor for HF. After controlling for other risk factors, a unit increase in BMI increase the risk of HF by 5% in men and 7% in women [19]. Visceral fat, insulin resistance and increased age are also important risk factors for the development of obesity-associated heart failure [21,88]. Co-occurring diseases such as CAD, diabetes mellitus, hypertension, obstructive sleep apnea, kidney disease and cardiac conduction abnormalities increase the risk of developing HF [39]. In addition to OCM-specific risk factors, classical risk factors for cardiovascular diseases may increase the risk for HF in obese patients. These factors include smoking, glucose dysmetabolism and sedentary lifestyle. However, the extent these classical risk factors contribute to an independent additional risk among morbid obese patients remains unclear [89,90].
Clinical Presentation: Obesity cardiomyopathy presents with a similar clinical spectrum of HF symptoms to other forms of cardiomyopathies. Frequently encountered symptoms are dyspnea, orthopnea, paroxysmal nocturnal dyspnea, wheezing, exercise intolerance and lower extremity edema [21]. These symptoms are attributable to mechanical consequences of the structural remodeling of the heart [21,26,19,91]. Obese patients may also present with reduced exercise time, functional capacity, larger LV mass, worse symptoms and labile obstructive hemodynamics [92]. Obesity may co-occur with hypertension, heart failure, atrial fibrillation, hypercholesterolemia, coronary artery disease, diabetes mellitus, obstructive sleep apnea, asthma, deep vein thrombosis and a variety of other medical conditions [34].
Clinical Diagnosis: The 2016 AHA Scientific Statement on Diagnostic and Treatment Strategies for specific forms of dilated cardiomyopathy lacks specific diagnostic criteria for OCM. Classified as a sub-type of DCM, the AHA proposes the diagnosis of OCM should be through the exclusion of other causes of DCM. Patients presenting with HF exclusively or predominantly due to obesity are considered to have OCM, usually with a BMI > 40 kg/m2 [93,94]. However, diagnosis of OCM remains challenging since obesity produces a wide spectrum of hemodynamic changes predisposing patients to pathologic alterations in cardiac morphology and ventricular functions including LV dilatation, eccentric or concentric LV hypertrophy, LV systolic and diastolic dysfunction, and RV dysfunction. These changes occur in all classes of obesity but more pronounced in morbidly obese patients [8,95].
Imaging Modalities: Several studies recent have suggested that non-invasive ejection indices such as LV fractional shortening and LV ejection fraction (LVEF) have been used in the assessment of LV function in obesity [95,96,97] [98,99,100,101]. However, even in studies reporting lower indices of LV systolic function in obese than in lean patients, LV ejection phase remained preserved or mildly impaired in a majority of patients with depressed values [8] [51,95]. Fewer studies report supra-normal LV systolic function in obese patients [96,97]. Despite research support for the concept of a true OCM, severe systolic dysfunction occurs infrequent in obesity alone, such that the presence of LV systolic dysfunction should trigger the exclusion of other potential etiologies before it is diagnosed as OCM [8].
On the other hand, LV diastolic filling due to obesity may mimic that of adverse loading conditions that correlate negatively with LV systolic function such as conditions producing increased afterload [96]. Long-standing obesity are associated with greater decrease in LV systolic function but the severity of obesity inversely relates to LV ejection phase [97]. Tissue Doppler Imaging [TDI] and speckle tracking imaging modalities have been recently reported to reveal significantly lower systolic annular velocities in obese patients compared to lean patients with a progressive decrease as severity of obesity increases. The two imaging modalities also reveal abnormal myocardial systolic deformation and abnormal longitudinal strain and strain rate usually with a compensatory increase in radial strain [8] [95,98,99]. Changes in myocardial function detected by TDI and speckle tracking are frequently observed in the absence of clinical HF and with normal LV ejection fraction, suggesting subclinical LV systolic dysfunction is common in obese patients.
Cardiac Biomarkers: In addition to imaging modalities, the AHA recommendations suggest changes in the levels of cardiac markers should provide complementary diagnostic clues. Patients with OCM have depressed levels of B-type Natriuretic Peptide (BNP) and NT-pro B-type Natriuretic Peptide (NT-Pro BNP) compared to lean patients. The levels correlate with the severity of obesity, with levels decreasing with increasing severity. However, the assessment of BNP levels are not confirmatory in the diagnosis of OCM since lower levels of BNP can also occur in both acute and chronic state of heart failure as well as in the setting of decompensated HF and elevated filling pressures [102,103]. Since clinical symptoms of dyspnea and edema are non-specific to HF in obese patients, hemodynamic confirmation is required in obese patients more than in lean patients for confirm clinical diagnosis [25].
Meta-Analysis of Diagnosis Parameters: Diagnosis of OCM has been challenging because of a frequent association with a wide spectrum of ventricular structural and functional changes. This meta-analysis combines findings from current studies on diagnosis of ventricular dysfunction in OCM patients compared to normal (control) patients. The objective is to identify the most frequently used clinical and imaging parameters in the diagnosis of ICM.
Search Strategy: Medical literature was reviewed to identify studies evaluating ventricular dysfunction in obese patients using echocardiographic imaging modalities: conventional and Tissue Doppler Imaging (TDI). A computerized search was performed in PubMed, OVID and EMBASE online databases. Studies were identified using a combination of the following key words: ventricular function, obesity, obese, body mass index (BMI) and echocardiography. Screening of bibliographies from qualifying studies and review articles complemented the online search. The inclusion criteria for studies included: (a) prospective and retrospective trials; (b) used echocardiography imaging for diagnosis of ventricular dysfunction; (c) published outcomes on alterations in both ventricular structure and function; and (d) compared data from obese and controls (lean patients). Data abstracted from the included studies to Microsoft Excel Worksheet included: patient population, mean age, mean BMI, LV structure (LVEDV, LVESV, RWT and LV mass), systolic dysfunction (LV ejection fraction and LV fractional shortening) and diastolic dysfunction (E/A ratio) and IVRT (Table 3).
Table 3. Summary of Findings on Diagnosis of Obesity Cardiomyopathy. (FS: Fractional Shortening: IVRT: Isovolumic Relaxation Time; LVED: LV End-Diastolic; LVEF: LV Ejection Fraction; LVES: LV-End Systolic; E/A: Early Diastolic and Atria Ratios; RWT: Relative Wall Thickness).
1st Author (Year) |
Wong et al. (2004) [59] |
Peterson et al. (2004) [104] |
Pascual et al. (2003) [105] |
Morricone et al. (2002) [106] |
Iacobellis et al. (2002) [107] |
Parameters |
Obese |
Control |
Obese |
Control |
Obese |
Control |
Obese |
Control |
Obese |
Control |
Patient No. |
46 |
33 |
20 |
31 |
48 |
25 |
28 |
18 |
75 |
60 |
Mean Age |
43±10 |
46±10 |
32±4 |
30±5 |
28±10 |
28±8 |
43±11.9 |
46±9.5 |
33±11.9 |
33±13.2 |
BMI (kg/m2) |
46±11 |
23±1 |
37±5 |
24±4 |
40±3.5 |
21±2.2 |
37±5.4 |
22±1.7 |
38±5.5 |
23±1.4 |
LVEDV (ml) |
NS |
NS |
94±17 |
85±17 |
117±31 |
75±22 |
101±27.2 |
87±22.1 |
NS |
NS |
LVESV (ml) |
NS |
NS |
33±10 |
30±8 |
50±18.1 |
29±11.4 |
NS |
NS |
NS |
NS |
RWT |
0.44±0.08 |
0.34±0.07 |
0.40±0.06 |
0.34±0.05 |
0.35±0.08 |
0.37±0.06 |
NS |
NS |
0.35±0.05 |
0.33±0.04 |
LV mass (g) |
229±59 |
137±40 |
161±26 |
128±32 |
NS |
NS |
240±85.3 |
163±37.5 |
167±50.3 |
148±26.5 |
LVEF (%) |
66±6.0 |
66±6.0 |
65±6 |
65±5 |
74±9.1 |
66±12.8 |
62±5.8 |
61±7.3 |
66±7.6 |
61±7.3 |
LV FS (%) |
NS |
NS |
37±5 |
38±5 |
37.5±8.0 |
30.8±7.4 |
37.8±7.3 |
36.1±7.0 |
40±5 |
43±4 |
E/A Ratio |
1.4±0.4 |
1.4±0.4 |
1.76±0.51 |
2.02±0.55 |
1.7±0.6 |
1.7±0.4 |
1.06±0.29 |
1.35±0.30 |
1.38±0.6 |
2.12±0.4 |
IVRT (ms) |
99±18 |
86±15 |
92±13 |
88±11 |
72.0±15.8 |
80±22.1 |
81±17.8 |
76±8.9 |
121±10.4 |
89±8.8 |
Results
Based on the inclusion criteria, five studies [59,104-107] were included in the final review and meta-analysis. Analysis of the included data comprised of 217 obese patients (BMI > 35 kg/m2) and 167 control or lean patients (BMI < 30kg/m2). The mean age was comparable between the two study cohorts – obese (36 years) and control patients (37 years). The obese group had a significantly higher weighted mean BMI of 40 kg/m2, range 37 to 46 kg/m2, while the control group had BMI 23 kg/m2 range 21 to 24 kg/m2. All the five included studies used both conventional echocardiography and Tissue Doppler Imaging (TDI) imaging modalities to assess LV structural changes and LV diastolic/systolic function. The most assessed echocardiographic parameters related to ventricular structure: LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), relative wall thickness (RWT) and LV mass. The most common echocardiographic parameters for assessing alterations in LV systolic function were LV ejection fraction (LVEF) and LV fractional shortening (LVFS) while the most common parameters for assessing changes in LV diastolic function were early diastolic and atria ratios (E/A ratio) and isovolumic relaxation time (IVRT). Parameters such as deceleration time, early diastolic myocardial velocities and late diastolic myocardial velocities, LV end diastolic diameters and left atrial diameter were reported less frequently.
The assessment of obesity-associated changes in LV structure was common in all studies. The mean LV mass was higher in obese patients (199 g, range 161 to 240 g) compared to control (144 g, range 128 to 163 g). LV mass was significantly higher in obese patients in all the five studies. Left ventricular (LV) end-diastolic volume (LVEDV) was also significantly higher in obese patients (104 ml) versus lean patients (82 ml). However, although LVESV was higher in obese patients (42 ml) compared to control (30 ml) the difference was not significant. Differences in RWT between obese patients and control were also not significant. LV ejection fraction (LVEF) and LV fractional shortening (LVFS) were the most common measures of LV systolic function. However, both obese and control patients did not reveal any significant differences in LVEF (67%) compared to control (64%) and LVFS (38% vs. 37%). Altered diastolic function was evident in obese vis-à-vis control patients. The E/A ratio was significantly decreased in obese patients (1.46) compared to control (1.72) while IVRT was significantly prolonged in obese patients (96 ms) compared to control (82 ms).
Morbid obesity-associated pathophysiological changes are injurious to almost all body systems hindering clinical diagnosis and making diagnostic processes challenging. The objective of the present meta-analysis was to describe structural and functional alterations of the LV in morbid obese patients leading to the development of cardiomyopathy. The findings associate morbid obesity with pathological alterations in both LV structure and function. The presence of increased LV mass, end-diastolic and systolic volume (and diameter) suggest enlarged (hypertrophied) LV structure. Decreased E/A ratio and prolonged IVRT are important diagnostic clues for LV diastolic dysfunction common in obese patients. However, LV systolic dysfunction is not always present or easily demonstrable in morbid obese patients. The two common echocardiography indices – LVEF and LVFS – show similar values with non-obese individuals.
Consistent with the present findings, several authors have already reported a significant association between morbid obesity (BMI > 40kg/m2) and echocardiography-defined alterations in LV structure and function predisposing to obesity-associated cardiomyopathy [38,50,53-57]. The chronic nature of obesity supports the use of non-invasive imaging, which is both safe and effective for repeat assessment of LV performance. Since echocardiography is a well-established modality in the assessment of LV function and morphology and readily available, it is the preferred non-invasive imaging modality for assessing LV performance in OCM patients [108]. Several studies have already reported greater incidences of LV hypertrophy (enlargement) and diastolic dysfunction in morbid obese patients. Left ventricular (LV) hypertrophy occurs in between 62% [109] and 82% [110] of morbid obese individuals while diastolic dysfunction is common in between 55% [110] and 60% [111]. However, unlike LV structural alterations and diastolic dysfunction, systolic dysfunction is uncommon affecting fewer morbid obese individuals estimated at between 11% [110] and 25% [111].
The present analysis also reports fewer diagnosis of LV systolic dysfunction. However, LV systolic dysfunction maybe present in the subclinical phase of OCM but not easily demonstrable using conventional echocardiography. In addition, in the early stages of the developing LV dysfunction, compensatory LV hypertrophy modulates LV wall stress and preserves systolic function, which may explain the slightly reduced or normal LVEF and LVFS values in obese patients [21,61,62]. Prolonged morbid obesity with increased circulating blood volume and LV stroke work may reduce or eliminate compensatory effect of LV hypertrophy to cause systolic dysfunction [38,56,57]. Similar echocardiographic changes may also occur in overweight patients (BMI = 25-29 kg/m2) demonstrating subclinical clues of LV diastolic dysfunction even before the development of risk factors such as increased LV mass (hypertrophy) [112].
Clinical management: Obesity cardiomyopathy lacks specific clinical management guidelines. Current treatment protocols target the underlying causes of obesity (weight loss) and heart failure. The 2016 AHA Scientific Statement for Diagnostic and Treatment Strategies for Specific DCM recommends management of obesity and comorbidities such as diabetes mellitus, hypertension and metabolic syndrome. For patients with OCM and overt heart failure, the AHA guidelines recommend the inclusion for conventional treatment for LV systolic dysfunction and symptomatic HF as outlined in the 2013 ACCF/AHA Guidelines for the Management of Heart Failure [113].
Lifestyle Modification: Lifestyle modification in the form of exercise (increased physical activities) and dietary management (caloric restriction) remains the mainstay of chronic treatment for OCM. The therapeutic target is to cause weight loss and reverse obesity-associated hemodynamic and structural abnormalities. Weight loss has favorable clinical effect in preventing the development of comorbidities that potentiate or accelerate the risk of HF as well as producing long-term improvement of cardiovascular function [114]. The largest exercise intervention trial – Heart failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) reported a non-significant trend towards a reduction in mortality or hospitalization and significant improvement in health status. Exercise intervention also caused a slightly higher degree of weight reduction in obese patients compared to lean patients [115]. Although weight loss changes may be minimal, weight reduction changes as low as 7% sustained for a mean of 2.8 years decreases the risk of diabetes mellitus by 58% in patients with impaired glucose tolerance compared with control group under diabetes management program [116]. Exercise intervention that achieves weight reduction reverses the adverse profile of circulating cytokines leading to favorable profile with increased secretion of adiponectin and reduced secretion of TNF [114]. While weight reduction increases plasma adiponectin, exercise intervention in the absence of weight loss improves insulin sensitivity but does not increase adiponectin [114]. Aerobic exercise further contributes to decreasing systolic blood pressure in both hypertensive and normotensive patients. It should be included as a complementary therapy to exercise intervention [116].
Bariatric Surgery: Bariatric surgery refers to all medical procedures utilized to achieve a reduction of excess weight. It is categorized into restrictive, malabsorptive or a combination of the two [117]. The restrictive type refers to surgical reduction of the size of the stomach leading to food intolerance and subsequently weight loss. Malabsorptive type on the other hand refers to operations to bypass segments of bowel to cause malabsorption of nutrients. However, the gold standard of bariatric surgery is a combination of restrictive and malabsorptive surgical procedures [117]. Bariatric surgery is a widely accepted indication for morbidly obese patients with HF and/or clinically pronounced alterations in cardiac hemodynamic and structural or with comorbidities. The primary criteria for indication is the presence of high-risk comorbidities and severe obesity (BMI > 35 kg/m2) [32]. The clinical role of bariatric surgery in OCM patients is a reduction of excess weight and subsequently the risk of cardiac failure (Figure 3).
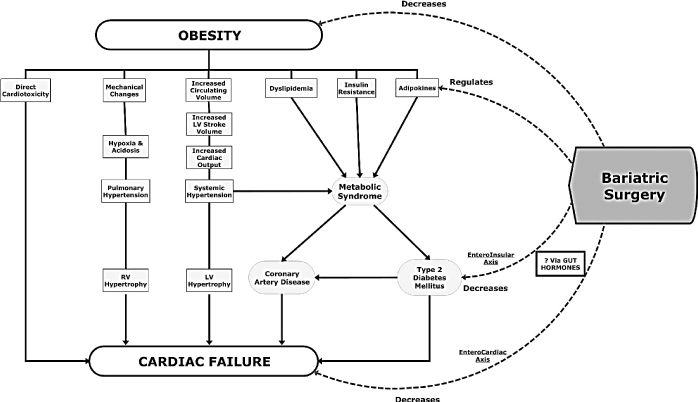
Figure 3. Figure 3: Role of Bariatric Surgery in Management of Obesity Cardiomyopathy [32]. Bariatric surgery causes substantial weight loss to directly decrease obesity and cardiac failure as well as indirectly decrease metabolic syndrome and comorbidities, and reverse ventricular remodelling and dysfunction.
Extensive research evidence has demonstrated the role of bariatric surgery on the management of OCM. The evidence shows bariatric surgery achieves substantial reversal of obesity-associated hemodynamic and structural alterations by leading to a larger magnitude of weight loss compared to exercise or dietary interventions [118-126]. It improves insulin resistance and leads to resolution of diabetes mellitus, improve hyperlipidemia and hypertension and may eliminate obstructive sleep apnea [57,127]. In morbid obese patients with altered ventricular function, bariatric surgery causes a reversal of ventricular remodeling [127,128]. The findings reveal the safety and efficacy of bariatric surgery as a weight loss intervention for OCM patients or a bridge to recovery or to heart transplantation.
Heart Failure Therapy: Lifestyle modification and bariatric surgery targets weight reduction as a prophylactic and curative therapy for OCM. However, for OCM patients having significant LV systolic dysfunction and symptomatic heart failure, conventional heart failure therapy as outlined in the 2013 ACCF/AHA Guidelines for the Management of HF should be considered [113]. The guidelines classified HF in to Stages A to D and recommends specific therapy for each stage, which include conventional HF therapy for OCM patients should include prophylactic (healthy living - targeting weight loss), pharmacological support, device therapy, and heart transplantation (Figure 4).
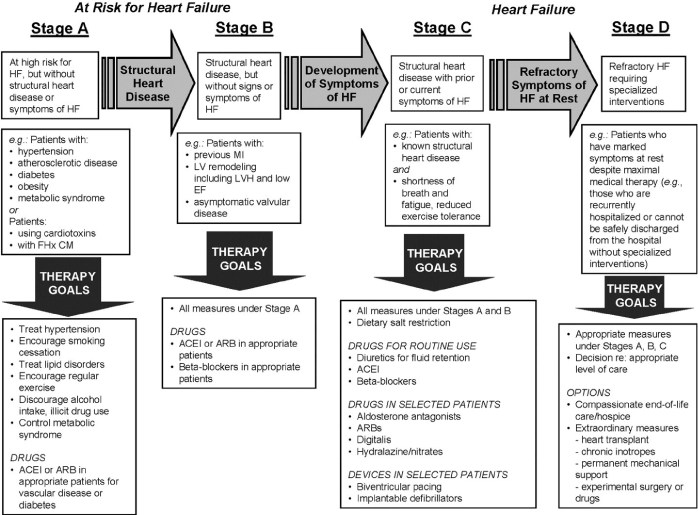
Figure 4. Development Stages of Heart Failure and their Treatment Strategies [113]. The 2013 ACCF/AHA Guidelines for the Management of Heart Failure. HF is classified into Stages A to D, with specific treatment protocols for each stage.
Meta-Analysis of Bariatric Surgery: Weight loss interventions remain the mainstay of the treatment of OCM. The clinical target is to reduce myocardial adiposity and consequently achieve a reversal in ventricular remodeling and minimize the risk of developing comorbidities. Current studies and meta-analysis have shown bariatric surgery achieves a greater weight loss in comparison to non-surgical (dietary or pharmacological) interventions. This meta-analysis goes further to combine recent research findings to determine the efficacy of bariatric surgery in reducing BMI and its effect on cardiac structure and function.
Literature Search: The search for relevant literature on bariatric surgery was performed on PubMed, EMBASE, Ovid and Cochrane online databases. A combination of search terms were used: ‘bariatric surgery’ OR ‘metabolic surgery’ OR ‘weight loss surgery’ OR ‘obesity surgery’ AND ‘echocardiography’ OR ‘magnetic resonance imaging’ OR ‘cardiac imaging’ OR ‘cardiac dimensions’ OR ‘ventricular dimensions’. Criteria for inclusion included studies that: (a) were prospective or retrospective cohort clinical trials; (b) recruited obese patients symptomatic of heart failure; (c) followed patients for at least three (3) months; (d) investigated bariatric surgery; and (e) provided quantifiable outcomes including at least one of the following. Pre and post BMI, pre and post LV function (LVEF), and pre and post LV structure (LV mass index). The exclusion criteria included (a) non-human studies; (b) non-surgical weight loss interventions; (c) case reports; (d) letters and comments; and (e) studies with follow-up of less than one month. There was no restriction on publication language or study population (both children and adults were eligible for inclusion). Additional studies identified from screening bibliographies and review articles on bariatric surgery.
Electronic copies of all articles identified in the online search were reviewed against the inclusion/exclusion criteria. All qualifying studies were then screened to determine they had sufficient data for analysis including weight loss and the effect on cardiac function and structure reported as a mean of the sample size. Selected articles were then abstracted and entered into a data abstraction form to extract all the salient information for analysis. Data abstracted included: (a) first author name; (b) publication year; (c) patient sample size: (d) mean age of recruited patients; (e) percentage of female patients; (f) BMI; (g) LVEF; (h) LV mass index; and mean follow-up period. In the case of more than one follow-up period, the longest period was considered (Table 4).
Table 4. Summary of Findings on Bariatric Surgery in Obesity Cardiomyopathy. (NS: Not Stated; BMI: Body Mass Index; LV: Left Ventricular; LVEF: Left Ventricular Ejection Fraction; F/Up: Follow-up).
1st Author Name [Ref] |
Year |
Study Design |
Patient Sample Size |
Mean Age (yrs.) |
% Female |
BMI (kg/m2) |
LVEF (%) |
LV Mass Index (g) |
Mean F/Up (Months) |
Pre |
Post |
Pre |
Post |
Pre |
Post |
Vest et al. [129] |
2014 |
Retrospective |
42 |
52 |
50 |
48.2 |
35.5 |
36.2 |
41.3 |
NS |
NS |
12 |
Kaier et al. [130] |
2014 |
Prospective |
52 |
44 |
NS |
42.4 |
31.5 |
59.0 |
67.0 |
NS |
NS |
6 |
Graziani et al. [131] |
2013 |
Prospective |
51 |
NS |
NS |
47.9 |
35.7 |
55.9 |
59.2 |
55.3 |
49.4 |
24 |
Damiano et al. [132] |
2012 |
Prospective |
26 |
41 |
50 |
49.7 |
39.9 |
59.0 |
61.0 |
NS |
NS |
8 |
Koshino et al. [133] |
2013 |
Prospective |
28 |
52 |
71 |
51.0 |
37.0 |
62.0 |
62.0 |
NS |
NS |
23 |
Cavarretta et al. [134] |
2013 |
Prospective |
16 |
46 |
75 |
44.8 |
31.2 |
62.1 |
64.5 |
NS |
NS |
16 |
Luaces et al. [135] |
2012 |
Prospective |
41 |
40 |
83 |
47.4 |
30.3 |
65.7 |
65.1 |
NS |
NS |
12 |
Lin et al. [136] |
2011 |
Prospective |
30 |
44 |
100 |
44.0 |
29.0 |
61.0 |
61.0 |
NS |
NS |
3 |
Valezi et al. [137] |
2011 |
Prospective |
43 |
34 |
72 |
41.8 |
28.4 |
70.2 |
72.9 |
NS |
NS |
12 |
Owan et al. [138] |
2011 |
Prospective |
338 |
42 |
81 |
47.9 |
32.2 |
63.0 |
65.0 |
44.0 |
38.0 |
24 |
Hsuan et al. [139] |
2010 |
Prospective |
66 |
31 |
65 |
43.3 |
34.1 |
NS |
NS |
50.0 |
39.0 |
3 |
Algahim et al. [140] |
2010 |
Prospective |
15 |
49 |
100 |
46.7 |
32.0 |
NS |
NS |
50.0 |
37.0 |
24 |
Rider et al. [141] |
2009 |
Prospective |
30 |
44 |
80 |
39.7 |
32.2 |
69.0 |
71.0 |
59.0 |
58.0 |
12 |
Jhaveri et al. [142] |
2009 |
Prospective |
17 |
45 |
100 |
44.1 |
37.1 |
68.1 |
63.0 |
55.0 |
49.0 |
17 |
Ramani et al. [143] |
2008 |
Retrospective |
12 |
41 |
75 |
53.0 |
38.0 |
21.7 |
35.0 |
NS |
NS |
12 |
Ippisch et al. [144] |
2008 |
Prospective |
38 |
16 |
69 |
60.0 |
40.0 |
NS |
NS |
54.0 |
42.0 |
10 |
Di Bello et al. [145] |
2008 |
Prospective |
13 |
31 |
85 |
47.0 |
36.0 |
72.0 |
70.6 |
56.2 |
41.8 |
24 |
McCloskey et al. [146] |
2007 |
Retrospective |
14 |
NS |
29 |
50.8 |
36.8 |
23.0 |
32.0 |
50.8 |
36.8 |
6 |
After reading title, abstract and full article of all qualifying studies, eighteen (18) were identified that fulfilled the inclusion criteria [129-146]. All the studies used non-invasive cardiac imaging methods – echocardiography or cardiac magnetic resonance (CMR) imaging – for prognostication and assessment of treatment efficacy of bariatric surgery on OCM patients. Fifteen (15) of these studies recruited a prospective cohort while three (3) recruited a retrospective cohort. The 18 studies produced a pooled data set of 872 OCM patients, mean age 41 years, range 16 [144] to 52 [129] [133] years. There was a gender bias, with women accounting for 74% of the combined study population. The high participation of females may be attributed to three studies [136,140,142] recruiting exclusively women (100%). The baseline mean BMI for the 872 patients was 47 kg/m2, range 39.7 [141] to 60.0 [144] kg/m2.
Bariatric surgery achieved a significant decrease of weighted BMI mean of 12.7 kg/m2 from 47.0 kg/m2 to 34.3 kg/m2 within a mean of 13.8 months follow-up period. The decrease was consistent in all the 18 studies. Pooled data from nine studies [131,138-142,144-146] demonstrated significant alterations in LV structure (LV reverse remodeling). The absolute LV mass in the nine studies reported a significant decrease in LV weighted mean of 9.3g from 52.7g at baseline (presentation) to 43.4g after 13.8 months follow-up. Systolic function measured using LVEF in 15 studies [129-138,141-143,145,146] demonstrated a slight insignificant increase in weighted mean of 2.9% from 56.5% to 59.4%. However, most of the recruited patients had preserved LV systolic function at baseline (LVEF > 45%) except three studies that recruited patients with significant LV dysfunction (LVEF = 21.7% [143] and LVEF = 23.0% [146] and LVEF = 36.2% [129]).
Overall, the present analysis demonstrates bariatric surgery improves both cardiac structure and function in OCM patients. These changes include statistically significant improvement in LV structure and systolic function. The clinically beneficial effects were demonstrated after significant reductions in weight (BMI reduction by 12.7 kg/m2) and echocardiography and CMR-defined reduction in LV mass (hypertrophy). Although current research on bariatric surgery in morbid obese patients with or without overt heart failure consist of small size studies, the present findings confirm and extend previous evidence of surgical weight loss interventions on LV structure and LV systolic/diastolic function. Studies using CMR imaging demonstrated increased preciseness compared to echocardiography studies but were fewer because of the ready availability and ease of use of echocardiography.
The present results reveal that bariatric surgery for morbid obese patients with cardiomyopathy or overt heart failure are feasible, safe and efficacious. The present findings are consistent and extend those of previous meta-analyses reporting that bariatric surgery achieves significant weight loss in morbid obese patients [147,148]. Another meta-analysis study [149] revealed that bariatric surgery compared with non-surgical weight loss interventions (dietary and pharmacological) achieves a greater weight loss and higher remission rates of Type 2 Diabetes and metabolic syndrome, which are significant risk factors for developing OCM. However, the results on superiority of surgical to non-surgical weight loss interventions were limited to two years follow-up and depended on outcomes of a small number of studies (11 studies) [149]. With increased experience in bariatric surgery, American Society for Bariatric Surgery and the American Society for Bariatric Surgery Foundation report mortality rates as low as 0.5% demonstrating the safety of bariatric surgical procedures [150].
Further, consistent with a review of bariatric surgery in OCM patients, Iyengar and Leier [151] report bariatric surgery causes a reversal in LV remodeling and improves LV function. LV remodeling contributes to the development of LV hypertrophy and LV diastolic dysfunction, which may result in biventricular enlargement and systolic dysfunction. The study also reports bariatric surgery causes a reduction in LV mass and wall stress, improves both diastolic and systolic function and depresses clinical manifestation of heart failure [151]. Further, while the benefits of bariatric surgery on BMI plateaus after sometimes, the effect on metabolism and LV mass remain sustained for longer suggesting it may be mediated by neurohumoral factors. In addition, declining LV mass likely contributes to improved long-term survival of morbid obese patients after bariatric surgery weight loss [148,149]. Moreover, McCloskey et al. [146] report that in addition to the protective effect of bariatric surgery on cardiac morphology and function, it provides a bridge to heart transplantation to patients initially contraindicated due to their morbid obesity.
Morbid obesity is a leading cause of lifestyle related diseases. Its extreme consequence is obesity cardiomyopathy (OCM), a form of non-ischemic dilated cardiomyopathy occurring in the setting of lipid accumulation in the cardiomyocytes causing alterations in cardiac structure and function not explained by structural heart diseases or systemic hypertension. Although recognized as a disease entity, controversy persists as to whether isolated obesity can cause cardiomyopathy. It is difficult to isolate the effect of obesity on cardiac function from that of other concomitant and synergistic comorbidities. Pathophysiologic mechanisms for the development of OCM include changes in cardiac hemodynamics, myocardial remodeling, ventricular dysfunction; metabolic disturbances; myocardial lipotoxicity, neurohumoral derangement; and small vessel disease. The presence of comorbidities such as hypertension, diabetes mellitus, dyslipidemia, insulin resistance, metabolic syndrome, obstructive sleep apnea and kidney diseases may accelerate or potentiate the development of OCM in morbid obese patients. Clinical presentation of OCM mimics that of other forms of cardiomyopathy and heart failure – dyspnea, orthopnea, paroxysmal nocturnal dyspnea, wheezing, exercise intolerance and lower extremity edema. The mainstay of diagnosis is non-invasive imaging using echocardiography and speckle tracking to characterize LV structural and functional changes. The assessment of cardiac biomarkers BNP and NT-Pro BNP provide complementary diagnostic clues but are non-specific to OCM. Finally, the cornerstone of clinical management of OCM is weight loss using exercise interventions or bariatric surgery. For OCM patients with significant LV systolic dysfunction and overt heart failure, management consists treatment of heart failure using conventional heart failure therapy.
References
- Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, et al. (2006) Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. New England Journal of Medicine 355: 763-778.
- Kenchaiah S, Pocock SJ, Wang D, Finn PV, Zornoff LA, et al. (2007) Body mass index and prognosis in patients with chronic heart failure: insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 116: 627-636.
- Kenchaiah S (2009) Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation 119: 44-52. [Crossref]
- Mozaffarian D (2015). Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 131: e29-e322.
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, et al. (2011) Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 377: 557-567.
- Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, et al. (2005) Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: a case-control study. The Lancet 366: 1640-1649. [Crossref]
- Alpert MA (1998) The heart and lung in obesity. Futura Publishing Company.
- Alpert MA (2014). Impact of obesity and weight loss on cardiac performance and morphology in adults. Progress in Cardiovascular Diseases 56: 391-400.
- Oliver T (1880) Post-mortem in a case of extreme obesity. Journal of anatomy and physiology 14: 343-345. [Crossref]
- MacLennan W (1898) On the treatment of obesity and myxoedema by a new preparation of thyroid (“thyroglandin”). British Medical Journal 2: 79.
- Perry AW (1903) Nature and treatment of obesity. California State Journal of Medicine 1: 356-359. [Crossref]
- Saphir O (1933) Fatty infiltration of the myocardium. Archives of Internal Medicine 52: 410-428.
- Smith HL (1933) Adiposity of the heart: A clinical and pathologic study of one hundred and thirty-six obese patients. Archives of Internal Medicine 52: 911-931.
- Alexander JK (1985) The cardiomyopathy of obesity. Progress in cardiovascular diseases 27: 325-334. [Crossref]
- Alpert MA (1997) Cardiac morphology and left ventricular function in normotensive morbidly obese patients with and without congestive heart failure, and effect of weight loss. American Journal of Cardiology 80: 736-740.
- Warnes CA (1984) The heart in massive (more than 300 pounds or 136 kilograms) obesity: analysis of 12 patients studied at necropsy. The American Journal of Cardiology 54: 1087-1091.
- De Divitiis O (1981) Obesity and cardiac function. Circulation 64: 477-482. [Crossref]
- Urbina EM (1995) Effect of body size, ponderosity, and blood pressure on left ventricular growth in children and young adults in the Bogalusa Heart Study. Circulation 91: 2400-2406. [Crossref]
- Kenchaiah S (2002) Obesity and the risk of heart failure. New England Journal of Medicine 347: 305-313. [Crossref]
- Garavaglia GE (1988) Myocardial contractility and left ventricular function in obese patients with essential hypertension. The American journal of cardiology 62: 594-597.
- Cruz CSD (2009) Role of obesity in cardiomyopathy and pulmonary hypertension. Clinics in Chest Medicine 30: 509-523. [Crossref]
- Hubert HB (1983) Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 67: 968-977.
- Ng M, Fleming T, Robinson M, Thomson B (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. The Lancet 384: 766-781.
- Pinto YM (2016). Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. European Heart Journal 37: 1850-1858.
- Bozkurt B (2016) Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation 134: e579-e646.
- Kasper EK (1992) Cardiomyopathy of obesity: a clinicopathologic evaluation of 43 obese patients with heart failure. The American Journal of Cardiology 70: 921-924.
- Szczepaniak LS (2007) Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circulation Research 101: 759-767.
- Zhang Y (2011) Role of cardiac steatosis and lipotoxicity in obesity cardiomyopathy. Hypertension 57: 148-150.
- Mandavia CH (2012) Over-nutrition and metabolic cardiomyopathy. Metabolism-Clinical and Experimental 61: 1205-1210.
- Goldberg IJ (2012) Lipid metabolism and toxicity in the heart. Cell metabolism 15: 805-812. [Crossref]
- Chan WC (2007) Polysarcia adiposa: morbid obesity. The American journal of forensic medicine and pathology 28: 249-254. [Crossref]
- Timoh T (2012) A perspective on obesity cardiomyopathy. Obesity Research and Clinical Practice 6: e181-e188.
- Waxman A (2003) Prevention of chronic diseases: WHO global strategy on diet, physical activity and health. Food and nutrition bulletin 24: 281-284. [Crossref]
- Maloney KF (2013) Obesity and Non-Atherosclerotic Cardiovascular Disease. Academic Forensic Pathology 3: 8-12.
- Aronne LJ (2002) Classification of obesity and assessment of obesity‐related health risks. Obesity: 10(S12). [Crossref]
- Foster WR (1985) National Institutes of Health Consensus Development Conference. Annals of Internal Medicine 103: 1073-1077.
- De Onis M (1996) Anthropometric reference data for international use: recommendations from a World Health Organization Expert Committee. The American Journal of Clinical Nutrition 64: 650-658
- Alpert MA (2001) Obesity cardiomyopathy: Pathophysiology and evolution of the clinical syndrome. The American Journal of the Medical Sciences 321: 225-236.
- Ebong IA (2014) Mechanisms of heart failure in obesity. Obesity Research and Clinical Practice 8: e540-e548.
- Wong C (2007) Obesity cardiomyopathy: Pathogenesis and pathophysiology. Nature Reviews Cardiology, 4: 433-436.
- Ather S (2012) Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. Journal of the American College of Cardiology 59: 998-1005.
- Ortega FB (2016) Obesity and cardiovascular disease. Circulation Research 118: 1752-1770. [Crossref]
- Alexander JK (1962) Blood volume, cardiac output, and distribution of systemic blood flow in extreme obesity. Cardiovascular Research Center Bulletin 1: 39-44.
- Alexander JK (1964) Obesity and cardiac performance. The American Journal of Cardiology14: 860-865. [Crossref]
- Backman L (1973) Cardiovascular function in extreme obesity. Journal of Internal Medicine 193: 437-446. [Crossref]
- De Divitiis O, Fazio SE, Petitto M, (1981) Obesity and cardiac function. Circulation 64: 477-482. [Crossref]
- Alaud-Din A (1990) Assessment of cardiac function in patients who were morbidly obese. Surgery 108: 809-818. [Crossref]
- Vasan RS (2003) Cardiac function and obesity. Heart 89: 1127-1129. [Crossref]
- Poirier P (2006) Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 113: 898-918.
- Alpert MA (2001) Management of obesity cardiomyopathy. The American Journal of the Medical Sciences 321: 237-241. [Crossref]
- Alpert MA (2005) Management of obesity cardiomyopathy. Expert Review of Cardiovascular Therapy 3: 225-230.
- Poirier P (2003) Waist circumference, visceral obesity, and cardiovascular risk. Journal of Cardiopulmonary Rehabilitation and Prevention 23: 61-169.
- Avelar E, Cloward TV (2007) Left ventricular hypertrophy in severe obesity: interactions among blood pressure, nocturnal hypoxemia, and body mass. Hypertension 49: 34-39.
- Kenchaiah S, Gaziano JM, Vasan RS (2004) Impact of obesity on the risk of heart failure and survival after the onset of heart failure. Medical Clinics of North America 88: 1273-1294.
- Eckel RH (1997) Obesity and heart disease: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation 96: 3248-3250.
- Qureshi MY (2012) The relationship of childhood obesity with cardiomyopathy and heart failure. In Pediatric Metabolic Syndrome (pp. 199-215). Springer, London.
- Huffman C (2010, November) Reversible cardiomyopathies: A review. In Transplantation Proceedings, 42: 3673-3678.
2021 Copyright OAT. All rights reserv
- Ashrafian H (2008) Effects of bariatric surgery on cardiovascular function. Circulation 118: 2091-2102.
- Wong CY (2004) Alterations of left ventricular myocardial characteristics associated with obesity. Circulation 110: 3081-3087.
- Berkalp B (1995) Obesity and left ventricular diastolic dysfunction. International Journal of Cardiology 52: 23-26.
- Lavie CJ (1986) Cardiovascular adaptation to obesity and hypertension. Chest 90: 275-279. [Crossref]
- Alexander JK (1993) Obesity and the heart. Heart Disease and Stroke: A Journal for Primary Care Physicians 2: 317-321.
- Ingelsson E (2005) Insulin resistance and risk of congestive heart failure. Jama 294: 334-341. [Crossref]
- Turer AT (2012) Adipose tissue biology and cardiomyopathy: Translational implications. Circulation research 111: 1565-1577.
- Drosatos K, Schulze PC (2013) Cardiac lipotoxicity: molecular pathways and therapeutic implications. Current Heart Failure Reports 10: 109-121.
- Banerjee S (2007) Myocardial metabolism and cardiac performance in obesity and insulin resistance. Current Cardiology Reports 9: 143-149.
- Schram K (2008) Implications of myocardial matrix remodeling by adipokines in obesity-related heart failure. Trends in Cardiovascular Medicine 18: 199-205.
- McGavock JM (2006) Adiposity of the heart, revisited. Annals of Internal Medicine 144: 517-524. [Crossref]
- Shibata R (2004) Adiponectin-mediated modulation of hypertrophic signals in the heart. Nature Medicine 10: 1384. [Crossref]
- Smith CC (2011) Adipocytokines, cardiovascular pathophysiology and myocardial protection. Pharmacology and Therapeutics 129: 206-219.
- Karmazyn M (2007) Leptin as a cardiac hypertrophic factor: a potential target for therapeutics. Trends in Cardiovascular Medicine 17: 206-211.
- Karmazyn M (2008) Signalling mechanisms underlying the metabolic and other effects of adipokines on the heart. Cardiovascular Research 79: 279-286.
- Trayhurn P (2005) Signaling role of adipose tissue: adipokines and inflammation in obesity. Biochemical Society Transactions 33: 1078-1081.
- Bahrami H (2008) Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. Journal of the American College of Cardiology 51: 1775-1783.
- Sacks HS (2011) Human epicardial fat: what is new and what is missing? Clinical and Experimental Pharmacology and Physiology 38: 879-887.
- Iacobellis (2003) Relationship of insulin sensitivity and left ventricular mass in uncomplicated obesity. Obesity 11: 518-524.
- Abel ED (2008) Cardiac remodeling in obesity. Physiological Reviews 88: 389-419. [Crossref]
- Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni‐D'Ambrosia G, Arbique D, et al. (2003) Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magnetic Resonance in Medicine 49: 417-423.
- Ebong IA (2013) Association of lipids with incident heart failure among adults with and without diabetes: the multi-ethnic study of atherosclerosis. Circulation: Heart Failure circheart failure-112.
- Schaffer JE (2003) Lipotoxicity: when tissues overeat. Current opinion in Lipidology 14: 281-287. [Crossref]
- Harte A (2005) Insulin-mediated upregulation of the renin angiotensin system in human subcutaneous adipocytes is reduced by rosiglitazone. Circulation 111: 1954-1961.
- Lavie CJ (2009) Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. Journal of the American College of Cardiology 53: 1925-1932.
- Kim JA, Montagnani M, Chandrasekran S, Quon MJ (2012) Role of lipotoxicity in endothelial dysfunction. Heart Failure Clinics 8: 589-607. [Crossref]
- Symons JD, Abel ED (2013) Lipotoxicity contributes to endothelial dysfunction: a focus on the contribution from ceramide. Reviews in Endocrine and Metabolic Disorders 14: 59-68.
- Gradman AH, Alfayoumi F (2006) From left ventricular hypertrophy to congestive heart failure: management of hypertensive heart disease. Progress in Cardiovascular Diseases 48: 326-341.
- Bauml, M. A., & Underwood, D. A. (2010). Left ventricular hypertrophy: an overlooked cardiovascular risk factor. Cleveland Clinic Journal of Medicine 77: 381-387.
- Glenn DJ (2011) A murine model of isolated cardiac steatosis leads to cardiomyopathy. Hypertension HYPERTENSIONAHA-110.
- Scherer PE (2016) Obesity, diabetes, and cardiovascular diseases: a compendium. Circulation Research 118: 1703–1705
- Van Gaal LF (2006) Mechanisms linking obesity with cardiovascular disease. Nature 444: 875. [Crossref]
- Kim SH (2015) Obesity and cardiovascular disease: friend or foe? European Heart Journal 37: 3560-3568. [Crossref]
- Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB, et al. (1999) Annual deaths attributable to obesity in the United States. JAMA 282: 1530-1538. [Crossref]
- Canepa M (2013) Comparison of clinical presentation, left ventricular morphology, hemodynamics, and exercise tolerance in obese versus nonobese patients with hypertrophic cardiomyopathy. American Journal of Cardiology 112: 1182-1189.
- American Diabetes Association (2013) Standards of medical care in diabetes-2013. Diabetes care, 36(Suppl 1): S11.
- Poirier P, Cornier MA, Mazzone T, Stiles S (2011) Bariatric surgery and cardiovascular risk factors: a scientific statement from the American Heart Association. Circulation 123: 1683-1701.
- Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, et al. (2013) Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail 1: 93-102. [Crossref]
- Alpert MA, Terry B (1993) Factors influencing left ventricular systolic function in nonhypertensive morbidly obese patients, and effect of weight loss induced by gastroplasty. The American Journal of Cardiology 71: 733-737.
- Alpert MA, Lambert CR, Panayiotou H (1995) Relation of duration of morbid obesity to left ventricular mass, systolic function, and diastolic filling, and effect of weight loss. American Journal of Cardiology 76: 1194-1197.
- Tumuklu MM, Etikan I (2007) Effect of obesity on left ventricular structure and myocardial systolic function: assesment by tissue Doppler imaging and strain/strain rate imaging. Echocardiography 24: 802-809.
- Barbosa MM (2011) Strain imaging in morbid obesity: insights into subclinical ventricular dysfunction. Clinical Cardiology 34: 288-293.
- Otto ME, Belohlavek M, Khandheria B, Gilman G, Svatikova A, et al. (2004) Comparison of right and left ventricular function in obese and nonobese men. Am J Cardiol 93: 1569-1572. [Crossref]
- Gulel O (2009) Evaluation of left atrial functions by color tissue Doppler imaging in adults with body mass indexes= 30 kg/m 2 versus those< 30 kg/m 2. The International Journal of Cardiovascular Imaging 25: 371-377.
- Van Veldhuisen DJ (2013) B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. Journal of the American College of Cardiology 61: 1498-1506.
- Christenson RH (2010) Impact of increased body mass index on accuracy of B-type natriuretic peptide (BNP) and N-terminal proBNP for diagnosis of decompensated heart failure and prediction of all-cause mortality. Clinical Chemistry 56: 633-641.
- Peterson LR (2004) Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. Journal of the American College of Cardiology 43: 1399-1404.
- Pascual M, Pascual DA, Soria F, Vicente T, Hernández AM, et al. (2003) Effects of isolated obesity on systolic and diastolic left ventricular function. Heart 89: 1152-1156. [Crossref]
- Morricone L (2002) Echocardiographic abnormalities in normotensive obese patients: Relationship with visceral fat. Obesity 10: 489-498.
- Iacobellis G, Ribaudo MC, Leto G, Zappaterreno A, Vecci E, et al. (2002) Influence of excess fat on cardiac morphology and function: study in uncomplicated obesity. Obes Res 10: 767-773. [Crossref]
- Nakajima TA, Fujioka SI (1985) Noninvasive study of left ventricular performance in obese patients: influence of duration of obesity. Circulation 71: 481-486.
- Catheline JM (2008) Preoperative cardiac and pulmonary assessment in bariatric surgery. Obesity Surgery 18: 271-277.
- Rocha IE (2007) Echocardiography evaluation for asymptomatic patients with severe obesity. Arquivos Brasileiros De Cardiologia 88: 52-58.
- Alawami M, Mustafa A (2013) Echocardiographic and Electrocardiographic Findings in Patients with Obesity Hypoventilation Syndrome. Heart, Lung and Circulation 22: 543-549.
- Russo C (2011) Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. Journal of the American College of Cardiology 57: 1368-1374.
- Yancy CW (2013) 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 62: e147-e239.
- Hamdy O (2005) Lifestyle modification and endothelial function in obese subjects. Expert Review of Cardiovascular Therapy 3: 231-241.
- Whellan DJ (2007) Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. American heart journal 153: 201-211.
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, et al. (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393-403. [Crossref]
- Ashrafian H, le Roux CW, Darzi A, Athanasiou T (2008) Effects of bariatric surgery on cardiovascular function. Circulation 118: 2091-2102. [Crossref]
- Alexander JK, Peterson KL (1972) Cardiovascular effects of weight reduction. Circulation 45: 310-318. [Crossref]
- Backman L (1979) Reversibility of Cardiovascular Changes in Extreme Obesity. Journal of Internal Medicine 205: 367-373.
- Reisin E (1983) Cardiovascular changes after weight reduction in obesity hypertension. Annals of Internal Medicine 98: 315-319.
- Karason K (1997) Effects of obesity and weight loss on left ventricular mass and relative wall thickness: survey and intervention study. British Medical Journal 315: 912-916.
- Jhaveri RR (2009) Cardiac remodeling after substantial weight loss: a prospective cardiac magnetic resonance study after bariatric surgery. Surgery for Obesity and Related Diseases 5: 648-652.
- Algahim MF (2010) Progressive regression of left ventricular hypertrophy two years after bariatric surgery. The American journal of medicine 123: 549-555.
- De las Fuentes L (2009) Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. Journal of the American College of Cardiology 54: 2376-2381.
- Rider OJ (2009) Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. Journal of the American College of Cardiology 54: 718-726.
- Hsuan CF (2010) The effect of surgical weight reduction on left ventricular structure and function in severe obesity. Obesity 18: 1188-1193.
- Alsabrook GD (2006) Gastric bypass for morbidly obese patients with established cardiac disease. Obesity surgery 16: 1272-1277.
- Ashrafian H (2008) Effects of bariatric surgery on cardiovascular function. Circulation 118: 2091-2102.
- Vest AR (2016) Clinical and echocardiographic outcomes after bariatric surgery in obese patients with left ventricular systolic dysfunction. Circulation: Heart Failure 9: e002260.
- Kaier TE (2014) Ventricular remodelling post-bariatric surgery: is the type of surgery relevant? A prospective study with 3D speckle tracking. European Heart Journal–Cardiovascular Imaging 15: 1256-1262.
- Graziani F (2013) Effects of bariatric surgery on cardiac remodeling: clinical and pathophysiologic implications. European Heart Journal, 34(suppl_1).
- Damiano S (2012) Effect of bariatric surgery on left ventricular geometry and function in severe obesity. Obesity Research and Clinical Practice 6: e189-e196.
- Koshino Y (2013) Changes in myocardial mechanics in patients with obesity following major weight loss after bariatric surgery. Obesity 21: 1111-1118.
- Cavarretta E (2013) Cardiac remodeling in obese patients after laparoscopic sleeve gastrectomy. World Journal of Surgery 37: 565-572.
- Luaces M (2012) Anatomical and functional alterations of the heart in morbid obesity. Changes after bariatric surgery. Revista Espanola de Cardiologia (English Edition) 65: 14-21.
- Lin CH (2011) Myocardial oxygen consumption change predicts left ventricular relaxation improvement in obese humans after weight loss. Obesity 19: 1804-1812.
- Valezi AC (2011) Morphofunctional evaluation of the heart of obese patients before and after bariatric surgery. Obesity Surgery 21: 1693-1697.
- Owan T (2011) Favorable changes in cardiac geometry and function following gastric bypass surgery: 2-year follow-up in the Utah obesity study. Journal of the American College of Cardiology 57: 732-739.
- Hsuan CF (2010) The effect of surgical weight reduction on left ventricular structure and function in severe obesity. Obesity 18: 1188-1193.
- Algahim MF (2010) Progressive regression of left ventricular hypertrophy two years after bariatric surgery. The American Journal of Medicine 123: 549-555.
- Rider OJ (2009) Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. Journal of the American College of Cardiology 54: 718-726.
- Jhaveri RR (2009) Cardiac remodeling after substantial weight loss: a prospective cardiac magnetic resonance study after bariatric surgery. Surgery for Obesity and Related Diseases 5: 648-652.
- Ramani GV (2008) Safety and efficacy of bariatric surgery in morbidly obese patients with severe systolic heart failure. Clinical Cardiology 31: 516-520.
- Ippisch HM (2008) Reversibility of cardiac abnormalities in morbidly obese adolescents. Journal of the American College of Cardiology 51: 1342-1348.
- Di Bello V (2008) Effects of bariatric surgery on early myocardial alterations in adult severely obese subjects. Cardiology 109: 241-248.
- McCloskey CA (2007) Bariatric surgery improves cardiac function in morbidly obese patients with severe cardiomyopathy. Surgery for Obesity and Related Diseases 3: 503-507.
- Garb J, Welch G, Zagarins S, Kuhn J, Romanelli J (2009) Bariatric surgery for the treatment of morbid obesity: a meta-analysis of weight loss outcomes for laparoscopic adjustable gastric banding and laparoscopic gastric bypass. Obesity Surgery 19: 1443-1447.
- Chang SH (2014) The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surgery 149: 275-287.
- Gloy VL (2013) Bariatric surgery versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomised controlled trials. BMJ 347: f5934.
- Buchwald H (2005) Bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. Journal of the American College of Surgeons 200: 593-604.
- Iyengar S, Leier CV (2006) Rescue bariatric surgery for obesity-induced cardiomyopathy. The American Journal of Medicine 119: e5-e6.




