Abstract
The aim of this study was to assess the nitrate and nitrite content in 46 samples of meat products (sausage, ham, mortadella, sausage and dried meat) and 107 samples of cheese (Minas frescal, mozzarella, Parmesan and prato) sold in Minas Gerais between 2013 and 2017, in order to verify the compliance of these products with current legislation. According to the standard of identity and quality for meat products, the maximum permitted limit for the addition of sodium or potassium nitrate in meat and meat products is 0.03 g/100 g, while the maximum limit for sodium or potassium nitrite is 0.015 g/100 g. Sodium or potassium nitrates can be added to cheeses up to a maximum limit of 50 mg/kg, except for cheeses classified as high moisture (46.0 to 54.9%) or very high moisture (greater than or equal to 55%). For nitrites, the acceptance criterion is absence. Spectrophotometric and reflectometric methods were used. The methods were validated, showing limits of quantification in the range of 0.0005 g/100 g to 0.0067 g/100 g of the sample. All the Minas frescal cheese samples complied with the legislation for both preservatives. Unsatisfactory nitrate contents were observed for mozzarella (9.4%), prato (9.4%) and parmesan (7.7%). For meat products, non-compliance for nitrate and nitrite was only found for sausages (16.7%) and restricted to 2015. Overall compliance was 94.4 per cent for cheese and 97.8 per cent for meat products.
Keywords
food preservatives, health surveillance, inspection, public health
Introduction
Nitrates and nitrites in food
The largest source of dietary nitrate is derived from vegetables. Lettuce, chard, arugula, spinach and beetroot are the vegetables with the highest nitrate content, with values above 250 mg/100 g on a dry basis [1-4].
Among these vegetables, beetroot stands out for having a high content, with an average of 2056 mg of nitrate in conventional cultivation [5]. Other products have lower concentrations of nitrate, such as mushrooms and corn, with values below 50 mg/Kg [2].
The amount of nitrate present in vegetables is directly related to cultivation, being influenced by the nitrate content in the soil, nitrogen-based fertilizers and the period of day and year of harvest [6]. The agricultural use of nitrogen fertilizers can lead to nitrate contamination of water [7].
With regard to nitrite, the largest source in the diet is derived from processed and cured meats, but the largest amount of nitrite present in the body is not derived from the diet, but rather from the reduction of nitrate by bacteria, or by the oxidation of Nitric Oxide (NO) produced endogenously [4]. Around 82% of total daily nitrite consumption originates from the bioconversion of nitrate into nitrite [8].
Human breast milk has a higher amount of nitrites compared to nitrates. In newborns, the reduction of nitrate to nitrite by bacteria is deficient, due to the non-development of the gastrointestinal tract, which makes breast milk important to make up for the nitrite deficiency [3].
Mediterranean and Japanese diet
In countries where the Mediterranean diet is consumed, studies have shown a decrease in the incidence and prevalence of chronic diseases, mainly cardiovascular diseases [9].
Japanese and Mediterranean diets are responsible for providing greater amounts of nitrate. In the Mediterranean diet, the association with antioxidant compounds contributes to reducing nitrate into nitrite and nitric oxide [10].
These diets are characterized by a high consumption of fish, wine, vegetables and fruits, which contributes to increasing the content of nitrite, polyphenols and Polyunsaturated Fatty Acids (PUFA) [11].
The phenolic compounds present in red wine (quercetin, resveratrol and catechins) and in white wine (n-tyrosol and hydroxytyrosol) have a potential antioxidant effect and, therefore, protect against ischemic reperfusion injury (IR) by inhibiting oxidative stress in the reperfusion.
Furthermore, polyphenols provide greater cardioprotection by stimulating the reduction of NO 2 to NO [11]. The intake of foods containing flavonoids increases the circulating concentration of bioactive NO and may represent an alternative for the treatment of coronary and peripheral arterial diseases [12].
According to studies by Nadtochiy and Redman [11] it was found that moderate wine consumption increased blood levels of Polyunsaturated Fatty Acids (PUFAs), such as Eicosapentaenoic Acid (EPA) and Docosahexanoic Acid (DHA) and that the reduction of NO 2 to NO in the stomach and oral cavity was dependent on wine, which confirms the interaction between PUFAs and nitric oxides. PUFAs also increased endothelial NO generation.
Nitrates and nitrites as food additives
According to Ordinance No. 540 (Brazil), [13] food additive is any ingredient intentionally added to food, without the purpose of nutrition, with the aim of modifying the physical, chemical, biological or sensory characteristics, during manufacturing, processing, preparation, treatment, packaging, packaging, storage, transportation or handling of food. Conservative additives are classified by this same ordinance as substances that prevent or delay the alteration of foods caused by microorganisms or enzymes.
Consumer demand for processed foods has caused the food additives industry to grow rapidly. Which explains the existence of around 2500 chemical products for this purpose, which give rise to around 5000 commercial brand products around the world. Each additive must present a technological need to be used in a food and undergo toxicity tests before approval [13].
Acceptable Daily Intake (ADI) and estimated daily intake (IDE) are two indices used to assess human exposure to food additives. The ADI is calculated from the No Observed Adverse Effect Level (NOAEL), while the IDE is a cumulative index derived from the average daily intake and the concentration of additives in foods. The sum of all EDIs must not be greater than the EDI of a food additive from all sources. These indices are important for evaluating carcinogenic and mutagenic effects [14].
In a review study carried out between 2000 and 2015 whose objective was to analyze the risks associated with the intake of food additives for human health and provide real data on consumption in all age groups, it was found that the intake of nitrates and nitrites was lower than the Acceptable Daily Intake (ADI) for all age groups analyzed and no age group was at risk [15].
Studies conducted by Simon (2003) with the aim of evaluating possible adverse effects related to the consumption of various food additives, including nitrates and nitrites, did not observe any association of hypersensitivity, such as angioedema, urticaria or anaphylaxis with their consumption [16].
Scotter and collaborators [14] discussed the interaction of nitrates and nitrites with other food additives and with components present in the food itself. Nitrates react with bisulfite producing compounds that inhibit the preservative action of the additives, which is why they are not used in combination.
Lecithin is commonly used as an emulsifying agent, as release agents and as dietary supplements. Heating it can lead to the production of trimethylamine, which after demethylation and in the presence of nitrite produces a carcinogen known as dimethylnitrosamine. Compounds derived from nitrite react with secondary amines to form N-nitrosamines, which may have nitrosation inhibited by the synergistic effect between the additives tocopherols and ascorbic acid, in concentrations of 500 to 1000 mg/kg and 100 to 500 mg/kg respectively [13].
Nitric Oxide
Endogenous Nitric Oxide (NO) is produced from the oxidation of L-arginine, by the enzymes Nitric Oxide Synthase (NOS). Its production is a physiological process, responsible for regulating immune, cardiovascular function and neurotransmission [3].
This nitric oxide production pathway was the only one known to the scientific community, until the discovery of the enterosalivary nitrate-nitrite-NO pathway (Figure 1), which is responsible for the production of NO and other nitrogen oxides from nitrate and nitrite [2,10,17].
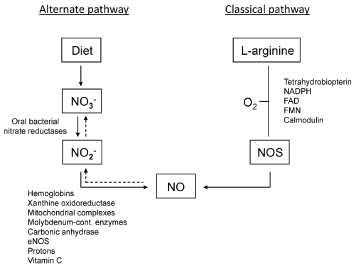
Figure 1. The classical pathway and the alternative pathway for nitric oxide generation [17]
This is possible due to the presence of the bacteria's nitrate reductase enzyme systems. Staphylococcus epi-dermidis, Veillonella, Nocardia and Staphylococcus aureus that live in the posterior epithelial fissures of the tongue and reduce nitrate to nitrite when they accumulate in saliva. Its subsequent reduction to nitric oxide occurs in acidic environments, such as the stomach or oral cavity [11]. It is interesting to note that the administration of antibiotics and mouthwashes containing antiseptics reduces the oral microbiota and therefore reduces the action of nitrate [10,18].
Nitric oxide, through the production of mucus and increased blood flow, plays an important role in protecting the gastric mucosa. In addition to these effects, acidified nitric oxide has bactericidal activity against the bacteria Yersinia, Salmonella and Escherichia coli [18].
Nitrite, at acidic pH, also exerts an antimicrobial effect against various fungi and bacteria, through its protonation to nitrous acid which decomposes into a variety of reactive nitrogen intermediates [19].
With the aim of verifying the bactericidal potential of nitrite in the stomach environment, the bacteria Yersinia enterocolitica ,Salmonella Enteritidis , Shigella sonneiand Escherichia coli were subjected for 30 minutes to 2 hours at a pH of 2.1 to 5.4 in the presence and absence of various nitrite concentrations. In the presence of acid alone, bacteria continued to grow, and in the presence of acidified nitrite, many bacteria died. Although there is no epidemiological evidence, and all studies on bactericidal effects have been performed in vitro, scientists speculate that nitrate ingestion may protect the stomach from colonization by H. Pylori [20].
Cardiovascular health
Cardiovascular health is directly affected by the decrease in the bioavailability or bioactivity of NO [21], since it influences blood flow and blood pressure, through the control of vascular tone.
Furthermore, NO contributes to vascular integrity and suppression of platelet aggregation, leukocyte migration, cell adhesion to the endothelium, and proliferation of vascular smooth muscle cells [2].
The formation of nitric oxide can also stimulate mitochondrial activity during physical activities, which is why supplements based on L-arginine, beet juice and nitrate salts have been proposed to improve performance in athletes and those who practice physical activity [22].
A clinical study demonstrated that ingesting 500 mL of beetroot juice was able to reduce systolic blood pressure by 10 mmHg after 2.5 hours, and reduce diastolic blood pressure by 8 mmHg after 3 hours in healthy individuals. This effect remained after 24 hours of ingesting the juice. The plasma nitrite peak was directly related to a greater reduction in BP [23].
Another study demonstrated that diets based on inorganic nitrate, such as beetroot juices, bread enriched with beetroot or supplements containing this mineral provide a protective effect against cardiovascular diseases, due to the reduction of blood pressure, prevention of endothelium and suppression of platelet aggregation [5].
Although there are studies that prove the reduction in systolic blood pressure, there is still no conclusive epidemiological evidence on controlling blood pressure through the consumption of fruits and vegetables [6]. KURTZ, et al. [18], suggest an increase in the intake of foods with high nitrate content as a supporting factor in reducing the risk of salt-induced hypertension.
Other observational epidemiological studies support the idea that the intake of nitrates and nitrites of plant origin are important in the physiology involving cardiovascular health and gastrointestinal immune function [24,25].
This is because the plant matrix is rich in phytochemical compounds capable of enhancing the reduction of nitrates to nitrites and nitric oxides, such as polyphenols, unsaturated fatty acids, vitamin C and alcohol [2].
Pulmonary hypertension
Pulmonary hypertension is a disease characterized by an increase in pulmonary vascular resistance, right ventricular overload, high pulmonary pressures (average pulmonary arterial pressures of 25 mmHg) and progressive and fatal clinical disorder of pulmonary circulation [26].
People with this disease have lower bioavailability of NO signaling and respond worse to factors that trigger vasodilation. For this reason, one of the most effective therapies for the disease involves targeting NO signaling. Therefore, supplementation with nitrates and nitrites, as well as the intestinal and oral microbiota are important in the treatment of pulmonary hypertension [26].
Chronic effects on ingestion of nitrates and nitrites
The risk of developing gastrointestinal cancer can be influenced by diet. High fat intake, for example, favors the production of bile acids that can damage DNA, due to reactive nitrogen and oxygen species [27,28].
High-fiber diets, on the other hand, may provide a protective effect against cancer risk because anaerobic bacteria present in the small intestine ferment carbohydrate residues into short-chain fatty acids. These bacteria provide a suitable substrate for epithelial cells and intestinal environments capable of suppressing inflammatory effects [24].
Diets with a high protein content favor the fermentation of undigested protein residues by the microbiota, which can lead to the formation of toxic and inflammatory nitrogenous metabolites [29].
These metabolites include nitrosamines and nitrosamides, known as N-nitroso compounds (NOC), which are formed from the reaction of nitrite with secondary amines and amides, or from catalytic nitrate intermediates such as N 2 O 3 or NO + [30].
Due to the ability of nitrates and nitrites to transform into N-nitroso compounds, the International Agency for Research on Cancer has classified these substances in group 2A, as probably carcinogenic to humans [31].
Nitrosamines can be produced endogenously or be present in some foods, such as smoked preserves, foods subject to drying by additives such as malt in the production of beer and whiskey, preserved foods and cured meat products [32].
Endogenously, NOCs can be influenced by several factors, including: gastric acidity, intestinal microbiota, average food residue time through the colon, medications such as proton pump inhibitors, antioxidants such as polyphenols and ascorbic acid, and red meat (KOBAYASHI [30].
Cancer
According to epidemiological data from case-control studies, there is evidence that associates the risk of developing gastric and esophageal cancer with the consumption of meat, processed meat, fish and preserved vegetables. This evidence was not consistent in cohort studies or for esophageal cancer. A positive relationship for the development of gastric cancer and intake of smoked foods was also observed [32].
In a prospective study, the association between the consumption of red meat, processed meat, nitrate, nitrite and cancer was evaluated. An association was found between the intake of these foods and colorectal cancer and a positive relationship between the consumption of nitrate and nitrite and other types of cancer, such as those occurring in the thyroid, ovary and kidneys [33].
Although it has increased, in some prospective studies, the associations between nitrate and nitrite intake with some types of cancer have not yet been consistent studies [34].
Song and collaborators [35], through a meta-analysis, suggested that the consumption of nitrates in the diet is related to a reduced risk of gastric cancer and that increased consumption of nitrite and N-nitrosodimethylamine (NDMA) could increase the risk of its development. The protective effect observed in nitrate consumption was justified by the presence of vitamin C, fiber and other antioxidants present in the largest source of nitrate in the diet, which are vegetables.
Thyroid
Antithyroid effects were observed at high doses of nitrate and nitrite in an animal study. However, this has not been confirmed in studies conducted on humans. In a meta-analysis of epidemiological studies, no positive association was found between nitrate intake and thyroid dysfunctions, such as hyperthyroidism, hypothyroidism and cancer. Only a significant association was found between the intake of high concentrations of nitrite and thyroid cancer [36].
Walton, et al. [37], in turn, suggests that nitrate concentrations exceeding 50 mg/liter in drinking water could be related to thyroid hypertrophy in humans, although the mechanism is still unclear.
In a cohort study conducted in Shanghai, China, with 73,317 women, aged between 40 and 70 years, in the period 1996 – 2000, no relationship was observed between nitrate intake and thyroid cancer. However, women who ingested a greater amount of nitrite from animal sources, especially from processed meats, had a two-fold increased risk of developing thyroid cancer [38].
Methemoglobinemia
Methemoglobinemia results in less oxygen release to the tissues, which can lead to tissue hypoxia and cyanosis, characterized by lethargy, dyspnea, headache and tachycardia. In babies, cyanosis is known as blue baby syndrome. Methemoglobinemia occurs when methemoglobin, which is the iron-containing form of hemoglobin (Fe 3+ ), reaches high concentrations, since its oxygen transport capacity is lower than that of ferrous hemoglobin (Fe 2+ ). A diet rich in nitrate favors the production of nitrite, which in turn oxidizes ferrous iron (Fe 2+ ) from the heme group to the ferric form (Fe 3+ ). Methemoglobin concentrations greater than 50% can be fatal due to the low amount of oxygen that reaches the tissues [39,40].
Diabetes
In recent years, the number of scientific evidence linking the consumption of red meat with the development of serious chronic diseases, such as type 2 Diabetes Mellitus (DM2), cardiovascular diseases and cancer, has increased. Processed meat differs from unprocessed meat in that it is subjected to treatments that aim to increase its shelf life. Processed meat generally has higher amounts of nitrites and nitrates than unprocessed meat [41].
The addition of these substances is intended to inhibit microbial growth, improve flavor and interfere with color stabilization, with nitrite being responsible for the red – pinkish color of these products [42].
Nitrates and nitrites are important in controlling the formation of neurotoxins from anaerobic bacteria, such as Clostridium botulinum , preventing the germination of their spores. Furthermore, such substances can inhibit the presence of other microorganisms responsible for gastrointestinal infections and necrotic enteritis, such as Staphylococcus aureus and Clostridium perfringens, respectively [42].
Nitric oxide produced during meat curing reacts with several muscle components and generates important products in the control of lipid oxidation [43].
Nitrites have been associated with the development of type 1 Diabetes Mellitus (DM1), although there is still no clear mechanism that explains this fact, values decreased after the regulation in 1978 on nitrite consumption [44].
Case-control studies have associated the development of DM1 with the ingestion of nitrates and nitrites, due to the toxic nitrosamines and nitrosamides that are formed endogenously. Furthermore, studies in rats have demonstrated an influence of water pH on the development of DM1, being favoured in more acidic pH than in neutral pH [45].
Prospective studies have correlated high consumption of red and processed meat and the risk of developing DM2 [42].
Nitric oxide produced from the metabolism of nitrate and nitrite, in the presence of superoxides, forms a strong oxidant, peroxynitrite (ONOO -). This compound is cytotoxic and can increase the potential for developing cardiovascular and neurodegenerative diseases, diabetes and their complications. Peroxynitrite can also modify methionine, cysteine, tyrosine and tryptophan residues, which can impair structural and catalytic integrity or interrupt regulatory pathways in cellular function [46].
Nitrosamines formed with the ingestion of nitrates and nitrites increase the risk of DM2 in animals as they are toxic to pancreatic beta cells. In adult rats, N-nitroso compounds, such as N-nitrosomethylurea and N-nitromethylurethane, and sodium nitrite, decreased insulin secretion [46].
Nitrosamines also damage DNA and contribute to the formation of reactive oxygen species, which are involved in lipid peroxidation, pro-inflammatory cytokine activation and protein adduct formation [41].
Multiple Sclerosis
Multiple Sclerosis (MS) is a disease whose etiology is not yet known, but it is known that environmental and genetic factors influence its development. Prospective cohort studies have revealed the importance of vitamin D deficiency, smoking and previous Epstein-Barro virus infection in the onset of the disease. The diet has also caught the attention of the scientific community and hypotheses have been raised about the consumption of meat, especially processed and smoked meats, associated with intestinal pathologies and viral diseases [47].
Monitoring
Food monitoring carried out in Brazil is carried out through microbiological, physical-chemical, microscopic and labelling analyzes in official laboratories of the Ministry of Health. The Minas Gerais Health Surveillance (VISA/MG), together with its State Monitoring Program of Food Quality (PROGVISA), has been monitoring the quality of various foods sold in the state of Minas Gerias annually since 2003. These foods are analyzed at the Instituto Octávio Magalhães/Fundação Ezequiel Dias (Central Public Health Laboratory of the State of Minas Gerais – LACEN/MG) [24].
The evidence presented demonstrates the importance of quantifying nitrates and nitrites in food and the need to carry out monitoring to guarantee the quality of the products consumed. This control allows us to identify failures and seek solutions and consequently improve the quality of life of the population, according to the work.
Material and Methods
Samples
The analyzes were carried out from 2013 to 2015 and 2017 at the Bromatological Chemistry Laboratory of the Ezequiel Dias Foundation - Funed/ Central Public Health Laboratory of the State of Minas Gerais (Lacen-MG). 46 samples of meat products (dried meat, sausage, ham and sausage) and 107 samples of cheese (Minas Fresal, mozzarella, parmesan and plate) were analyzed. The samples collected are part of the Health Surveillance food monitoring program and were obtained randomly in the different meso regions of the state.
Methodology
For the quantitative determination of nitrates and nitrites, the methodology described in Normative Instruction 68/2006 [48,49]. was used and for the qualitative analysis, Ordinance 1/1981 [50], also for the two preservatives (PART 1 – “In natura Beef”, items 8 and 9). The quantitative determination of nitrate and nitrite levels was made only for medium and low moisture cheeses, according to the identity and quality standards (PIQ's) specific to each type of cheese [51-54]. High moisture cheeses were only subjected to qualitative analysis, as the use of these additives in this type of cheese is not authorized.
Two analysis methods were used. The spectrophotometric method was used from 2013 to 2015, with all quantitative analyzes for meat products and dairy products being qualitative [50] and quantitative analyzes [24]. In 2017, all quantitative and qualitative analyzes were carried out using the reflectometric method [24,55]. The change in the choice of methods was due to the simplicity, practicality and speed that the reflectometric method allows. The spectrophotometric method takes longer and uses some toxic reagents.
Qualitative methods for nitrate and nitrite
See Annex I of Ordinance 1/1981, items 8 and 9 [50].
Spectrophotometric method - Quantitative determination (BRAZIL)[49]
In this method, the nitrates and nitrites present in the sample were hot extracted and the nitrates were reduced to nitrite by the action of spongy cadmium in an alkaline medium. The orange-colored alpha-naphthylamino-p-azobenzene-p-sulfonic acid was formed through the diazotization of nitrites with sulfanilic acid and subsequent binding with alpha naphthol. The reading was taken on a spectrophotometer at 480 nm.
Approximately 10 g of the sample was weighed for deproteinization and 5 mL of 5% borax and 40 mL of boiling water were added. The mixture was heated in a boiling water bath for 15 minutes with constant stirring. It was transferred to a volumetric flask where clarification was carried out using 2 mL of 0.25 M or 15% (m/v) potassium ferrocyanide and 2 mL of 1 M or 30% (m/v) zinc acetate. The volume was made up with ultrapure water and stirred vigorously. It was filtered through qualitative filter paper and the filtrate was collected in an amber glass bottle for subsequent determination of nitrates and nitrites.
To determine the original nitrite, 20 mL of the filtrate obtained from the deproteinized sample was transferred to a 100 mL amber volumetric flask. The volume was completed with ultrapure water at the time of analysis and the flasks were reserved for the color reaction.
For the determination of total nitrite (reduction of nitrate to nitrite), 20 mL of the filtrate obtained from the deproteinized sample was transferred and 5 mL of buffer pH 9.6 - 9.7 was added. Approximately 20 g of treated cadmium was weighed into the Erlenmeyer flask. The cadmium was stirred for 15 minutes and the cadmium was allowed to settle and filtered directly into a 100 mL volumetric flask.
Spectrophotometric method - Qualitative determination [24].
10 g of the sample were weighed, after homogenization and grinding in a blender, and 25 mL of 5% borax was added. 100 mL of boiling ultrapure water was added and heated for 15 minutes in a water bath. After cooling, 5 mL of 15% potassium ferrocyanide and 5 mL of 30% zinc acetate were added under stirring and left to rest for approximately 10 minutes at room temperature.
To test for nitrate, the sample was filtered and 5 mL of the filtrate was transferred in duplicate to a test tube. 1 drop of saturated sodium chloride solution and 4 mL of diphenylamine reagent were added. After leaving the tubes to rest for 30 minutes until the color reaction was observed, the negative control (white) and the positive control were performed, adding 0.15 mL of standard 100 mg/L sodium nitrate solution (stock solution A). to the test tube containing the filtrate aliquot and homogenized.
Reflectometric method - Quantitative determination [24,55]
The method consists of reducing nitrate ions to nitrite ions through the action of a reducing agent. Nitrite ions react with an aromatic amine to form a diazonium salt in the presence of an acidic buffer. The salt formed then reacts with N-(1-naphthyl)-ethylene diamine, which results in a red-violet azo dye that was carried out by reflectometry using a Color Meter-Minolta 200 colorimeter.
20g of processed sample were weighed in duplicate, using a semi-analytical balance. 150 mL of purified water was added and homogenized for 1 minute in a mixer. The mixture was heated to 80 °C and while still hot the contents were filtered through qualitative filter paper. After cooling, the reading was taken.
Results and Discussion
Cheeses
In accordance with the Cheese Identity and Quality Standard (PIQ), which appears in Ordinance No. 146 of 1996 from the Ministry of Agriculture, Livestock and Supply (MAPA) [56,57], sodium or potassium nitrates can be added to cheeses up to a maximum limit of 50 mg/kg, with the exception of cheeses classified as high moisture (46.0 to 54.9%) or very high humidity (greater than or equal to 55%). In the case of nitrites, they cannot be added to cheese, regardless of its moisture content. Therefore, its acceptance criterion is absence.
The number of samples, types of cheese and percentage of non-compliance with legislation regarding the nitrate and nitrite content in the samples varied according to Table 1.
Table 1. Assessment of nitrate and nitrite non-compliance in cheese samples collected in 2013, 2014 and 2015
Year |
Products |
Number of samples |
Nitrate non conformities (%) |
Nitrite non conformities (%) |
2013 |
Dish |
16 |
12.5 |
0 |
Parmesan |
3 |
0 |
0 |
Mozzarella |
12 |
0 |
0 |
2014 |
Dish |
11 |
0 |
0 |
Parmesan |
6 |
16.7 |
0 |
Mozzarella |
21 |
4.8 |
4.8 |
fresh mines |
9 |
0 |
0 |
2015 |
Dish |
5 |
20 |
0 |
Parmesan |
4 |
0 |
0 |
Mozzarella |
10 |
0 |
0 |
Minas Frescal |
10 |
0 |
0 |
Table 2. Assessment of non-conformity of nitrate and nitrite in samples of meat products collected in the years 2013, 2014, 2015 and 2017
Year |
Products |
Number of samples |
Nitrate non conformities (%) |
Nitrite non conformities (%) |
2013 |
Mortadella |
4 |
0 |
0 |
Sausage |
13 |
0 |
0 |
Desiccated meat |
5 |
0 |
0 |
2014 |
Ham |
3 |
0 |
0 |
Sausage |
3 |
0 |
0 |
Sausage |
4 |
0 |
0 |
2015 |
Sausage |
6 |
16.7 |
16.7 |
Sausage |
3 |
0 |
0 |
2017 |
Sausage |
4 |
0 |
0 |
Desiccated meat |
1 |
0 |
0 |
Table 3. Data in the literature on the percentage of non-compliance in the research of nitrates and nitrites in different types of food products
Authors |
Product type |
No. of samples |
% nitrate non-compliance |
% nitrite non-compliance |
Melo Filho, et al. [59] |
Sausage |
54 |
56 |
18 |
Oliveira, et al. [60] |
Freshchickensausage |
28 |
7.1 |
7.1 |
Freshhamsausage |
28 |
7.1 |
7.1 |
Gonçalves, et al. [61] |
Prato Cheese |
10 |
10 |
0 |
Parmesancheese |
11 |
18 |
0 |
Mozzarella Cheese |
27 |
0 |
0 |
Minas Frescal Cheese |
29 |
7 |
0 |
Scheibler, et al. [62] |
Mixed sausages |
16 |
37.5 |
37.5 |
Freitas, et al. [63] |
Dairy Drinks |
4 |
0 |
0 |
Monteiro, et al. [64] |
Vegetables |
71 |
0 |
0 |
Soups |
16 |
0 |
0 |
Adami, et al. [66] |
Sausage |
33 |
69.7 |
30.3 |
Colonial typecheese |
10 |
20 |
100 |
Prato typecheese |
14 |
42.9 |
100 |
De Almeida, et al. [48] |
Dairy beverage |
34 |
2.9 |
- |
Dish, Parmesan and Mozzarella |
260 |
3.8 |
0.8 |
Minas/Ricotta Cheese |
156 |
24.8 |
16.5 |
Sousa, et al. [67] |
Pepperoni sausage |
4 |
100 |
100 |
Araldi, et al. [68] |
Salami |
9 |
- |
0 |
In 2013, nitrate levels were above the maximum limit permitted by legislation in 12.5% of cheese samples, with two samples outside specification. In 2015, the percentage of non-compliance increased to 20% for the same analysis, with a sample reaching 92.9 mg/kg, a value well above the maximum allowed.
In 2015, the number of samples was much lower for this type of cheese compared to 2013, which may justify the percentage increase in non-compliance for nitrate. The results remained within compliance with the value established for nitrite analyzes in all samples.
In 2014, Parmesan cheese showed 16.7% non-compliance for nitrate analyses, with a sample reaching a value of 57.6 mg/kg, which exceeds the maximum allowed by legislation of 50 mg/kg. For this type of cheese, there were no non-conforming samples for nitrite analyzes in all years.
Minas fresco cheese, as it is classified as a very high moisture cheese by Ordinance No. 352 of 1997 [24], must have a total absence of nitrates and nitrites in its composition. Therefore, all analyzes are qualitative. In 2014 and 2015, all samples analyzed were in compliance with the established guidelines.
For mozzarella-type cheeses, after determining the moisture content, samples classified as high or very high moisture were sent for qualitative analysis. In 2014 there were seven samples, while in 2015 there were six samples classified as high or very high humidity.
In 2015, all analyzes were within the limits recommended by legislation, with values below 50 mg/kg for quantitative analyzes and absence for qualitative analyses. In 2014, there was non-compliance in 4.8% of nitrate and nitrite analyzes, representing a sample with high moisture content and whose analysis was qualitative, indicating the presence of these compounds.
Meat
According to the standard of identity and quality of meat products, present in Ordinance No. 1,004 of 1998 [58], the maximum limit allowed for adding sodium or potassium nitrate to meat and meat products is 0.03g/100g, while the Maximum limit for sodium or potassium nitrite is 0.015g/100g.
Table 2 shows the number of samples of meat products analyzed per year according to the type of product and the percentage of non-compliance for nitrate and nitrite analyzes.
In 2013, the method quantification limit (LQ) for nitrates and nitrites in meat products was 0.003g/100g. That year, the results varied from 0.004g/100g to 0.015g/100g for nitrates, that is, the highest value found corresponded to half the maximum allowed. Analyzing the results for nitrites, 19 samples were below the LQ and the other samples ranged from 0.004g/100g to 0.014g/100g, that is, only one sample was close to the maximum allowed of 0.015g/100g, but did not reach surpass it.
In 2014, the LQ for nitrates and nitrites was 0.0005g/100g. That year, one sample was below the LQ for nitrate levels and the others ranged from 0.0015g/100g to 0.0076g/100g. For nitrite analyses, six samples were below the LQ and the remainder ranged from 0.0005g/100g to 0.0039g/100g. This year, as the LQ was lower, fewer samples were below its limit, compared to 2013.
In 2015, the LQ value was 0.0005g/100g, and a sausage sample was unsatisfactory for the analysis of nitrate and nitrite, representing 16.7% of the total samples. The other samples were within compliance.
In 2017, the LQ for nitrate levels was 0.0067g/100g and for nitrites it was 0.0023g/100g. Of five samples analyzed, four were below the LQ for nitrates and one sample obtained a result of 0.01g/100g, a value 3 times lower than the maximum allowed. Considering the nitrite analyses, three samples were below the LQ and one sample obtained a result of 0.005g/100g while the other presented a value of 0.0028g/100g.
Therefore, in the years 2013, 2014 and 2017 there were no non-compliances in all samples analyzed, both for nitrate and nitrite. Only in 2015, of six samples analyzed, one sample was non-compliant for nitrates and nitrites, representing 16.7% of the total analyzed.
Ten studies were compared (Table 3) with the aim of evaluating whether the results of these studies in relation to nitrate and nitrite levels in meat products and cheeses reduced the percentages of non-compliance.
Melo Filho, Biscontini and Andrade [59] analyzed 54 samples of sausages sold in Recife-PE, and observed that 18% of the samples had residual nitrite levels above the specified limit, and that for nitrate levels this percentage was 56%. In another study, where 56 samples of chicken and ham sausages were analyzed collected randomly, between November/2001 and July/2002, with 4 different lots and 7 producers, 7.1% exceeded the legal standards for nitrate and nitrite [60].
Gonçalves and collaborators [61] analyzed 77 samples of cheese sold in the State of Minas Gerais in 2009, there was non-conformity in the nitrate research in 10% of Prato type cheeses, 18% of Parmesan cheeses and 7% of Minas Frescal cheeses. Mozzarella cheese was in compliance with legislation for nitrate and nitrite analysis, and the other types of cheese were in compliance with nitrite research.
Scheibler [62] carried out a study on artisanally produced mixed sausages sold in eight municipalities in the Vale do Taquari Region – RS. Of the total of 16 samples, 37.5% showed nitrate and nitrite levels that did not comply with current legislation.
Freitas, et al. [63] in the city of Lavras/MG, did not observe any non-compliance with the legislation, of the 4 samples of dairy drinks analyzed for nitrate and nitrite levels.
Monteiro [64], with the aim of evaluating the occurrence of nitrates and nitrites in vegetable products and soups intended for baby food in Lisbon, analyzed 71 samples of 25 different vegetable species, and for the preparation and analysis of soups, representative samples of a total of 18 vegetable species and 16 different soups made. No sample of vegetables and soups was larger than predicted by legislation.
It is worth noting that Brazil does not have legislation for the limits of nitrate content in vegetables, but Regulation (EU) no. 1258/2011 of the European Commission, of December 2, 2011 [65], establishes the maximum levels of nitrates present in certain leafy vegetables and foods intended for baby food. Due to the influence of climatic conditions on nitrate levels in horticultural products, the regulation establishes different maximum limits depending on the time of year. For example, from October 1st to March 31st, fresh lettuces grown in a greenhouse must have maximum levels of 5000 mg NO 3 /Kg and those grown in the field must have levels of up to 4000 mg NO 3 /Kg. From April 1st to September 30th, lettuce grown in a greenhouse must have levels of up to 4000 mg NO 3 /Kg and in the field up to 3000 mg NO 3 /Kg. The maximum limits for nitrates in fresh spinach must be 3500 mg NO 3 /Kg, and in preserved, frozen or deep-frozen spinach, 2000 mg NO 3 /Kg.
In the study by Adami [66], samples of sausages (mixed and pork types) and cheeses (colonial and plate types) collected from April to June 2013, produced in the Vale do Taquari region, in Rio Grande do Sul, were analyzed. In the cheese samples, nitrite levels were found above the limit specified by legislation, and 42.9% of non-conformity for the nitrate research in dish-type cheese and 20% for colonial-type cheese. Of the 33 sausage samples analyzed, the percentage of non-compliance was 69.7% for nitrates and 30.3% for nitrites.
Almeida [48], during his master's degree, stratified food data monitored by PORGVISA/MG from 2007 to 2013. Prato, Parmesão and Mozzarella cheeses were collected in all years of the study with the exception of 2007. There were 260 samples of these cheeses and the percentage of non-compliance with legislation was 3.8% for nitrates and 0.8% for nitrites.
Minas/Ricotta cheese was collected from 2009 to 2013, totalling 156 samples analyzed. For the nitrate survey there were no unsatisfactory results in 2012, and the total non-compliance was 24.8%. For nitrite research, in 2010 and 2012 there were no non-compliances and the percentage of unsatisfactory results for the other years was 16.5% [24].
Sousa, et al.[67], evaluated the nitrate and nitrite levels in Calabresa-type sausages sold in Picos – PI, and all four samples analyzed presented levels above those recommended by legislation. In the state of Santa Catarina, in the Municipality of Alto Vale do Rio do Peixe, Salame samples were collected in August and September 2016 from three local brands and three different batches. These samples were analyzed for nitrite content and did not find any content that did not comply with legislation [68].
Comparing the results of this work with the work of Gonçalves and collaborators [61], there was a decrease in non-compliance for parmesan and fresh cheese, however mozzarella cheese presented 9.4% and no non-conformity was previously found. For Prato cheese, the percentages of non-compliance were close to 10% in both studies. For nitrites, there was the presence of non-compliance, different from the previous study.
Conclusion
With the results presented, it was possible to conclude that more cheese samples did not comply with current legislation for nitrate levels compared to meat products. Prato and Parmesan cheeses showed a higher percentage of non-compliance, highlighting the importance of inspection for this type of product, mainly because it is widely sold in the state. With regard to nitrite levels, a sample of meat product and a sample of cheese were non-compliant in accordance with current legislation. The results reinforce the importance of continuous monitoring of nitrates in meat products and cheeses, always seeking to offer the population the best health care, prioritizing prevention.
References
- Lundberg JO, Weitzberg E, Cole JA, Benjamin N (2004) Nitrate, bacteria and human health. Nat Rev Microbiol 2: 593-602. [Crossref]
- BONDONNO CP, CROFT KD, HODGSON JM (2016) Dietary Nitrate, Nitric Oxide, and Cardiovascular Health. Crit Rev Food Sci Nutr 56: 2036-2052. [Crossref]
- BRYAN NS, IVY JL (2015) Inorganic nitrite and nitrate: Evidence to support consideration as dietary nutrients. Nut Res 35: 643-654. [Crossref]
- Jones JA, Hopper AO, Power GG, Blood AB (2015) Dietary intake and bio-activation of nitrite and nitrate in newborn infants. Pediatr Res 77: 173-181. [Crossref]
- D’EL-REI J, Cunha AR, Trindade M, Neves MF (2016) Beneficial effects of dietary nitrate on endothelial function and blood pressure levels. Int J Hypertens [Crossref]
- GEE LC, AHLUWALIA A (2016) Dietary Nitrate Lowers Blood Pressure: Epidemiological, Pre-clinical Experimental and Clinical Trial Evidence. Curr Hypertens Rep 18: 14. [Crossref]
- KARR C (2011) Addressing Environmental Contaminants in Pediatric Practice. Pediatr Rev 32: 190-200. [Crossref]
- Cockburn A, Brambilla G, Fernández ML, Arcella D, Bordajandi LR, et al. (2013) Nitrite in feed: From animal health to human health. Toxicol Appl Pharmacol 270: 209-217. [Crossref]
- Milkowski A, Garg HK, Coughlin JR, Bryan NS (2010) Nutritional epidemiology in the context of nitric oxide biology: A risk-benefit evaluation for dietary nitrite and nitrate. Nitric Oxide 22: 110-119. [Crossref]
- BRYAN NS (2018) Functional Nitric Oxide Nutrition to Combat Cardiovascular Disease. Current Atheroscler Rep 20: 21. [Crossref]
- NADTOCHIY SM, REDMAN EK (2011) Mediterranean diet and cardioprotection: the role of nitrite, polyunsaturated fatty acids, and polyphenols. Nutrition 27: 733-744. [Crossref]
- Balzer J, Heiss C, Schroeter H, Brouzos P, Kleinbongard P, et al. (2006) Flavanols and cardiovascular health: Effects on the circulating NO pool in humans. J Cardiovasc Pharmacol 47: 122-127. [Crossref]
- BRAZIL (1997) Ministry of Agriculture Livestock 24 and Supply. National Secretariat for Agricultural Defense. Ordinance No. 540 of October, 1997. Approves the Technical Regulation for Food Additives, definitions, classification and use. Official Gazette [of] the Federative Republic of Brazil. Brasília. 19694- 19695.
- SCOTTER MJ, CASTLE L (2004) Chemical interactions between additives in foodstuffs: A review. Food Addit Contam 21: 93-124. [Crossref]
- RANGAN C (2008) Food Additives and Sensitivities. Medical Toxicology of Natural Substances: Foods, Fungi, Medicinal Herbs, Plants, and Venomous Animals 55: 22-3.
- JAIN A, MATHUR P (2015) Evaluating Hazards Posed by Additives in Food : A Review of Studies Adopting a Risk Assessment Approach 3: 243-255.
- SIMON RA (2003) Adverse reactions to food additives. Current Allergy and Asthma Reports 3: 62-66.
- Kurtz TW, DiCarlo SE, Pravenec M, Morris RC (2018) Functional foods for augmenting nitric oxide activity and reducing the risk for salt-induced hypertension and cardiovascular disease in Japan. J Cardiol 72: 42-49. [Crossref]
- GLADWIN MT (2004) Haldane, hot dogs, halitosis, and hypoxic vasodilation: The emerging biology of the nitrite anion. J Clinical Invest 113: 19-21. [Crossref]
- Lundberg JO, Weitzberg E, Cole JA, Benjamin N (2004) Nitrate, bacteria and human health. Nat Rev Microbiol 2: 593-602. [Crossref]
- ARCHER DL (2002) Evidence that Ingested Nitrate and Nitrite Are Benecial to Health. Journal of Food Protection 65: 872-875.
- ISHIBASHI T, YOSHIDA J, NISHIO M (1999) Evaluation of NOx in the Cardiovascular System: Relationship to NO-related Compounds In Vivo. Jpn J Pharmacol 81: 317-323. [Crossref]
- POORTMANS JR, GUALANO B, CARPENTIER A (2015) Nitrate supplementation and human exercise performance: Too much of a good thing? Curr Opin Clin Nutr Metab Care 18: 599-604. [Crossref]
- WEBB AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, et al. (2008) Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 51: 784-790. [Crossref]
- HORD NG, TANG Y, BRYAN NS (2009) Food sources of nitrates and nitrites: the physiologic contact for potential health benefits. Am J Clin Nutr 90: 1-10. [Crossref]
- Koch CD, Gladwin MT, Freeman BA, Lundberg JO, Weitzberg E, et al. (2017) Enterosalivary nitrate metabolism and the microbiome: Intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic Biol Med 105: 48-67. [Crossref]
- Vipperla K, O’Keefe SJ (2013) Study of teduglutide effectiveness in parenteral nutrition-dependent short-bowel syndrome subjects. Expert Rev Gastroenterol Hepatol 7: 683-687. [Crossref]
- Lee WJ, Hase K (2014) Gut microbiota–generated metabolites in animal health and disease. Nat Chem Boil 10: 416-424. [Crossref]
- Hughes R, Magee EAM, Bingham S (2000) Protein degradation in the large intestine: relevance to colorectal cancer. Current Issues Intest Microbiol 1: 51-58. [Crossref]
- KOBAYASHI J (2018) Effect of diet and gut environment on the gastrointestinal formation of N-nitroso compounds: A review. Nitric Oxide 73: 66-73. [Crossref]
- LACHENMEIER DW (2009) Carcinogens in Food: Opportunities and Challenges for Regulatory Toxicology. The Open Toxicology Journal 3: 30-34.
- JAKSZYN P, GONZALEZ CA (2006) Nitrosamine and related food intake and gastric and oesophageal cancer risk: a systematic review of the epidemiological evidence. World J Gastroenterol 12: 4296-303. [Crossref]
- HABERMEYER M. et al. (2015) Nitrate and nitrite in the diet: How to assess their benefit and risk for human health. Molecular Nutrition & Food Research 59: 106-128.
- Bryan NS, Alexander DD, Coughlin JR, Milkowski AL, Boffetta P (2012) Ingested nitrate and nitrite and stomach cancer risk: An updated review. Food Chem Toxicol 50: 3646-3665. [Crossref]
- Song P, Wu L, Guan W (2015) Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: a meta-analysis. Nutrients 7: 9872-9895. [Crossref]
- Bahadoran Z, Mirmiran P, Ghasemi A, Kabir A, Azizi F, et al. (2015) Is dietary nitrate/nitrite exposure a risk factor for development of thyroid abnormality? A systematic review and meta-analysis. Nitric Oxide 47: 65-76. [Crossref]
- Walton K, Walker R, Van De Sandt JJM, Castell JV, Knapp AGAA, et al. (1999) The application of in vitro data in the derivation of the acceptable daily intake of food additives. Food Chemical Toxicol 37: 1175-1197. [Crossref]
- Aschebrook-kilfoy B, Shu XO, Gao YT, Ji BT, Yang G, et al. (2012) Thyroid cancer risk and dietary nitrate and nitrite intake in the Shanghai women’s health study. Int J Cancer 132: 897-904. [Crossref]
- BASULTO J, MANERA M, BALADIA E (2014) Ingesta dietética de nitratos en bebés y niñosespañoles y riesgo de metahemoglobinemia. Pediatria de Atencion Primaria 16: 65-68.
- MENSINGA TT, SPEIJERS GJ, MEULENBEL TJ (2003) Health implications of exposure to environmental nitrogenous compounds. Toxicol Rev 22: 41-51. [Crossref]
- WOLK A (2017) Potential health hazards of eating red meat. J Intern Med 281: 106-122. [Crossref]
- CELADA P, SÁNCHEZ-MÚNIZ YFJ (2016) Are meat and meat product consumptions harmful? Their relationship with the risk of colorectal cancer and other degenerative diseases | ¿Es el consumo de carne y derivados peligroso para la salud? Relación con el riesgo de cáncer colorrectal y otras enfer. Anales de la Real Academia Nacional de Farmacia 82: 1.
- SKIBSTED LH (2011) Nitric oxide and quality and safety of muscle based foods. Nitric Oxide 24: 176-183. [Crossref]
- WHITE D, COLLINSON A (2013) Red Meat, Dietary Heme Iron , and Risk of Type 2 Diabetes : The Involvement of Advanced. Adv Nutr 4: 403-411. [Crossref]
- NIELSEN DS, Krych L, Buschard K, Hansen CHF, Hansen Ak (2014) Beyond genetics. Influence of dietary factors and gut microbiota on type 1 diabetes. FEBS Lett 588: 4234-4243. [Crossref]
- KIM Y, KEOGH J, CLIFTON P (2015) A review of potential metabolic etiologies of the observed association between red meat consumption and development of type 2 diabetes mellitus. Metabolism 64: 768-779. [Crossref]
- LAUER K (2014) Notes on the Epidemiology of Multiple Sclerosis, with Special Reference to Dietary Habits. Int J Mol Sci 15: 3533-3545. [Crossref]
- ALMEIDA (2015) GPB Food quality monitored by PROGVISA/MG from 2007 to 2013. Dissertation (Master's in Animal Science) – Veterinary School, Federal University of Minas Gerais. Minas Gerais.
- BRAZIL (2006) Ministry of Agriculture, Livestock and Supply. National Secretariat for Agricultural Defense. Normative Instruction No. 68 of December 12, 2006. Establishes the official physicochemical analytical methods for controlling milk and dairy products. Official Gazette [of] the Federative Republic of Brazil, Brasília, 8.
- BRAZIL (1981) Ministry of Agriculture, Livestock and Supply. National Secretariat for Agricultural Defense. National Animal Reference Laboratory. Ordinance No. 01 of October 7, 1981. Approves the official analytical methods for controlling products of animal origin and their ingredients. II – Physical and Chemical Methods. Official Gazette [of] the Federative Republic of Brazil, Brasília, DF, 1.
- BRAZIL (1997) Ministry of Agriculture, Livestock and Supply. National Secretariat for Agricultural Defense. Ordinance No. 352 of September 4, 1997. Approves the Technical Regulation for Establishing the Identity and Quality of Minas Frescal Cheese. Official Gazette [of] the Federative Republic of Brazil. Brasília, 19684.
- BRAZIL (1997) Ministry of Agriculture, Livestock and Supply. National Secretariat for Agricultural Defense. Ordinance No. 353 of September 4, 1997. Approves the Technical Regulation for Establishing the Identity and Quality of Parmesan Cheese, Parmesan, Reggiano, Reggianito and Sbrinz. Official Gazette [of] the Federative Republic of Brazil. Brasília, 19684.
- BRAZIL (1997) Ministry of Agriculture, Livestock and Supply. National Secretariat for Agricultural Defense. Ordinance No. 358 of September 4, 1997. Approves the Technical Regulation for Establishing the Identity and Quality of Prato Cheese. Official Gazette [of] the Federative Republic of Brazil. Brasília, 19690.
- BRAZIL (1997) Ministry of Agriculture Livestock 24. and Supply. National Secretariat for Agricultural Defense. Ordinance No. 364 of September 4, 1997. Approves the Technical Regulation for Establishing the Identity and Quality of Mozzarella Cheese (Mozzarella or Mozzarella). Official Gazette [of] the Federative Republic of Brazil. Brasília, 19694- 19695.
- MERCK (2016) Merck Reflectoquant® Test Nitrates Kit Insert, no. 1.16971.0001.
- MERCK (2013) Merck Reflectoquant® Test Nitrites Kit Insert, no. 1.16973.0001.
- BRAZIL (1996) Ministry of Agriculture, Livestock and Supply. National Secretariat for Agricultural Defense. Ordinance No. 146 of March 7, 1996. Approves the technical regulations on the identity and quality of dairy products. Official Gazette [of] the Federative Republic of Brazil. Brasília, 3977.
- BRAZIL (1998) Ordinance No. 1,004, of December 11, 1998. Technical Regulation for the Function Attribution of Additives and their Maximum Use Limits for Category 8 - Meat and Meat Products. Official Gazette of the Union, Brasília.
- MELO FILHO, AB, BISCONTINI TMB, ANDRADE SAC (2004) Nitrite and nitrate levels in sausages sold in the metropolitan region of Recife. Food Science and Technology 24: 390-392.
- OLIVEIRA MJ DE, ARAÚJO WMC, BORGO LA (2006) Quantification of nitrate and nitrite in fresh sausages. Food Science and Technology 25: 736-742.
- GONÇALVES JF, et al. (2011) Occurrence of nitrates and nitrites in Minas Frescal, Mozzarella, Parmesan and Prato cheeses. Adolfo Lutz Institute Magazine (Print) 70: 193-198.
- SCHEIBLER JR, MARCHI MI, DE SOUZA CFV (2013) Analysis of the nitrite and nitrate content of sausages produced in municipalities in Vale do Taquari-RS. Academic Highlights Magazine 5: 4.
- FREITAS DF, et al. (2014) Assessment of nitrate and nitrite levels in samples of dairy drinks sold in the city of Lavras/MG. Vale do Rio Verde University Magazine 12: 85-89.
- MONTEIRO APA (2014) Occurrence of nitrogen derivatives in some products intended for baby food: nitrates and nitrites. Master's thesis.
- European Communities Commission (2011) Regulation (EU) No 1258/2011 of 2 December 2011. Official Journal of the European Union, Brussels.
- ADAMI FS (2016) Nitrate and nitrite content and microbiological analysis in sausages and cheeses. 2015, 66 f., Thesis (Doctorate) UNIVATES University Center.
- SOUSA V, de SC, et al. (2016) Quantification of nitrate and nitrite used in pepperoni sausages sold in Picos-PI. Intertox Journal of Toxicology, Environmental Risk and Society 9: 2.
- ARALDI EZ, et al. (2017) Study of microbiological conditions and nitrite levels in salami produced in Alto Vale do Rio do Peixe – Santa Catarina, Brazil. Evidence - Science and Biotechnology 16: 131.

