Abstract
In our setting, the frequency of gastrointestinal stromal tumors (GIST) deficient in succinate dehydrogenase (SDH) is 5-7% of 600 cases diagnosed per year. Drug treatments for SDH-mutated GISTs may be ineffective. Knowing your mutations preoperatively can provide surgeons with more information with which to make an effective surgical treatment plan. Woman with moderate cognitive impairment, diagnosed with leukodystrophy, possibly ischemic, who consulted for an abdominal tumor. On physical examination, he presented a palpable tumor in the right hypochondrium. By CT, the tumor is located in the gastric wall. In addition, there is a uterine cystic mass. Gastric tumor biopsy demonstrates a GIST tumor, with negative biomarkers for this tumor by immunohistochemistry (IHC).
Linear gastric resection including the tumor is done and a cholecystectomy for cholelithiasis. Three months later, a hysterectomy with bilateral salpingo-ophorectomy is performed. The uterine tumor has positive IHC expression for SDH fraction B (SDHB) and does not show expression for the proto-oncogene proteins c-KIT and Anoctamin-1(DOG-1). The gastric tumor analyzed by DNA sequencing shows two mutations in PDGFRA (platelet-derived growth factor receptor alpha) and two pathogenic events in SDHB. According to the different databases consulted, the variant c.319_329del (p.Thr107Ter) found in PDGFRA has not been previously described. In SDHB, the p.Arg90Ter mutation expresses a truncated protein. As variants in the number of copies, a chromosomal aberration with loss of the chr region has been detected 1p, which includes the SDHB. Molecular analysis as a diagnostic method has be requested prior to surgery. From a surgical perspective, surgery it is based on diagnostic imaging characteristics, molecular data, and risk of rupture and perforation.
Keywords
gastric GIST, mutation, PDGFRA, SDHB, next generation sequencing, precision surgery
Abbreviations
DOG-1: UniprotKB protein Q5XXA6 (ANO1_HUMAN) Anoctamin-1. Gene HGNC:21625 ANO -1; KIT: protein ID UniprotKB P10721 (KIT_HUMAN). Mast/stem cell growth factor receptor Gene HGNC: 6342, KIT receptor tyrosine kinase; c-KIT: proto-oncogene; PDGFRA: protein ID UniprotKB P16234. Platelet-derived growth factor receptor alpha. ID HGNC (8803) Platelet-derived factor receptor alpha (PDGFRA) gene located on chromosome 4q12. Tyrosine kinase gene; SDHx: Any of the four genes or four proteins that make up the succinate dehydrogenase complex; SDHB: protein ID UniProtKB P21912. Iron sulfide subunit B of the mitochondrial succinate-dehydrogenase complex. Gene ID HGNC (10681) has a cytogenetic location on chromosome 1p36.13. The MIM gene locus is (185470). It is a tumor suppressor gene; Ki-67: protein ID UniProtKB P46013. proliferation marker. HGNC:7107; NGS: next generation DNA sequencing; HGNC: gene nomenclature committee; KEGG: Kyoto Encyclopedia of Genes and Genomes; CD56: Unipot protein KBP13591 (NCAM1). Neural cell adhesion protein 1. Gene ID HGNC: 7656; CD34: protein ID UniProtKB-P28906. Hematopoietic progenitor cell antigen CD34. Gene ID HGNC: 1662.; S-100-B: UniProtKB protein - P04271. S100-B protein. HGNC gene: 10500. Calcium-binding protein B S100; SOX-10: protein ID UniProtKB-P56693.transcription factor protein. Gene HGNC:11190. SRY-box transcription factor 10; WT1: Wilms Tumor Protein; α-SMA: alpha smooth muscle actin; Desmin: cytoskeleton marker; Keratin: cytoskeleton marker; Melan A: melanoma marker; IHC: immunohistochemical analysis; FISH: Fluorescent in situ hybridization; JAZF1-SUZ12 fusion: Atlas of Genetics and Cytogenetics in Oncology and Hematology Id 107452. JAZF1 gene name, location 7p15.2. SUZ12 gene name, location 17q11.2; SDHAF4: HGNC 20957 gene. Assembly factor 4 of the succinate dehydrogenase complex. Its cytogenetic location is on chromosome 6q13. The SDHAF4 protein is an assembly factor for SDH and coordinates its synthesis.
Introduction
The large number of molecular types and subtypes described for gastrointestinal stromal and uterine endometrial tumors have an impact on surgery for these diseases. The report that we present shows the difficulties encountered in making decisions for the diagnosis and treatment of these tumors. This is a woman with moderate cognitive impairment, diagnosed with leukodystrophy, possibly ischemic, who consulted for an abdominal tumor. On physical examination, he presented a palpable tumor in the right hypochondrium.
Surgical resection of the gastric tumor has been performed. In the gastric stromal tumor, an IHC analysis has performed that shows negative SDHB expression and subclass the case presented as GIST with SDH deficiency. Three months later, was performed a hysterectomy with bilateral salpingo-ophorectomy. Unlike GIST, the uterine tumor has positive IHC expression for SDHB, and does not show expression for c-KIT. With GIST sections embedded in paraffin, a sequencing study (NGS-DNA) has been performed, finding molecular variations in PDGFRA and SDHB. Precision surgery in oncology has been based on the understanding of research principles that include, among others, the following domains of knowledge: a) surgical technique and oncological results, b) biochemistry and molecular biology, and c) biostatistics and data processing in surgery [1].
Case report
This is a 74-year-old woman, diagnosed by brain MRI and spectra of leukodystrophy of probable ischemic etiology. Loss of appetite and weight loss for 6 months. Physical examination: palpable tumor in the right hypochondrium that moved to the left hypochondrium. Digital rectal examination: uterine cystic tumor imprinting on the anterior rectal wall. Analysis: CEA: 2.42 ng/ml, Ca.19-9: 56.10 U/ml. Ca 125: 127 U/ml.
Gastroscopy reports a sub-epithelial lesion of 12 x 15 mm, which marks the greater gastric curvature. Gastric endoscopic ultrasonography shows a solid submucosal lesion measuring 8 x 5.5 cm, with a small amount of perilesional fluid, without lymphadenopathy. Gallbladder with cholelithiasis. The trans-gastric biopsy described a stromal tumor with negative expression by immunohistochemistry of GIST biomarkers.
In Figure 1a and 1b, it shows the abdominal-pelvic CT scan performed preoperatively
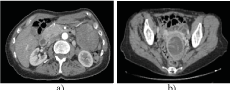
Figure 1. Abdominal-pelvic CT scan. a) Tumor in the left hypochondrium, ventral to the kidney and cranial to the gastro-epiploic artery tumor, b) cystic tumor of the uterine wall.
The incision is by midline laparotomy, with local gastric resection. The tumor has been removed after dissection of the colic and gastro-epiploic vessels, without rupture of the tumor capsule. In addition, a cholecystectomy has been performed. The per-operative pathological describes a heterogeneous tumor of 8 x 5.5 cm, located on the external surface of the gastric wall. The tumor is compatible with GIST, with surgical margins free of tumor infiltration.
In the upper part of the image, the linear resection of the greater gastric curvature has been observed, with triple stapling and section with Echelon FlexTM Endopath ®. In the lower part of the same Figure, the resected mesenchymal tumor has been found, Figure 2.
Microscopic analysis shows a tumor of the muscular, with a diffuse pattern of mostly epithelioid cells and fusocellular areas. In addition, it shows anaplastic tumor dedifferentiation, extensive areas of necrosis, and a high mitotic index (>10 mitosis/5HPF). The edges of the resection piece are free of tumor.
IHC expression of the tumor is c-Kit, DOG-1, SDHB and CD34 negative, S-100 negative in epithelioid cells, positive in peripheral spindle cells and positive for SOX10.The definitive diagnosis is a malignant mesenchymal tumor with inconclusive IHC expression.
A targeted sequencing analysis (NSG-DNA) of the GIST tumor has been performed with the Action OncoPanel DX gene panel, from Imegen, Valencia (Spain), annex 1. It includes SDH, SDHB, PDGFRA and KIT, which allows ruling out mutations in negative genes previously analyzed by IHC.The molecular characteristics found show two mutations in PDGFRA and two pathogenic events in SDHB.
The uterine tumor has been resected 3 months later, having performed a hysterectomy with double adnexectomy. In the images shown below it has been observed the uterus with a cystic formation in its thickness, which protrudes on the surface (arrow). In b) uterus opened for its anatomo-pathological study, in which an encapsulated cystic tumor and with intramural septa has been observed. Figure 3a and 3b.
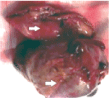
Figure 2. Image of the resection surgical piece that includes the gastric wall (upper left arrow) and the tumor (lower left arrow).
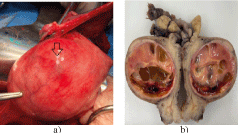
Figure 3. a) Image of the uterus during the surgical intervention. b) uterus sectioned for pathological analysis.
Z
The microscopic study has been observed how the cystic formation is formed by isomorphic cells, which resemble the endometrial stroma in the proliferative phase. The IHC profile of the tumor shows positive expression for estrogen α and progesterone receptors at the nuclear level. He is also positive for CD10, WT1 and SDHB. It is negative for α-SMA, Desmin, c-KIT, Dog-1, Keratin and Melan A.
With these clinical data, the primary objective is to apply the research principles of precision surgery to the treatment of GIST in this patient. In addition, the secondary objectives are: a) to analyze gastric GIST by NGS-DNA, and b) to integrate the clinical-pathological and molecular data related to GIST, ESS and leuko-encephalopathy and determine if these data can improve the precision of surgical treatment.
The genes from NGS-DNA have been analyzed in the Cosmic [2] databases, catalog of somatic mutations in cancer, Gene Ontology (GO) [3] and The Human Gene Mutation Database at the Institute of Medical Genetics in Cardiff (HGMD) [4]. The interpretation of the GIST genes variants has been classified according to the ClinVar [5] and Sanger [6] databases. The ACMG/ANP [7] criteria have also been used for this classification.
Results
The summary of the morphological and immunohistochemistry findings in gastric antral GIST and uterine endometrial stromal tumor has been shown in Table 1.
Table 1. Summary of the morphological and immunohistochemistry findings in antral gastric gist and endometrial stromal tumor of the uterus
Diagnosis |
Morphology |
Immunohistochemistry |
Other antibodies
analyzed |
|
|
Negative |
Positive |
Negative |
GIST antral |
Cells
epithelioid |
c-KIT
CD117
DOG-1
CD34
S100 |
αSMA
CD56 |
AE1/AE3
Chromogranin
Synaptophysin
S100
SOX 10
SDHB |
|
spindle cells |
|
S100
SOX 10 |
|
|
extensive necrosis |
|
|
Ki-67+,17% 10 mit/5HPF |
|
|
Negative |
Positive |
Negative |
Uterine endometrial stromal tumor |
Endometrial tumor with cystic degeneration |
c-KIT
DOG1 |
CD10
Estrogen receptors (ER)
Progesterone receptors (PR)
WT1
SDHB |
Cyclin D1
αSMA
Desmine
Keratin
Melan A |
Leukodystrophy |
White matter |
|
|
|
In the GIST tumor of the case presented and analyzed by NGS-DNA, two mutations in PDGFRA and two pathogenic events in SDHB has been detected. The somatic mutations, pathogenic variants, exons involved and allelic frequency of PDGFRA and SDHB in gastric GIST has been shown below, as well as some associated aspects, Table 2.
Table 2. Gastric GIST. NSG. Mutations in the PDGFRA and SDHB genes
Genes |
Somatic mutations |
Pathogenic variant |
Exon |
Allelic frequency |
Association |
PDGFRA (NM_006206.4) |
c.1682T>A (p.Val561Asp ) |
Constitutively with tyrosine kinase activity. |
12 |
34.80% |
Sensitive to tyrosine kinase inhibitors such as Imatinib or Sunetinib |
|
|
|
|
|
|
PDGFRA (NM_006206.4) |
c.319_329del (p.Thr107Ter ) |
Truncating deletion, which chemically modifies the protein expressed by this gene.
Inactive gene product. |
3 |
30.36% |
Uncertain functional significance for this tumor type. Possibly related to the anaplastic phenotype |
SDHB (NM_003000.3) |
c.268C>T (p.Arg90Ter) |
Two pathogenic events: truncating mutation with loss of function and decrease in the number of copies of the gene (loss of the chr.1p arm that contains it).
Mutation in the SDHB gene is commonly seen in GIST without a KIT mutation. However if you have mutation in PDGFRA. |
3 |
50.30% |
These cases respond poorly to treatment with tyrosine kinase inhibitors. The implication of SDHB in the germ line is ruled out, as this mutation was not found in the patient's blood sample. |
Here, we show a GIST that lacks a KIT mutation and has a somatic c.1682T>A (p.Val561Asp) mutation of intragenic PDGFRA receptor tyrosine kinase activation. The mutation has been localized in the juxta-membrane region l encoded in exon 12 [8].
The single nucleotide variant identified in the tumor analyzed in PDGFRA (NM_006206.4, KEGG 51569), mapped to the chr. 4q12, has been described in UniProt (UniProtKB, P16234), as dbSNP rs121908586, p.Val561Asp. The molecular consequence of this variation is an exceptional change, which results in V561D with an allelic frequency of 34.8%. This genomic variation consulted in ClinVar, in relation to human health, has contradictory interpretations on pathogenicity and its importance is uncertain [9]. PDGFRA has another genetic variant found in the tumor with an allele frequency of 30%. It is a deletion of a fragment comprising the nucleotides c.319_329del (p.Thr107Ter), leading to a stop codon at position 107.This variant has not been described in the different databases consulted [10]. The native PDGFRA protein is 1089 Aa, (UniprotKB Accession No: p16324), many variants and precursors have been described. Isoform 1 is the one selected in UniprotKB as canonical. The pathogenic variant found in the tumor is translated into a 106 Aa chain, lacking the cell membrane anchoring and tyrosine kinase activity domains (Aa 593-954). Predictably, this fragment is inactive and/or will be destroyed by the proteasome. Its clinical significance is uncertain and has been related with the anaplastic areas of the tumor.
The genetic variant/mutation SDHB (NCBI 6390) and in UniProtKB (p 21912), reported for NM_003000.3 (SDHB) exon 3 in the analyzed tumor, is called c.268C>T (p. Arg90Ter) and has an allelic frequency of 50.3%. The native protein has 280 Aa. The truncating mutation p.Arg90Ter generates a protein of 89 Aa, which does not seem to have an assigned function. This c.268C>T variant has no proven clinical relevance for this type of tumor. However, in the 1p36.13 location it is been related to paraganglioma – hereditary pheochromocytoma. The nonsense SDH mutation R22X found in these tumors likewise expresses a 21 Aa (instead of 159) truncated SDHD protein (the D subunit of SDH), leading to loss of electron transfer in the complex. II and to the activation of the signaling pathway HIF-1ɑ.Since SDHx mutations may have a germinal origin, the presence of the c.268C>T (p.Arg90Ter) variant in the patient's blood sample has been analyzed and ruled out. In addition, as variants in the number of copies, a loss of the region of chr.1p 95000-121250000 has been detected, which includes the SDHB gene and has been classified as probably pathogenic (Annex 2).
Discussion
The wild-type GIST (WT-GIST) is a subtype without activating mutations in receptor tyrosine kinases c-KIT or PDGFRA. The deficiency of the activity SDH is the most frequent molecular alteration in WT-GIST [11]. In the presented case, the tumor does not have c-Kit expression by IHC. However, by sequencing, a double mutation in PDGFRA, has been observed.
In the second variant c.319_329del (p.Thr107Ter) the expression of the protein is truncated, which would condition the functional and clinical manifestation of the oncogenic variant c.1682T>A (p.Val561Asp) in the event that both mutations coincide in the same tumor clone. In any case, this mutation modifies the chemical structure of the protein, which predictably prevents or disturbs the control of cell proliferation. This gene variant has probably contributed to the phenotype with areas of anaplastic dedifferentiation of GIST.
After analyzing both pathogenic events in SDHB, the one designated c.268C>T (p. Arg90Ter) leads to the expression of a truncated protein. This pathogenic variant has not demonstrated clinical relevance for this type of tumor.
The variant analysis of copy of number demonstrates a substantial loss of the chr.1p region, which includes SDHB and has been considerate as probably pathogenic. The concurrence of these two events, a truncating mutation with an allelic frequency of 50.3% and a 1p chromosomal aberration, suggests the existence of a deficiency in the SDH activity of the GIST studied.
During the discussion the following questions have been considered for discussion: a) what clinical significance could SDH deficiency have in this case?, b) if the negative IHC for GIST-specific biomarkers should have led us preoperatively to molecular analysis, and c) if the surgery performed was adequate once all the data had been integrated.
SDH-deficient and PDGFRA-mutated GISTs are characterized by increased expression of neural markers, activation of the fibroblast growth receptor (FGF) and several biological pathways (hypoxia and epithelial-mesenchymal transition), related to invasion and tumor progression [12]. Therefore, it is possible that the gastric GIST in the case used these pathways for its development. If so, the field of treatment of these tumors with biological therapy could be get better. On the other hand, the factors that determine whether SDH dysfunction leads to an oncological or neurological disease are due to the loss of enzymatic activity and the disturbance in the formation of complex II [13]. From a clinical point of view, the leukoencephalopathy in this case could be in the spectrum of isolated deficiency complex II and SDHB [14] deficiency. If leukodystrophy progresses during the clinical evolution, observed by MRI and Spect in the patient, a diagnostic option would be sequencing. A whole exome sequencing in patients with white matter abnormalities may decrease the number of undiagnosed cases [15].
In the WHO classification (2020), endometrial stromal tumors have been divided into four categories: endometrial stromal nodules (ESN), low-grade endometrial stromal sarcomas (LG-ESS), high-grade endometrial stromal sarcomas (HG-ESS) and undifferentiated uterine sarcomas (UUS) [16]. The differential diagnosis between NSS and LG-ESS is difficult from the anatomical-pathological point of view, as has occurred in the case presented [17]. Chromosomal rearrangements that create gene fusions are characteristic of endometrial stromal tumors [18] and are drivers responsible for the development of low-grade endometrial stromal sarcoma (ESS).
JAZF1-SUZ12 is the most common gene fusion and is the ESS [19] cytogenetic hallmark. In the case presented, whit the aim of confirming presence of gene fusion, one option would have been to perform FISH with probes for JAZF1–SUZ12. If positive result, the recommendation indicated would have been to carry out an NGS-DNA. In low-grade tumors, genes related to inflammation and immunity are downregulated. In these, the expression level of estrogen receptors ESR1 are significantly higher, as in the case presented.
The decision for surgical treatment of gastric GIST has been based on the morphological appearance of the tumor tissue stained with hematoxylin/eosin and observed under light microscopy (epithelial cells) with areas of anaplastic dedifferentiation), on inconclusive IHC for GIST biomarkers and on the high risk.
Surgery is the standard of care for localized GIST of the stomach, and complete resection of the primary tumor with an intact pseudo-capsule is necessary to cure the disease [20]. Laparoscopic cradle resection, cooperative laparoscopic and endoscopic surgery, and the technique called third-space endoscopic and robotic surgery provide an adequate oncological result for all tumor sizes [21,22].
In recent years, the results of surgical treatment of pediatric patient’s whit wild-type GIST (WT-GIST) indicate that formal anatomical resections (total or subtotal gastrectomy) be avoided, as they are associated with decreased of disease-free survival [23].
In surgical resection of SDH-deficient WT-GIST in adults, data from retrospective analyzes to support surgical treatment guidelines are lacking. There is no evidence of clinical guidelines that support aggressive resections in these tumors [24].
Generally, in surgical decision-making, surgical morbidity is equate with the benefits of resection. However, at the time of surgery we were unaware that the tumor had undifferentiated areas and it was a wild-type GIST with an SDHB mutation. Due to this circumstance, a recommendation on guidelines for surgical management of GIST not done made if the mutations and protein expressions in these tumors they are unknown.
It is possible that, if the FISH fusion analysis of the JAZF1-SUZ12 genes in the uterine tumor tissue could has been carried out, the tumor subtype could have been known more precisely. In LG-ESS, not performing an oophorectomy has a higher risk of recurrence but does not influence survival [25]. In conclusion, molecular analysis is diagnostic method prior to surgery. The cornerstones of surgical resection are imaging features, molecular data, and risk of rupture and perforation.
Acknowledgments
Our thanks to the Institute of Genomic Medicine SL Imegen of Valencia (Spain) for their help in the determination and interpretation of the oncogenomic results (Sample code 131580 (21B05517 M/G).
And to Prof. Begoña Ochoa (Department of Physiology, Faculty of Medicine and Nursing, University of the Basque Country UPV/EHU) for their help in interpreting the molecular analyzes and critical reading of the manuscript.
References
- Maier-Hein L, Vedula SS, Speidel S, Navab N, Kikinis R, et al. (2017) Surgical data science for next-generation interventions. Nat Biomed Eng 1: 691-696. [Crossref]
- Forbes SA, Beare D, Boutselakis H, Bamford S, Bindal N, Tate J, et al. (2017) COSMIC: somatic cancer genetics at high resolution. Nucleic Acids Res 45: D777-D783. [Crossref]
- Gene Ontology Consortium (2015) Gene Ontology Consortium: going forward. Nucleic Acids Res 43: D1049-56. [Crossref]
- http://www.hgmd.cf.ac.uk/ac/index.php
- Landrum MJ, Chitipiralla S, Brown GR, Chen C, Gu B, et al. (2020) ClinVar: improvements to accessing data. Nucleic Acids Res 48: D835-D844. [Crossref]
- Beck TF, Mullikin JC, NISC Comparative Sequencing Program, Biesecker LG (2016) Systematic Evaluation of Sanger Validation of Next-Generation Sequencing Variants. Clin Chem 62: 647-54. [Crossref]
- Richards S, Aziz N, Bale S, Bick D, Das S, et al. (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17: 405-424. [Crossref]
- Heinrich MC, Corless CL, Duensing A, McGreevey L, et al. (2003) PDGFRA activating mutations in gastrointestinal stromal tumors. Science 299: 708-710. [Crossref]
- https://ncbi.nlm.nih.gov/search/all/?term=c,319_329del%20(p.thr107Ter%20)
- Boikos SA, Pappo AS, Killian JK, LaQuaglia MP, Weldon CB, et al. (2016) Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol 2: 922-928. [Crossref]
- Indio V, Schipani A, Nannini M, Urbini M, Rizzo A, et al. (2021) Gene Expression Landscape of SDH-Deficient Gastrointestinal Stromal Tumors. J Clin Med 10: 1057. [Crossref]
- Alston CL, Davison JE, Meloni F, van der Westhuizen FH, He L, et al. (2012) Recessive germline SDHA and SDHB mutations causing leukodystrophy and isolated mitochondrial complex II deficiency. J Med Genet 49: 569-577.
- Moreno C, Santos RM, Burns R, Zhang WC (2020) Succinate Dehydrogenase and Ribonucleic Acid Networks in Cancer and Other Diseases. Cancers (Basel) 12: 3237. [Crossref]
- Vanderver A, Simons C, Helman G, Crawford J, Wolf NI, et al. (2016) Whole exome sequencing in patients with white matter abnormalities. Ann Neurol 79: 1031-1037. [Crossref]
- Female Genital Tumours (2020) WHO Classification of Tumours. (5th edn), IARC Publications: Lyon, France.
- Akaev I, Yeoh CC, Rahimi S (2021) Update on Endometrial Stromal Tumours of the Uterus. Diagnostics (Basel) 11: 429. [Crossref]
- Koontz JI, Soreng AL, Nucci M, Kuo FC, Pauwels P, et al. (2001) Frequent fusion of the JAZF1 and JJAZ1 genes in endometrial stromal tumors. Proc Natl Acad Sci U S A 98: 6348-6353. [Crossref]
- Hrzenjak A (2016) JAZF1/SUZ12 gene fusion in endometrial stromal sarcomas. Orphanet J Rare Dis 11: 15.
- Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, et al. (2018) Gastrointestinal stromal tumours: Esmo-Euracan Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29: Siv267.
- Kim JJ, Lim JY, Nguyen SQ (2017) Laparoscopic resection of gastrointestinal stromal tumors: Does laparoscopic surgery provide an adequate oncologic resection? World J Gastrointest Endosc 9: 448-455.
- Shi F, Li Y, Pan Y, Sun Q, Wang G, et al. (2019) Clinical feasibility and safety of third space robotic and endoscopic cooperative surgery for gastric gastrointestinal stromal tumors dissection: A new surgical technique for treating gastric GISTs. Surg Endosc 33: 4192-4200.
- Weldon CB, Madenci AL, Boikos SA, Janeway KA, George S, et al. (2017) Surgical Management of Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Pediatric and Wildtype GIST Clinic. J Clin Oncol 35: 523-528. [Crossref]
- Kim BJ, Kays JK, Koniaris LG, Valsangkar NP (2017) Understanding the critical role for surgery in the management of wild-type gastrointestinal stromal tumor (GIST). Transl Gastroenterol Hepatol 2: 91. [Crossref]
- Feng W, Hua K, Malpica A, Zhou X, Baak JP (2013) Stages I to II WHO 2003-defined low-grade endometrial stromal sarcoma: how much primary therapy is needed and how little is enough? Int J Gynecol Cancer 23: 488-493. [Crossref]



