Background: Over diagnosis and overtreatment of indolent prostate cancer (PC) is a serious health issue. There is an unmet clinical need for noninvasive, easy to administer, diagnostic assay to help assess whether a prostate biopsy is warranted.
Objective: The performance of a novel, serum-based multiplexed autoantibody assay was assessed on a new, more sensitive Magneto-sensing assay using eight (8) autoantibodies plus standard of care (SOC) (PSA and age) vs. SOC alone for discriminating prostate cancer risk on biopsy as well as detecting high- grade prostate cancer on biopsy (Gleason Score (GS) 7 or greater).
Methods: Using the magneto-sensing technology, the levels of eight (8) autoantibodies were determined among 250 men with PSA levels 2-20 ng/mL using retrospective serum samples from two academic and one community clinical sites. Eligible men were PC-free, >40 years old, undergoing prostate biopsy due to suspicious digital rectal examination finding and/or elevated PSA levels. We evaluated the predictive ability of the autoantibody assay plus SOC using the area under receiver operating characteristic curve (AUC) to predict PC vs. no cancer on biopsy and high-grade PC defined as GS7 or greater from GS6/no cancer on biopsy. Analyses were repeated restricting to those with PSA 2-10 ng/ml and stratifying by age (<65 vs. ≥65).
Results: Among 250 men (median age 62 years; median PSA 5.5 ng/mL), 139 had PC; 42 with GS6, 74 with GS7 and 21 with GS8 or higher. The autoantibody assay plus SOC demonstrated an AUC of 0.74 (95%CI 0.68-0.80) vs. an AUC for SOC of 0.51 (95%CI 0.44-0.59) (p<0.001). Similarly, discrimination was improved for detecting GS7 and higher vs. GS6 or lower/no cancer on biopsy (AUC 0.68, 95% CI 0.61, 0.75) vs. SOC alone (AUC 0.58, 95% CI 0.51, 0.65) (p=0.009). A test developed with these biomarkers detected GS7 or higher PC with 95% sensitivity and 34% specificity. Performance was similar in men with PSA 2-10 ng/ml (n=223), with an AUC of 0.74 using the antibody assay plus SOC vs. 0.54 with SOC. In men age <65 (n=156), the AUC of the biomarkers plus SOC was 0.75 and SOC alone was 0.52. In men age ≥ 65 (n=67), the AUC of the biomarkers plus SOC was 0.76 and SOC alone was 0.58.
Conclusions: Magneto-sensing serum-based detection of autoantibodies significantly improved identification of patients with PC and particularly higher-grade PC vs. the current SOC. This antibody assay outperformed SOC alone in both older and younger men and within the diagnostic grey zone (PSA 2-10). The Magneto-sensing autoantibody assay can be used in a point of care setting in a primary care office and could potentially reduce the total number of unnecessary prostate biopsies.
prostate cancer, prostate biopsies, Magneto-sensing autoantibody
In the United States, prostate cancer is the most common non-skin related cancer in among men, with an estimated 161,360 new cases and 26,730 deaths in 2017 [1].
Since the 1980’s, widespread screening with serum prostate-specific antigen (PSA) levels and digital rectal examination (DRE) have facilitated early detection and we have seen a significant decline in prostate cancer mortality [2]. However, PSA testing lacks specificity for high-grade disease, leading to a high rate of false-positive results and unnecessary, repeat biopsies, which pose the risk for bleeding, infection, and pain [2-4]. In addition, harms of over diagnosis and treatment, stemming from PSA testing include, infection, blood loss requiring transfusion, pneumonia, erectile dysfunction, and incontinence [5]. Furthermore, patient anxiety may result from false-positive results of PSA testing as well as having to undergo prostate biopsy [6].
Since the advent of screening, the incidence of prostate cancer has increased dramatically [3]. PSA testing has led to the diagnosis of clinically insignificant tumors that would not have been life threatening [2].
Almost 80% of cases are detected at clinically localized sage III, and more than half are expected to be low-risk tumors [7-9]. Such tumors are an infrequent case of death, and the men affected are more likely to die of other causes [8,9]. As a result, many men are subjected to unnecessary prostate biopsies and overtreatment of indolent cancer in order to save one man from dying of prostate cancer [8,9].
The initial dilemma in the management of clinically localized prostate cancer stems from prostate cancer’s heterogeneity, as evidenced by its natural history [2]. While many prostate cancer cases will not progress, or will progress slowly and remain asymptomatic during a lifetime, select cases are aggressive and warrant early detection and treatment [2].
The limitations of the PSA test as well as controversy surrounding the 2012 U.S. Preventive Services Task Force (USPSTF) recommendation and have prompted research on novel serum and tissue biomarkers to identify patients at risk for intermediate- or high-risk prostate cancer. Several other serum, urine, or biopsy tissue-derived biomarkers are available to aid in prostate cancer diagnosis, but unlike the serum- based, novel, multiplexed autoantibody assay described herein, many are based in some way on measurement of PSA. Efforts toward the development of screening tests for prostate cancer have generally depended on single biomarker molecules, primarily PSA, as well as PCA3 [10]. Current technologies have been disappointing and have not resulted in diagnostic tests sufficiently reliable or convenient to apply to clinical practice for detection of early-stage prostate cancer [11]. Consequently, there is a need for new biomarkers that can identify prostate cancer at any state of prostate cancer progression while limiting the number of false positives [12].
The discovery that patients with cancer produce detectable autoantibodies against antigens in their tumors suggests that these biomarkers could have diagnostic and prognostic value [13]. Building upon these findings, researchers identified a panel of eight autoantibodies that are released by the immune system in response to the presence of prostate cancer and developed an algorithm that can be used to indicate a relatively high or low risk of prostate cancer, particularly for patients with intermediate (4.0 to 10 ng/mL) PSA levels [12].
This cancer-specific, non-PSA blood test has demonstrated efficacy in identifying men at high risk of prostate cancer in a variety of clinical studies [12]. The autoantibody assay has the potential to aid clinicians in determining the most appropriate candidates for an initial or repeat biopsy and is intended for use in patients with prostate cancer risk factors to help provide additional insight to support a more informed clinical decision about performing a prostate biopsy.
A cancer-specific and non-PSA blood test (APIFINY®) helps decision making in a patient with a rising PSA and correctly identifies a patient’s risk for prostate cancer after biopsy, repeat biopsy and MRI were inconclusive or indicated low risk.
Patient
In 2013, an otherwise healthy 58-year-old Caucasian man with no family history of prostate cancer was referred to a urologist because of a history of a rise in PSA. The PSA at the time of this evaluation was 4.1 ng/mL. By physical examination, the DRE revealed prostate asymmetry but with no evidence of nodularity. Because of an increase in his PSA and an abnormal DRE, a transrectal ultrasound (TRUS)- guided prostate biopsy was performed without complications and the pathology report returned prostate atypia, but no prostate adenocarcinoma. A PSA was performed 14 months later which showed that it increased to 4.5ng/mL. Because of the previous history of prostate atypia and a further rise in PSA, the patient underwent a repeat 12-core TRUS-guided biopsy. The pathology reports this time indicated benign prostatic hyperplasia. The patient elected no treatment.
In 2017, with a PSA of 9.0 ng/mL, the patient presented to a second urologist for an additional opinion. He initially refused a third biopsy. As part of the clinical work up, a 3T prostate magnetic resonance imaging (MRI) test was ordered to help determine whether a prostate biopsy was necessary. The MRI showed a normal prostate with no evidence of any suspicious lesions. Still concerned about the patient’s risk for clinically significant disease due to the doubling of his PSA, the urologist ordered an APIFINY® test.
Use of the novel, serum-based, multiplexed, autoantibody assay test result
A simple, non-invasive blood draw in the lab was conducted. The autoantibody assay returned a score of 99 out of 100 and this was sent to the ordering clinician within a few days (Figure 1). This score indicated this patient had a higher risk for harboring prostate cancer. Based on this new information furnished by the autoantibody assay, the patient and urologist decided to proceed with a transrectal ultrasound-guided prostate biopsy. This revealed prostatic adenocarcinoma Gleason 6 cancer in four of 12 cores (Table 1).

Figure 1. APIFINY patient report.
Table 1. Clinical and test result information.
PSA History |
August 2013 |
4.1 ng/mL |
October 2014 |
4.5 ng/mL |
February 2016 |
5.2 ng/mL |
April 2017 |
9.0 ng/mL |
Clinical and test result information - June 2017 |
PSA |
9.0 ng/mL |
DRE |
normal |
MRI |
normal |
Previous Bx |
Atypia |
Previous Repeat Bx |
normal |
APIFINY Score |
99 (out of 100) |
TRUS biopsy |
Gleason Score 3+3=6 |
The autoantibody assay test is a valuable cancer-specific diagnostic non-PSA blood test for men who have an abnormal PSA or DRE and who are being considered for prostate biopsy or in those men who have had a negative prior biopsy, but there is still concern that prostate cancer was missed.
In this clinical case, the assay provided important, additional information that modified this patient’s management. Although the patient had an elevated PSA, his DRE was normal, and a MRI and two biopsies were all negative for prostate cancer. However, the multiplexed autoantibody assay test result was 99 (out of 100) indicating a likelihood of a higher risk of prostate cancer. This finding helped the patient and urologist make a better-informed decision to move forward with the prostate biopsy. Fortunately for the patient, the indolent cancer detected can now be actively monitored.
Clinical updates using a novel magneto-sensing (Magarray) autoantibody assay to detect high-grade prostate cancer
Identification of 8 prostate cancer-specific autoantibodies
The autoantibody assay was developed based on research demonstrating that patients with cancer produce detectable autoantibodies against antigens in their tumors [13]. For example, mutant forms of the p53 protein elicit anti-p53 antibodies in 30% to 40% of patients with various cancers types [14,15]. As articulated by Schipper and colleagues [2], multiple complex molecular events characterize prostate cancer initiation, unregulated growth, invasion, and metastasis. Distinct sets of genes and proteins dictate progression from precursor lesion to localized disease and finally to metastatic disease [12].
Biomarkers that detect prostate cancer in any of these states of progression would be ideal as we now more fully understand there is an immune response to cancer in humans which has been demonstrated by the identification of autoantibodies against a number of intracellular antigens in patients with various tumor types [12]. This phenomenon is known as the humoral response and the detection of such autoantibodies has been shown to have diagnostic and prognostic value in the detection of cancer and the ability to predict the course of the disease [12]. In addition, it has been shown that most antigens from tumor cells that elicit a response are not just products of mutated genes [12]. These proteins are often differentiation antigens or other proteins over-expressed in cancer [12].
Using iterative biopanning and phage-protein microarrays, Wang et al. [13] developed an assay whereby multiple autoantibody biomarker can be used to screen for prostate cancer. An algorithm was developed to discern healthy from diseased individuals independent of PSA [12]. Clinical studies show that relying on multiple immunogenetic prostate-cancer peptides appears to be a significant improvement over a single biomarker such as PSA [12]. Such markers will more accurately identify patients who are most likely to benefit from referral to a urologist for further evaluation, biopsy and, potentially, treatment for early prostate cancer while reducing inaccurate readings, unnecessary invasive testing in healthy men, and associated morbidity and healthcare costs.
Building upon these findings, researchers identified a panel of eight autoantibodies that that are upregulated or altered early in prostate cancer progression, and developed an algorithm that can be used to indicate a relative high or low risk of prostate cancer, particularly for patients with intermediate (4.0-10 ng/mL) PSA levels [12]. The autoantibody markers span a range of biological functions integral to prostate cancer progression (Table 2). Three of the biomarkers are associated with androgen response regulation, four biomarkers are related to cellular structural integrity, and one biomarker has been associated with prostate cancer progression and a variety of cellular functions ranging from cellular signaling for numerous protein kinases to regulating cell cycle and cellular division [12].
Table 2. Prostate cancer specific biomarkers. Data extracted from Schipper et al. [12].
Biomarker |
Function |
ADP-Ribosylation Factor 6 (ARF 6) |
regulates actin cytoskeleton remodeling vesicle shedding by tumor cells |
NK3 homeobox 1 (NKX3-1) |
regulates androgen response genes (BMI1) |
5’-UTR-BMI1 |
androgen response gene |
Centrosomal Protein 164kD (CEP 164) |
responsible for spindle pole integrity at centrosome |
3’- UTR- Ropporin |
responsible for ciliary movement in spermatozoa through dynein regulation |
Desmocollin |
|
Aurora Kinase Interacting Protein 1 (AURKAIP-1) |
regulates androgen response genes (TWIST1) |
Casein Kinase 2, alpha prime polypeptide (CSNK2A2) |
regulates cell cycle and cellular division |
Magneto-sensing technology accurately detects prostate cancer specific autoantibodies
Many autoantibody assays are limited by high levels of nonspecific signal. Research on a new more sensitive magneto-sensing (MagArray) assay demonstrates the ability of this assay to accurately detect the 8 Apifiny autoantibodies. In a pilot study, researchers used the magneto-sensing assay to analyze serum samples from 10 patients with biopsy confirmed prostate cancer and 10 patients with negative biopsies [16]. Patients in this study ranged from 52 to 77 years of age and had PSA levels of 1.3 to 24 ng/mL. The serum samples were titrated (1/100-1/400 diluation) on the magneto-sensing platform. In patients with high-grade prostate cancer, the signal detected on the magneto-sensing assay was higher than that for patients without prostate cancer [16]. In addition, patients with Gleason Scores of ≥7 had higher signals than those with Gleason Scores of 6. The individual 8 autoantibodies gave unique and titratable signals, according to the researchers.
Magneto-sensing assay predicts high-grade prostate cancer
In a larger multicenter study, researchers compared the predictive ability of measuring the eight (8) autoantibodies using the magneto-sensing assay plus standard of care (PSA and age) with that of standard of care alone in 250 men undergoing prostate biopsy due to suspicious digital rectal examination findings (median age, 62 years; PSA level, 2-20 ng/mL) [17]. Of this group, 139 men had prostate cancer: 42 patients had GS6, 74 patients had GS7, and 21 patients had GS8 or greater.
The magneto-sensing assay plus standard of care was significantly better at predicting prostate cancer compared to standard of care alone, as measured using the area under receiver operating characteristic curve (AUC; 0.74 vs. 0.51; P<0.001) [17]. Similarly, the magneto-sensing assay was significantly better at discriminating between biopsies with Gleason Score ≥7 vs Gleason Score of 6 or negative biopsy (AUC, 0.68 vs. 0.58; P=0.009). The assay’s sensitivity and specificity in detecting prostate cancer with a Gleason Score of ≥7 was 95% and 34%, respectively. The negative predictive value at the cut point of 59 was 95.4%. In a subgroup analysis of men with PSA levels of 2 to 10 ng/ml (n=223), the magneto-sensing assay plus standard of care performed better at predicting prostate cancer than standard of care alone (AUC, 0.74 vs. 0.54) [17]. Analysis by age group also showed similar benefit for the magneto-sensing assay: in men <65 years (n=156) the AUC for the MagArray assay plus standard of care versus standard of care alone was 0.75 versus 0.52. In men age ≥65 (n=67), the AUC was 0.76 versus 0.58, respectively.
Magneto-sensing assay detects prostate cancer in men with normal PSA levels
Traditionally, men with PSA levels of <4 ng/mL have been considered to be at low risk for prostate cancer. However, in the landmark Prostate Cancer Prevention Trial (PCPT) [14], Thompson et al. [14] reported the diagnosis of prostate cancer in 15.2% of men with a PSA level ≤4 ng/mL. Of these men, 14.9% harbored high-grade disease.
A multicenter study suggests that the magneto-sensing assay can improve detection of high-grade prostate cancer in patients with PSA levels <4 ng/dL [18]. The study involved 71 men (median age, 61 years) with PSA levels ranging from 0.1 to 4 ng/mL (median PSA=3.1 ng/mL) who were scheduled to undergo prostate biopsy due to suspicious digital rectal exam findings, family history, and/or PSA levels.
The magneto-sensing assay plus standard of care showed greater discrimination between men with prostate cancer from those with negative biopsy compared with standard of care alone (AUC, 0.87 vs 0.56; P<0.0001) [18]. The sensitivity and specificity of the magneto-sensing assay plus standard of care is shown in the Figure 2. At a sensitivity of 95%, the assay had a specificity of 50% and a negative predictive value of 89% (with only two cases of prostate cancer being missed with this test). In addition, the assay plus standard of care was better able to discriminate between high-grade prostate cancer and low-grade/negative biopsy than standard of care alone (AUC, 0.80 vs 0.63; P=0.037).
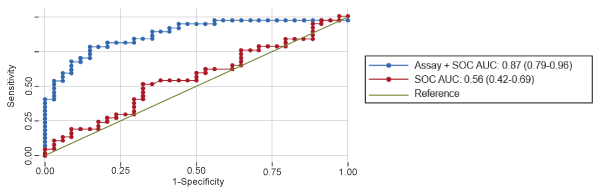
Figure 2. Area under the curve (AUC) for Magneto-Sensing assay plus standard of care (PSA plus age) versus standard of care alone. Reprinted with permission from Freedland et al. [18].
The current study has several limitations, including the need to more fully assess the test in all races, as well as to determine how other conditions (such as obesity and its pro-inflammatory state, or steroid use) may affect the assay’s performance. Moreover, the outcome of this study was ≥G7 on biopsy; it is known some G7 disease (especially low volume G7 (3+4)) can be indolent, and some, high-volume G6 can be clinically meaningful. Finally, this study’s outcome was biopsy; it is well documented that approximately 25% of biopsies will return a false-negative result [7]. Ideally, long-term follow-up including prostate cancer death are needed to verify this as a predictor of prostate cancer. Finally, future clinical studies are warranted to further elucidate the value of this testing, which has the potential to more accurately identify patients who are most likely to benefit from prostate biopsy and early treatment, while reducing the rate of false-positive results and unnecessary biopsy and treatment, as well as associated morbidity and healthcare costs.
Employing new magneto-sensing technology, this study evaluated a novel, cancer-specific, non-PSA biomarker assay based on autoantibody signatures that can be used as a noninvasive biomarker tool for clinicians in the assessment of risk for the presence and aggressiveness of prostate cancer. When results of this assay are combined with traditional clinical risk factors, risk stratification and biopsy decision making are improved compared to current methods in clinical practice. We hypothesize the assay will significantly reduce costs to the healthcare system while further improving patient’s quality of care. Providers and their patients may consider using this novel assay prior to proceeding with prostate biopsy.
We are indebted to Arul Chinnaiyan, M.D., Ph.D. for his continued encouragement of this work, to Dr. Robert Reinhardt for his guidance, and to the entire Armune Bioscience and MagArray Inc staff for their assistance in the laboratory.
MagArray Inc and Armune Bioscience provided all financial support for the work reported in this article. Dr. Hafron is a consultant and speaker for Armune Bioscience. Dr. Rajan is a speaker for Armune Bioscience.
- American Cancer Society (2017) Key Statistics for Prostate Cancer. [https://www.cancer.org/cancer/prostate- cancer/about/key-statistics.html]. Accessed March 23, 2017.
- Moyer VA; U.S. Preventive Services Task Force (2012) Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 157: 120-134. [Crossref]
- Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, et al. (2010) Mortality results from the Goteborg randomised population- based prostate-cancer screening trial. Lancet Oncol 11: 725-732. [Crossref]
- Rosario DJ, Lane JA, Metcalfe C, Donovan JL, Doble A, et al. (2012) Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ 344: d7894. [Crossref]
- Ilic D, Neuberger MM, Djulbegovic M, Dahm P (2013) Screening for prostate cancer. Cochrane Database Syst Rev : CD004720. [Crossref]
- Carlsson S, Aus G, Wessman C, Hugosson J (2007) Anxiety associated with prostate cancer screening with special reference to men with a positive screening test (elevated PSA) - Results from a prospective, population-based, randomised study. Eur J Cancer 43: 2109-2116. [Crossref]
- Banerji JS, Wolff EM, Massman JD 3rd, Odem-Davis K, Porter CR, et al. (2016) Prostate Needle Biopsy Outcomes in the Era of the U.S. Preventive Services Task Force Recommendation against Prostate Specific Antigen Based Screening. J Urol 195: 66-73. [Crossref]
- Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM (2016) Increasing incidence of metastatic prostate cancer in the United States (2004-2013). Prostate Cancer Prostatic Dis 19: 395-397. [Crossref]
- Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, et al. (2013) Early detection of prostate cancer: AUA Guideline. J Urol 190: 419-426. [Crossref]
- Jacobsen SJ, Katusic SK, Bergtralh EJ, Oesterling JE, Ohrt D, et al. (1995) Incidence of prostate cancer diagnosis in the eras before and after serum prostate-specific antigen testing. JAMA 274: 1445-1449. [Crossref]
- Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, et al. (2009) Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst 101: 374-383. [Crossref]
- Schipper M, Wang G, Giles N, Ohrnberger J (2015) Novel prostate cancer biomarkers derived from autoantibody signatures. Transl Oncol 8: 106-111. [Crossref]
- Wang X, Yu J, Sreekumar A, Varambally S, Shen R, et al. (2005) Autoantibody signatures in prostate cancer. N Engl J Med 353: 1224-1235. [Crossref]
- Thompson IM, Pauler DK, Goodman PJ, et al. (2004) Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med 350: 2239-2246. [Crossref]
- Soussi T (2000) p53 Antibodies in the sera of patients with various types of cancer: a review. Cancer Res 60: 1777-1788. [Crossref]
- Hafron J, Sing S, Yu H. Development of a novel magneto-sensing (MagArray) autoantibody assay to detect high-grade prostate cancer. [Data on file].
- Freedland S, Howard L, Singh S. A novel serum based magneto-sensing autoantibody assay to predict high-grade prostate cancer at biopsy. [Data on file].
- Freedland S, Howard L, Singh S. A novel serum based magneto-sensing autoantibody assay predict high-grade prostate cancer at biopsy in patients with PSA less than 4 ng/ml. [Data on file].
Editorial Information
Editor-in-Chief
Jinyong Peng
Article Type
Case Report
Publication history
Received date: September 11, 2017
Accepted date: October 10, 2017
Published date: October 13, 2017
Copyright
© 2017 Hafron JM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Hafron JM, Yu H, Juang A, Vuong D, Kamer S, et al. (2017) New developments in prostate cancer screening using a novel cancer-specific, non-PSA biomarker assay derived from autoantibody signatures. J Med Therap 1: DOI: 10.15761/JMT.1000119


