Wireless telecommunication sources working with frequencies ranging from 0.9 to 2.5 GHz are still increasing rapidly. Among these are also digitally enhanced cordless telecommunication (DECT) phones which emit only a weak radiation when an active DECT base and its handset are separated from each other. To address this topic, we investigated the cellular effects of DECT base radiation and its possible compensation by specially designed novel resonance devices. Connective tissue fibroblasts (L-929) were exposed to the radiation of an active commercially available DECT base ± single resonance devices or combination of them. Unexposed cells in another incubator without any DECT base radiation served as corresponding controls. Cell vitality was checked by measurement of the enzymatic activity of mitochondrial dehydrogenases by the color change of the sodium salt 2,3-bis[2-methoxy-4-nitro-5-sulfo-pheny]-2H-tetrazolium-5-carboxyanilide (XTT).
The results clearly demonstrate that exposure to DECT base radiation caused a significantly reduced cell vitality which was accompanied by marked morphological changes in the cells such as intracellular vacuolization, rounding and detachment. Approximately 15% of the reducing potential was related to microwave-related warming. Reduction in cell vitality after DECT base radiation exposure was partially neutralized by use of one of the novel resonance devices alone or in combination. Especially resonance device type A-1 was very effective and its potential for neutralization could be increased to 95% by additionally using two crossed resonance devices type RD-B with their plus end directed towards the cells.
electromagnetic radiation, digitally enhanced cordless telecommunication (DECT), microwaves, health effects, cell death, cell culture
Abbreviations
DECT: digitally enhanced cordless telecommunication; RD-A, RD-B: resonance device types
Mobile phones, digitally enhanced cordless telecommunication (DECT) phones, routers and others belong to a group of wireless telecommunication sources which have caused a dramatic increase in environmental levels of electromagnetic radiation [1,2]. All these sources emit radiation with different characteristics in a wide spectrum of frequencies ranging from 0.9 to 2.5 GHz. Although the energy of this type of radiation is quite weak, recent research studies have provided strong evidence that electromagnetic radiation influences human wellbeing and health by affecting biological and biochemical processes [3-8]. Due to its world-wide importance with more than 5 billion users [9], mobile phone technology has been extensively investigated for its health effects at the cellular, experimental animal, and epidemiological level. Epidemiological and experimental research on DECT base and handset radiation exposure which might be also potentially harmful to millions of people is very limited [10].
Given the limited available data, the objective of the present study was to investigate whether newly created resonance devices, either alone or in combination, might be able to neutralize DECT base radiation.
DECT phone
The active base of a commercially available DECT phone (Gigaset 4010 Classic; Siemens, Germany) was used for the experiments described here. Analysis of the frequency characteristics gave a sharp peak at 1.885 GHz with - 46.47 dBm. For further details, see Dartsch and Dochow [11].
Resonance devices
Basically, the resonance devices consist of passive elements or compartments with a length of 20 to 40 cm and a tube diameter of 5 cm without any electronic parts. The devices are filled with layers of material of iron, zinc, copper, magnetized metal parts, wood (cardboard), carbon or carbon related materials and varying quartz minerals. The use of hollow conductor elements was assumed from the usual high frequency electromagnetic signal transmission [12,13].
Three different resonance devices of type A were used for the experiments presented here: (1) Type A1 (RD-A1) consisted of two small copper hollow conductors with two crossing copper wires. These copper hollow conductors were surrounded by a bigger zinced iron hollow conductor. Both hollow conductors were made by copper and zinced iron and were filled up with different mixed quartz granulates and two compartment separating layers based on carbon materials. At the end of this resonance device unit with its internal passing power supply wires, a ring of rose quartz and a ring magnet element was placed around both wires. In addition, the round copper hollow conductors, which were separated in the middle of the tube in two compartments were made by a fixed copper tongue. (2) The second type A2 (RD-A2), was made by two small separated copper hollow conductors which surround the two wires independently. These two hollow conductors were used instead of one and the copper hollow conductor compartments as used in RD-A1. (3) The third type (RD-A3) was assembled identically as RD-A2, but had a reversed magnet ring south/north polarization.
The second resonance device type was RD-B [14] which consisted of a tube filled with different layers or sheeting materials as in RD-A (cardboard, iron/zinc, copper magnetized metal parts, quartz, carbon (plastic) as granulate. As depicted in Figure 1, its central element was a copper hollow conductor filled with quartz granulate mixtures. The conductor inlay was divided in two compartments by a central layer of carbon granulate. Both tubes were closed up by a bigger rose quartz piece.
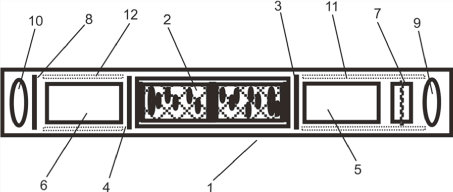
Figure 1. Schematic presentation of a resonance device (type RD-B) consisting of a housing (1), a copper hollow conductor filled up with varying quartz granulates (2), carbon and zinced iron sheet (3), copper sheet (4), tube elements filled with quartz (5,6), magnet element (7), zinced iron sheet (8), rose quartz pieces (9,10) and some cardboard anti-shake elements (11,12). RD-A consists of two additional AC power supply wires inside from right (input) to left (output) crossing the element number (2) as a copper/iron hollow conductor plus its wire surrounding elements (7,10).
Cell culture and test procedure
In the present study, cultured connective tissue fibroblasts (cell line L-929; Leibniz-Institut, Deutsche Sammlung für Mikroorganismen und Zellkulturen, Braunschweig, Germany) as a standard cell line for toxicological studies were taken at passages 22 to 83 over a total experimental period of 8 months. Cells were routinely cultivated in the moist atmosphere of an incubator at 37°C and gassed with 5% CO2 and 95% air to yield a constant pH value. Culture medium was RPMI 1640 with 10% fetal calf serum and standard amounts of penicillin and streptomycin. All cell culture reagents were from GE Healthcare Life Sciences; Freiburg, Germany.
For the tests, cells were seeded from 80 to 90% confluent mass cultures at a density of 20,000 cells/well into 16 wells in the middle part of a 96 well-plate (200 µL culture medium/well). After 24 hours to ensure cell attachment and metabolization, culture medium was exchanged to 250 µL/well of Leibowitz L-15 medium (Biochrom; Berlin, Germany) containing 10% fetal calf serum and standard amounts of penicillin and streptomycin. This culture medium guarantees a pH value at 7.4 even at normal atmospheric conditions. Plates were transferred to a Cultura M mini incubator and cultivated at 37 ± 1°C without CO2-gassing.
The active DECT base was directly placed on the lid of the culture plate and cells were exposed to the DECT base radiation at continuous operation for the next 24 hours. Approximately 10 meters distance in the same laboratory rooms, a second Cultura M mini incubator was taken for the untreated control cells in a 96-well plate at the same cultivation conditions.
After 24 hours of continuous exposure, cell vitality was checked by morphological observation of the cell cultures and by enzymatic activity. For this purpose, cell culture medium was removed and replaced by 180 µL fresh culture medium and 20 µL XTT (Xenometrix AG, Allschwil, Switzerland) and incubated for 120 minutes in the incubator at 37°C.
XTT is the sodium salt of 2,3-bis[2-methoxy-4-nitro-5-sulfo-pheny]-2H-tetrazolium-5-carboxyanilide and has a yellowish color. Mitochondrial dehydrogenases of viable cells cleave the tetrazolium ring of XTT to yield orange formazan crystals which are soluble in aqueous solutions. The intensity of the resulting orange solution correlates directly with cell vitality and metabolic activity [15,16].
After 120 minutes, optical density was measured as a differential measurement of ΔOD = 450 minus 690 nm after a 4 second shaking interval using an ELISA reader (BioTek Slx808). The experiments were performed in several independent test series as indicated in the legends of tables. Statistical analysis was conducted using Wilcoxon-Mann-Whitney test.
As shown in Table 1, exposure of connective tissue fibroblasts to the active DECT base for 24 hours caused a reduced cell vitality in all experiments when compared to untreated control cells (cell vitality was set as 100%). When the results of the single experiments are taken together, cell vitality was reduced to 44.06 ± 5.93% (mean value ± standard error of the mean), i.e., that about two third of the cells have lost their vitality. In order to block the influence of emitted microwaves from the active DECT base [17] which might cause a local rise of temperature and, thus, might also account for the observed loss of cell vitality, the same experiments were conducted with a corrugated card board between the DECT base and the cell layers. As shown in Table 1, the setup caused a marked reduction in cell vitality to a level of 58 ± 0.1% (mean value ± standard error of the mean). Both of these values, with and without corrugated card board, differed significantly from untreated control cells as checked by Wilcoxon-Mann-Whitney test (P ≤ 0.01). Moreover, the values demonstrate that only approximately 15% of the cellular effects caused by DECT base radiation were related to the emission of microwaves. The reduced cell vitality after DECT base radiation exposure also resulted in a largely altered morphology of connective tissue fibroblasts with intracellular vacuolization and rounding of cells with long cytoplasmic protrusions or even detachment (Figures 2a, 2b).
Table 1. Experimental data of each experiment with connective tissue fibroblasts exposed to DECT base radiation ± corrugated card board. Cell vitality of control cells is set as 100 %. Number of independent experiments = 3 and 2. S.E.M. = Standard error of the mean.
Description of
experimental setup |
Number of
data points |
Cell vitality
in % (mean value of
all data points) |
± |
S.E.M
(of all data
points in %) |
Cell vitality in %
(mean value of
all experiments) |
± |
S.E.M
(of all
experiments in %) |
Exposure to DECT base radiation |
2´ 14 |
54.18 |
± |
1.84 |
44.06 |
± |
5.93 |
Exposure to DECT base radiation |
2´ 20 |
44.34 |
± |
6.10 |
Exposure to DECT base radiation |
2´ 20 |
33.66 |
± |
3.05 |
Exposure to DECT base radiation and corrugated card board |
2´ 20 |
57.87 |
± |
3.58 |
58.00 |
± |
0.10 |
Exposure to DECT base radiation and corrugated card board |
2´ 20 |
58.12 |
± |
3.48 |
The fact that wireless telecommunication sources might cause unwanted health effects is still under controversial discussion. Mobile phones have become the main wireless telecommunication source worldwide, but wireless DECT phones are still in use in millions of domestic homes and at workplaces. Although DECT phones are considered to emit only a weak radiation when an active DECT base and handset are separated from each other, this radiation is able to reduce cell vitality by about 65% as shown in this study. Only 15% of this radiation causing cell death are related to microwaves; the other amount of radiation seems to be due to the frequency of 1.885 GHz. However, the cellular effects as observed here are in accordance with previous studies on other cell types [18,19] and seem to be related to oxidative stress [18-22].
One might argue that an active DECT base for a continuous period of 24 hours and a distance of only some centimeters between cells and DECT base might be not a realistic situation. However, there are numerous people who have a DECT base nearby and the handset placed on the table nearly every day. Under these circumstances, the cellular effects of an active DECT base as described in this study become more prominent.
The use of the resonance devices RD-A1, RD-A2 and RD-A3 during 24 hours of exposure of the cells to DECT base radiation resulted in a reduction of cell vitality which was markedly different from the experiments without the device (Table 2). Depending on the type of resonance device used, cell vitality was 86.8 ± 4.3 (RD-A1), 75.36 ± 5.27 (RD-A2) and 65.71 ± 5.02 (RD-A3). All data are mean values ± standard error of the mean. Thus, a significant part of the DECT base radiation could be neutralized by the resonance devices. The efficiency of RD-A1 to neutralize DECT base radiation was also visualized microscopically as the use of this device resulted in a cell morphology which did not differ markedly from the morphology seen for control cells (Figure 2c).
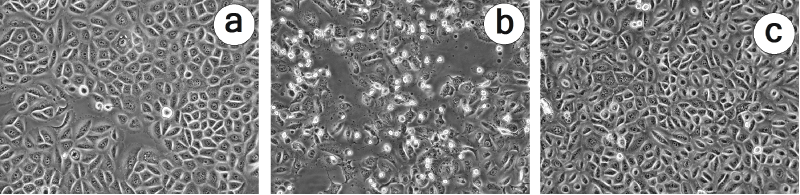
Figure 2. Micrographs illustrating the alterations in cell morphology of connective tissue fibroblasts which were exposed to DECT base radiation for 24 hours (b) in comparison to untreated control cells (a) or cells which were treated with DECT base radiation + resonance device RD-A1 (c). Phase contrast microscopy at an Olympus IX50 inverted microscope equipped with an Olympus 20x Planachromate and an Olympus E-10 digital camera at 4 megapixels.
Table 2. Experimental data of each single experiment with connective tissue fibroblasts exposed to DECT base radiation and resonance devices RD-A1, RD-A2 and RD-A3. Cell vitality of control cells is set as 100 %. Number of independent experiments = 4 and 3. S.E.M. = Standard error of the mean.
Description of experimental setup |
Number of
data points |
Cell vitality in %
(mean value of all
data points) |
± |
S.E.M (of all
data points in %) |
Cell vitality in %
(mean value of
all experiments) |
± |
S.E.M
(of all
experiments in %) |
Exposure to DECT base radiation + Resonance Device (RD) A1 |
2´ 14 |
78.64 |
± |
2.32 |
86.80 |
± |
4.30 |
Exposure to DECT base radiation + Resonance Device (RD) A1 |
2´ 14 |
96.34 |
± |
1.79 |
Exposure to DECT base radiation + Resonance Device (RD) A1 |
2´ 20 |
85.19 |
± |
5.43 |
Exposure to DECT base radiation + Resonance Device (RD) A1 |
2´ 20 |
86.63 |
± |
3.64 |
Exposure to DECT base radiation + Resonance Device (RD) A2 |
2´ 14 |
84.64 |
± |
2.92 |
75.36 |
± |
5.27 |
Exposure to DECT base radiation + Resonance Device (RD) A2 |
2´ 20 |
66.29 |
± |
1.73 |
Exposure to DECT base radiation + Resonance Device (RD) A2 |
2´ 20 |
75.15 |
± |
4.27 |
Exposure to DECT base radiation + Resonance Device (RD) A3 |
2´ 20 |
62.46 |
± |
6.90 |
65.71 |
± |
5.02 |
Exposure to DECT base radiation + Resonance Device (RD) A3 |
2´ 20 |
61.09 |
± |
8.30 |
Exposure to DECT base radiation + Resonance Device (RD) A3 |
2´ 20 |
60.53 |
± |
4.30 |
Exposure to DECT base radiation + Resonance Device (RD) A3 |
2´ 20 |
78.77 |
± |
1.86 |
When executing the experimental setup in a more complex way by using RD-A1 or RD-A2 in combination with two crossed RD-B with their minus end directed towards the cells, the positive effects of RD-A1 and RD-A2 were completely erased and cell vitality did not differ from the levels which were obtained without the device (Table 3).
Table 3. Experimental data of each experiment with connective tissue fibroblasts exposed to DECT base radiation + two different resonance devices RD-A1 and RD-A2 in combination with two crossed resonance devices RD-B with their minus end directed towards the cells. Cell vitality of control cells is set as 100 %. Number of independent experiments = 2. S.E.M. = Standard error of the mean.
Description of experimental
setup |
Number of
data points |
Cell vitality in %
(mean value of all
data points) |
± |
S.E.M
(of all data
points in %) |
Cell vitality in %
(mean value of
all experiments) |
± |
S.E.M
(of all
experiments in %) |
Exposure to DECT base radiation +RD-A1+ two crossed RD-B with their minus end directed towards the cells |
2´ 20 |
38.74 |
± |
2.68 |
45.44 |
± |
6.72 |
Exposure to DECT base radiation +RD-A1+ two crossed RD-B with their minus end directed towards the cells |
2´ 20 |
52.14 |
± |
5.55 |
Exposure to DECT base radiation +RD-A2+ two crossed RD-B with their minus end directed towards the cells |
2´ 20 |
48.16 |
± |
4.52 |
41.63 |
± |
6.55 |
Exposure to DECT base radiation +RD-A2+ two crossed RD-B with their minus end directed towards the cells |
2´ 20 |
35.09 |
± |
7.87 |
When having the same basic setup as above with the resonance devices RD-A1 and RD-A2, but with two crossed RD-B with their plus end directed towards the cells, an even stronger neutralizing effect of RD-A1 and RD-A2 in comparison to the basic setup without crossed RD-B was observed (Table 4). Under these conditions, RD-A1 was able to neutralize the DECT base radiation to yield a vitality level of 95.22 ± 5.92% (mean value ± standard error of the mean) and RD-A2 yielding a vitality level of 79.33 ± 20.85% (mean value ± standard error of the mean). When conducting Wilcoxon-Mann-Whitney test, there was no longer a statistically relevant difference between control cells and RD-A1 in combination with two crossed RD-B directed with their plus end towards the cells. For RD-A2 and the two crossed RD-B directed with their plus end towards the cells, at least a slightly lower reduction of cell vitality was obtained when compared with RD-A2 alone.
Table 4. Experimental data of each experiment with connective tissue fibroblasts exposed to DECT base radiation + two resonance devices RD-A1 and RD-A2 in combination with two crossed resonance devices RD-B with their plus end directed towards the cells. Cell vitality of control cells is set as 100 %. Number of independent experiments = 3 and 2. S.E.M. = Standard error of the mean
Description of experimental
setup |
Number of
data points |
Cell vitality in %
(mean value of all
data points) |
± |
S.E.M
(of all data
points in %) |
Cell vitality in %
(mean value of
all experiments) |
± |
S.E.M
(of all
experiments in %) |
Exposure to DECT base radiation +RD-A1+ two crossed RD-B with their plus end directed towards the cells |
2´14 |
94.78 |
± |
1.87 |
95.22 |
± |
5.92 |
Exposure to DECT base radiation +RD-A1+ two crossed RD-B with their plus end directed towards the cells |
2´20 |
89.52 |
± |
3.10 |
Exposure to DECT base radiation +RD-A1+ two crossed RD-B with their plus end directed towards the cells |
2´20 |
101. 35 |
± |
3.25 |
Exposure to DECT base radiation +RD-A2+ two crossed RD-B with their plus end directed towards the cells |
2´14
|
94.07
|
±
|
2.18 |
79.33 |
±
|
20.85 |
Exposure to DECT base radiation +RD-A2+ two crossed RD-B with their plus end directed towards the cells |
2´20 |
64.59 |
± |
2.70 |
Quite surprising in its straightforwardness were the results when one or more resonance devices were used to neutralize DECT base radiation. As shown here in different independent experiments, the setup was able to neutralize the unwanted cellular effects of the radiation at a different extent. Why especially resonance device type A-1 was most effective and its potential for neutralization was increased to 95% by additionally using two crossed resonance devices type RD-B with their plus end directed towards the cells, is currently unknown. However, the results presented here can be used as a basis for further investigations in this field.
- Ahlbom A, Feychting M (2003) Electromagnetic radiation. Br Med Bull 68: 157-165. [Crossref]
- Sage C, Carpenter DO (2009) Public health implications of wireless technologies. Pathophysiology 16: 233-246. [Crossref]
- Diem E, Schwarz C, Adlkofer F, Jahn O, Rüdiger H (2005) Non-thermal DNA breakage by mobile-phone radiation (1800 MHz) in human fibroblasts and in transformed GFSH-R17 rat granulosa cells in vitro. Mut Res/Gen Toxicol Environ Mutagen 583: 178-183. [Crossref]
- Blank M, Goodman R (2009) Electromagnetic fields stress living cells. Pathophysiology 16: 71-78. [Crossref]
- Hardell L, Carlberg M (2009) Mobile phones2021 Copyright OAT. All rights reservain tumours. Int J Oncol 35: 5-17. [Crossref]
- Kundi M, Hutter HP (2009) Mobile phone base stations-Effects on wellbeing and health. Pathophysiology 16: 123-135. [Crossref]
- Levitt BB, Lai H (2013) Biological effects from exposure to electromagnetic radiation emitted by cell tower base stations and other antenna arrays. Environ Rev 18: 369-395.
- Vijayalaxmi, Scarfi MR (2014) International and national expert group evaluations: biological/health effects of radiofrequency fields. Int J Environ Res Public Health 11: 9376-9408. [Crossref]
- Davis DL, Kesari S, Soskolne CL, Miller AB, Stein Y (2013) Swedish review strengthens grounds for concluding that radiation from cellular and cordless phones is a probable human carcinogen. Pathophysiol 20: 123-129. [Crossref]
- Redmayne M, Inyang I, Dimitriadis C, Benke G, Abramson MJ (2010) Cordless telephone use: implications for mobile phone research. J Environ Monit 12: 809-812. [Crossref]
- Dartsch PC, Dochow T (2017) Cellular effects following exposure to wireless DECT base radiation and presentation of a device for their compensation. J Complement Alternat Med Res 3: 1-9.
- König FM (2017) Neutralisierung ungünstig einwirkender, technischer Wechselfelder. Raum & Zeit 206: 2-7.
- König FM (2016) Vorrichtung zur Reduzierung der Belastung von Organismen durch elektromagnetische Wechselfelder (EMF) elektrischer Verbraucher. Patent Application No. DE10201600497.6
- König FM (2016) Vorrichtung zur Reduktion der EMF-Belastung von Organismen. Patent Application No. DE102016001982.4
- Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL (1991) An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods 142: 257-265. [Crossref]
- Brosin A, Wolf V, Mattheus A, Heise H (1997) Use of XTT-assay to assess the cytotoxicity of different surfactants and metal salts in human keratinocytes (HaCaT). A feasible method for in vitro testing of skin irritants. Acta Dermato-venereologica 77: 26-28. [Crossref]
- Hamnerius Y Uddmar T (2000) Microwave exposure from mobile phones and base stations in Sweden. In: Proceedings of the International Conference on Cell Tower Sitting 52-63, 2000.
- Lixia S, Yao K, Kaijun W, Deqiang L, Huajun H, et al. (2006) Effects of 1.8 GHz radiofrequency field on DNA damage and expression of heat shock protein 70 in human lens epithelial cells. Mutat Res 602:135–142. [Crossref]
- Hou Q, Wang M, Wu S, Ma X, An G, et al. (2015) Oxidative changes and apoptosis induced by 1800-MHz electromagnetic radiation in NIH/3T3 cells. Electromagn Biol Med 34:85-92. [Crossref]
- Lantow M, Lupke M, Frahm J, Mattsson MO, Kuster N, et al. (2006) ROS release and Hsp70 expression after exposure to 1800 MHz radiofrequency electromagnetic fields in primary human monocytes and lymphocytes. Radiat Environ Biophys 45:55-62. [Crossref]
- Lantow M, Schuderer J, Hartwig C, Simko M (2006) Free radical release and HSP70 expression in two human immune-relevant cell lines after exposure to 1800 MHz radiofrequency radiation. Radiat Res 165:88–94. [Crossref]
- Dasdag S, Akdag MZ (2016) The link between radiofrequencies emitted from wireless technologies and oxidative stress. J Chem Neuroanat 75: 85-93. [Crossref]


