Introduction
Graves’ Disease (GD) or von Basedow disease, is a syndrome characterized by an enlarged and overactive thyroid gland (Graves’ hyperthyroidism), ocular abnormalities (Graves’ orbitopathy; GO) and localized dermopathy (pretibial myxoedema; PTM) [1,2].Graves is the most common cause of hyperthyroidism worldwide, it affects ~2% of women and 0.2% of men in the world (female to male ratio ~10:1), clinical triad documented in only 10-15% of cases. Epidemiological studies indicate that the incidence of GD is ~20–40 cases per 100,000 annually. GD is most common in adults aged between 20 and 50 years and it is assumed that the majorityof patients older than 40 years with hyperthyroidism have GD[1].
GD can present within two extremes — an asymptomatic mild form, usually identified by decreased serum TSH levels on routine thyroid function testing, or as a severe, life-threatening ‘thyroid storm’ with a high mortality, which is an accelerated hyperthyroidism, that can present with delirium, tachycardia, increased blood pressure, high fever, etc. Thyroid disorders are known to cause neuropsychiatric manifestations some of which are depression, dementia, mania, and autoimmune Hashimoto encephalopathy.However, these manifestations are often overlooked, especially in the 15% of patients who debut with T3-toxicosis or in older adults with so-called apathetic hyperthyroidism[2].
Moreover, it is acceptedthat mental disorders merge highly with thyroid diseases as indicated by terms such as“Basedow psychosis” and “myxoedema psychosis”. This connections are debatable however, because only patients with potential mental conditions and who were introduced to psychiatry were previously analyzed[2,3]. Previous research investigating the association of depression with medical conditions have indicated that hypothyroidism and hyperthyroidism show a 56% and 31% merging rate, respectively[3].
According to studies, during depressive states, there have been shown alterations in serum TSH levels, which suggestsa merge between mental alterations and the hypothalamus pituitary thyroid axis (HPT axis)[1], and its direct correlationwith the state of the illness. Depression can also lead to an aggravation of Graves’ hyperthyroidism[4,5].Thus it could be implied that depression-anxiety and thyroid have a bidirectional causality [Figure 1].
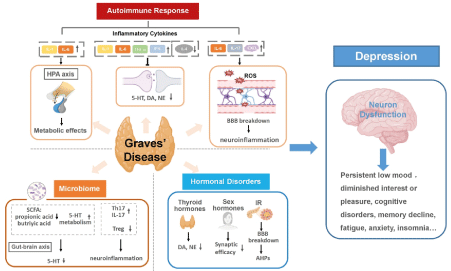
Figure 1: Graves’ disease as a mechanistic driver of depression (and psychosis). Specific pathways of autoimmune responses, hormonal disorders and microbiota dysbiosis in GD trigger neuron dysfunction in the brain and promote the development of depression. Autoimmune responses in GD cause altered inflammatory cytokines levels. Continuous activation of the HPA axis induced by persistent inflammatory cytokines stimulation can damage neurons and activate continuing central inflammation, along with affecting the expression and action of hormone receptors. Inflammatory cytokines can also disrupt monoamine neurotransmitter metabolism, resulting in lower accessibility to these neurotransmitters. The increase of blood–brain barrier (BBB) permeability in the inflammatory state of GD allows inflammatory cytokines to enter the brain and may lead to neuroinflammation. Modified from [5]
The presence of TSHR autoantibodies and TSHR-specific T cells on TSHR expressed in non-thyroidal tissues, in particular fibroblasts and adipocytes can induce the development of the Graves´ Triad (goiter,GO and PTM). It is remarkable that GO is clinically apparent in ~5% of patientsand it is precisely these patients who tend to have higher levels of TSH receptor stimulating autoantibodies (TRAb)[2,6].
The HPT axis has pathways hypothalamic and a malfunction in any of them can lead to behavioral changes. TRH stimulates the pituitary gland to secreteTSH , which regulates the secretion of thyroid hormones; the system is stimulated and down regulated by thyroid hormones. Cortisol, which is stimulated by the hypothalamus pituitary adrenal axis, stress, and depression, inhibits TRH and TSH[6].Cortisol elevation causes a decrease in T3 up to 20% in the cerebral cortex, predominantly in the fraction produced by the thyroid gland, not dependent on peripheral cleavage from T4[4-6].
Mostof theT4 enters the brain via large transporters such as transthyretin (TTR). Deiodization occurs in glial cells. In glial cells, T4 is converted to T3. The action of T3 is mediated by binding to the thyroid hormone nuclear receptor (THRs)[3-6]. THR-αis highly expressed in the adult brain and constitutes 70–80% of the THR distribution.A blunted reaction of TSH to a TRH load stimulation has been reported in depressed patients without thyroid diseases[7].
GD triggers neuron dysfunction in the brain and promote the development of depression. Depressed patients compared to healthy controls, have shown higher concentration levels of TSH receptor antibodies (TRAb)[8]. Autoimmune responses in GD cause altered inflammatory cytokines levels. Continuous activation of the HPA axis induced by persistent inflammatory cytokines stimulation can damage neurons and activate continuing central inflammation.Cytokines can lead to various neuropsychiatric effects, such as disrupting neurotransmitter metabolism, inducing blood–brain barrier (BBB) dysfunction[5].The increase of BBB permeability in the inflammatory state of GD allows inflammatory cytokines to enter the brain and may lead to neuroinflammation[7,9].
Multiple hormonal disorders can develop as a result of GD hyperthyroidism, includinginsulin and sex hormones. The increase of thyroid hormones can reduce the secretions of dopamine (DA) and norepinephrine (NE). Insulin Resistance (IR) in GD can increase both blood and intracellular glucose level, inducing neuronal tissue damage and afterhyperpolarization. Thus, it is concluded that multiple mechanisms are involved in both psychosis and affective disorders associated with Graves' disease, many perhaps related to intracellular signaling pathways mediated by T3, the most metabolically active thyroid hormone, making this section of the clinical spectrum the "hairs of the jellyfish" in the Graves' disease setting[Figures 2,3] [10,11].

Figure 2: “The hairs of the jellyfish”is a metaphor related to mental disorders of organic originin some academic contexts. Image taken from [10]
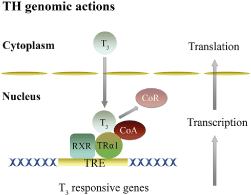
Figure 3: Genomic mechanisms of thyroid hormones action in the brain. Genomic actions of T3 are dependent on gene transcription mediated by its binding to nuclear TRα and TRβ, and the formation of heterodimer complex with RXR (RXR-TR) that binds to a TRE, located at the regulatory region of T3 target genes. This activity is regulated by an exchange of CoR for CoA. CoA, Co-activator; CoR, Co-repressor; RXR, Retinoid X receptor; TR, Thyroid hormone receptor; TRE, Thyroid response element; T3, 3,5,3′ -triiodo-L-thyronine. Adapted from [11]
Finally, by way of closing, the following poem:
Visible, invisible,
A fluctuating charm,
An amber-colored amethyst
Inhabits it; your arm
Approaches, and
It opens and
It closes;
You have meant
To catch it,
And it shrivels;
You abandon
Your intent—
It opens, and it
Closes and you
Reach for it—
The blue
Surrounding it
Grows cloudy, and
It floats away
From you.
Marianne Moore (1887 – 1972)[12]
References
- Davies TF, Andersen S, Latif R, Nagayama Y, Barbesino G, et al. (2020) Graves' disease. Nat Rev Dis Primers 6: 52. [Crossref]
- Bunevicius R, Prange AJ Jr (2006) Psychiatric manifestations of Graves' hyperthyroidism: pathophysiology and treatment options. CNS Drugs 20: 897-909. [Crossref]
- Fukao A, Takamatsu J, Arishima T, Tanaka M, Kawai T, et al. (2019) Graves' disease and mental disorders. J Clin Transl Endocrinol 19: 100207. [Crossref]
- Lekurwale V, Acharya S, Shukla S, Kumar S (2023) Neuropsychiatric Manifestations of Thyroid Diseases. Cureus 15: e33987. [Crossref]
- Song Y, Wang X, Ma W, Yang Y, Yan S, et al. (2023) Graves' disease as a driver of depression: a mechanistic insight. Front Endocrinol (Lausanne) 14: 1162445. [Crossref]
- Jurado-Flores M, Warda F, Mooradian A (2022) Pathophysiology and Clinical Features of Neuropsychiatric Manifestations of Thyroid Disease. J Endocr Soc 6: bvab194. [Crossref]
- Bunevicius R, Prange AJ Jr (2010) Thyroid disease and mental disorders: cause and effect or only comorbidity? Curr Opin Psychiatry 23: 363-368. [Crossref]
- Bode H, Ivens B, Bschor T, Schwarzer G, Henssler J, et al. (2022) Hyperthyroidism and clinical depression: a systematic review and meta-analysis. Transl Psychiatry 12: 362. [Crossref]
- Ren X, Chen H (2022) Changes in Th9 and Th17 lymphocytes and functional cytokines and their relationship with thyroid-stimulating hormone receptor antibodies at different stages of graves' disease. Front Immunol 13: 919681. [Crossref]
- Lucas CH, Graham WM, Widmer C (2012) Jellyfish life histories: role of polyps in forming and maintaining scyphomedusa populations. Adv Mar Biol 63: 133-196. [Crossref]
- Talhada D, Santos CRA, Gonçalves I, Ruscher K (2019) Thyroid Hormones in the Brain and Their Impact in Recovery Mechanisms After Stroke. Front Neurol 10: 1103. [Crossref]
- Hicok B (2000) To Work “Lovingly”: Marianne Moore at Bryn Mawr, 1905-1909. Journal of Modern Literature 23: 483-501.



