A complete spinal cord injury (SCI) is the complete sensory and motor loss below the site of spinal cord injury following acute or chronic destruction, compression, or ischemia of the spinal cord. Initially, this may present as spinal shock, which is an acute physiological loss or depression of spinal cord function. It presents as a flaccid areflexic paralysis below the level of the injury with autonomic features (e.g., hypotension and bradycardia). After some days to weeks the spinal shock wears off and a complete spinal cord injury may remain. It presents with spastic paresis, hyperreflexia, and continued sensory loss. The differentiation and regeneration potential of stem cells has been well studied in both preclinical and clinical investigations. A traumatic spinal cord injury triggers a complex local inflammatory reaction capable of enhancing repair and exacerbating pathology (Figure 1).
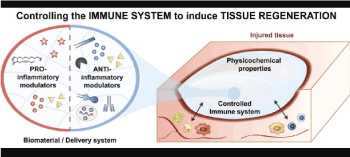
Figure 1. Local inflammatory reaction and immune response: tissue regeneration
The composition and effector potential of the post-injury cellular and molecular immune cascade changes as a function of time and distance from the lesion [1]. In recent years, a series of experiments have revealed that replacement of damaged or defective cells by exogenously administered stem cells is critical in achieving their therapeutic effects in various diseases including spinal cord injuries [2]. The production along this time- space continuum of cytokines, proteases, and growth factors establishes dynamic environments that lead to the death, damage, repair or growth of affected neurons and glia [1].
The current clinical report is meant to demonstrate the beneficial changes with the use of Bioquantine® and its administration in a patient with a complete spinal cord injury (Figure 2) offering a possible optional translational therapy and potential neuroregeneration in his clinical condition.
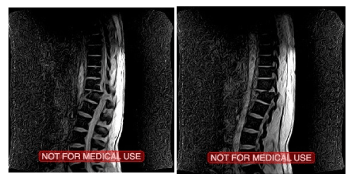
Figure 2.Patient’s initial MRI before the vertebral fixation procedure on 11/ 02/2016
After the decision was made by the medical team and the patient’s consent, the application of a translational protocol based on reported clinical evidence; we applied allogeneic MSCs combined with Bioquantine® in situ at the intrathecal (subdural) pathway. Bioquantine®, same miRNA demonstrated by Sergei Paylian and its evidence of neuroregeneration on murine models [3-5] on its purified form (intra and extra-oocyte liquid phases of electroporated oocytes) [6-8], has been well tolerated by the patient without any anaphylactic reaction. Mesenchymal stem cells (MSCs) are ideal for cell-based therapy in various inflammatory diseases because of their immunosuppressive and tissue repair properties. Moreover, their immunosuppressive properties and low immunogenicity contribute to a reduced or weakened immune response elicited by the implantation of allogeneic MSCs compared with other cell types, allogeneic MSCs are an option because of their low immunogenicity and immunosuppressive and tissue repair capabilities [4].
The importance of immunity in tissue repair and regeneration is now evident. Thus, promoting tissue healing through immune modulation is a growing and promising field. Targeting microRNAs (miRNAs) is an appealing option since they regulate immunity through post-transcriptional gene fine-tuning in immune cells. Indeed, miRNAs are involved in inflammation as well as in its resolution by controlling immune cell phenotypes and functions [9]. As demonstrated and discussed by Celeste Piotto, et al. [9], the immunoregulatory role of miRNAs during the restoration of tissue homeostasis after injury, is mainly focused on neutrophils, macrophages and T lymphocytes. While numerous regulators are known to coordinate the immune response following injury, microRNAs (miRNAs) have emerged as important actors. Therefore, targeting the immunoregulatory roles of miRNAs could become an attractive option for regenerative therapies. miRNAs are well conserved among species and are involved in a variety of key biological processes by controlling post-transcriptional gene expression through decreasing mRNA stability and/or inhibiting translation [9].
In this review and after 8 intrathecal applications of allogeneic MSCs and Bioquantine® in situ combined on percutaneous
procedures in 28 months we have experienced different positive outcomes but also experienced some negative findings throughout the evolution of our protocol for spinal cord injury (SCI). On the last application our patient showed persistent clonus 72hrs after the subdural application, also reported extreme sweating and muscle ache. These similar inflammatory reactions have been reported by Jessica M. D’Amico, et al.[5], in their review it is summarized in both animal and human studies how motoneurons and their activation by sensory pathways become hyperexcitable to compensate for the reduction of functional activation of the spinal cord and the eventual impact on the muscle. Specifically, decreases in the inhibitory control of sensory transmission and increases in intrinsic motoneuron excitability were described. Our evidence based medicine protocol is targeting as a possible option for severe SCI patients [8]. Facing this important reaction in our combinatorial biologics translational protocol and presented for the first time in our patient, was clear for the medical team that the persistent clonus episode needed to be analyze, decided to order different blood work, MRI and spinal fluid test.
An improvement in sensitivity and strength in both striated and smooth muscle was reported by the patient on the first 48hrs, the day after our patient described the intensity of the clonus as uncontrollable intermittent and exhausting. Excessive sweating was followed after the clonus episodes that lasted for about 30 to 90 seconds each and happened approximately every two to four hours.
Requested the MRI did not show any major structural change or additional image report (Figure 3) just a brief comment regarding to a minor subchondral edema post procedure.
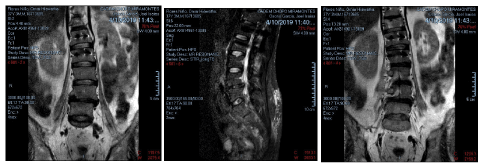
Figure 3. Patients post procedure MRI 04/10/2019
The following day the collected cerebrospinal fluid was sent to the laboratory to do the cytology test and to culture it before a possible neuroinfection. Getting the report days after, did not show positive to bacterial infection (Figures 4 and 5), but the cerebrospinal fluid cytology reported non-specific inflammatory changes.

Figure 4. Cerebrospinal fluid culture report

Figure 5. Cerebrospinal fluid cytology report
We experienced what Celeste Piotto, et al. mentioned: Tissue injury is followed by a cascade of processes leading to the restoration of tissue homeostasis and it is well recognized that the immune system is strongly involved in tissue healing [9].
According to Ilse Bollaerts, et al. [10-14]; the acute inflammatory response that takes place rapidly after traumatic CNS lesions is put forward as one of the major elements affecting the regenerative outcome [10]. Microglia, the main mediators of inflammation in the CNS, are among the first cells to respond to damage. They become activated, thereby changing their morphology from ramified to amoeboid, proliferate, migrate to the injury site, and start to produce a variety of pro- and anti-inflammatory cytokines [11]. Furthermore, neutrophils and macrophages from the periphery are recruited to the injured area, and, together with reactive astrocytes, microglia/macrophages will contribute to the formation of a regeneration-inhibiting glial scar [12]. Traditionally, the acute inflammatory response has been viewed as a detrimental orchestrator in CNS damage and pathology. After spinal cord injury, depletion of peripheral macrophages enhances axonal regeneration and improves functional recovery [13]. The damage to the spinal cord is followed by an acute inflammatory response. Resident microglia are activated, and neutrophils, macrophages, and lymphocytes infiltrate the lesion site. Also here, this inflammatory reaction eventually becomes chronic, and reactive astrocytes form a regeneration-inhibiting glial scar [13]. In the search for the exact contribution of inflammation to the regenerative potential, most studies in the field of spinal cord regeneration have focused on the role of microglia and blood-borne macrophages. After a spinal cord injury, proinflammatory, M1-like macrophages are associated with secondary tissue damage, neuronal loss, axon retraction and demyelination, while anti-inflammatory, M2-like macrophages are assumed to be protective and promoting axon growth. In this regard, the balance between pro- and anti-inflammatory macrophages could be a major factor determining the regenerative outcome [13-17]. Indeed, it has been demonstrated that most microglia/ macrophages in the injured spinal cord display an M1-like activation state, with only a limited and transient presence of M2-like cells. Moreover, evidence suggests that lesion-derived factors (cytokines, chemokines, etc.) affect the microglial/macrophage phenotype, thereby favouring the proinflammatory state.
According to different reports on multiple scientific reviews, and mainly due to the signs and symptoms showed by our patient, we decided to control the inflammatory reaction with Prednisone 20mg a day for 5 days when the increase of the clonus intensity and discomfort were referred as uncontrollable. After the recommended steroid dose controlled the inflammation the clonus was totally gone, and the patient continued with his physical rehabilitation program.
As we are promoting our safe combinatorial biologics method based on translational medical applications on SCI, we have also experienced some non positive reactions through our protocol’s evolution, thereby increasing the inflammatory response and facing clonus as a transitory reaction among others. These reactions have been reported on multiple reviews and articles on both animal and human models in some CNS traumatic injuries. The positive effects of our protocol and the remarkable evolution in our patient in only 28 months were not limited by the suffered neuroimmflamatory reaction, by the contrary; it has been reported that takes part of the tissue regeneration and immune adaptation according to multiple medical reviews.
- Alexander JK, Popovich PG (2009) Neuroinflammation in spinal cord injury: therapeutic targets for neuroprotection and regeneration. Prog Brain Res 175: 125-137. [Crossref]
- Shi Y, Cao J, Wang Y (2015) Rethinking regeneration: empowerment of stem cells by inflammation. Cell Death Differ 22: 1891-1892. [Crossref]
- Sergei Paylian (2016) Potential threapeutic applications of extract made from electroporated xenopus laevis frog oocytes in murine models of melanoma, traumatic brain injury and experimental skin wrinkling. BAOJ Pharm Sci 2: 024.
- Kim JS, Choi HW, Choi S, Do JT (2011) Reprogrammed pluripotent stem cells from somatic cells. Int J Stem Cells 4: 1-8.
- D'Amico JM, Condliffe EG, Martins KJ, Bennett DJ, Gorassini MA (2014) Recovery of neuronal and network excitability after spinal cord injury and implications for spasticity. Front Integr Neurosci 8: 36. [Crossref]
- Paylian S (2015) Co-electroporation with Xenopus laevis oocytes reprograms normal and cancerous human cells to resembel induced human pluripotent stem cells. BAOJ Cancer Res Ther 1: 1-13.
- Young W (2014) Spinal cord regeneration. Cell Transplant 23: 573-611.
- Joel Isaias Osorio Garcia (2019) The use of Bioquantine® and allogeneic Mesenchymal Stem Cells combined with a spinal cord stimulation system in a No Option patient with ASIA-A scale. American Journal of Translational Medicine 2474: 28-20.
- Piotto C, Julier Z, Martino MM (2018) Immune regulation of tissue repair and regeneration via miRNAs-new therapeutic target. Front Bioeng Biotechnol 6: 98. [Crossref]
- Benowitz LI, Popovich PG (2011) Inflammation and axon regeneration. Current Opinion in Neurology 24: 577-583.
- Czeh M, Gressens P, Kaindl AM (2011) The yin and yang of microglia. Developmental Neuroscience 33: 199-209.
- Lee-Liu D, Edwards-Faret G, Tapia VS, Larraín J (2013) Spinal cord regeneration: lessons for mammals from non-mammalian vertebrates. Genesis 51: 529-544. [Crossref]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, et al. (1999) Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol 158: 351-365. [Crossref]
- Bollaerts I, Van Houcke J, Andries L, De Groef L, Moons L (2017) Neuroinflammation as fuel for axonal regeneration in the injured vertebrate central nervous system. Mediators Inflamm 2017: 9478542. [Crossref]
- Zhang J, Huang X, Wang H (2015) The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res Ther 6: 234. [Crossref]
- Karantalis V, Schulman IH, Balkan W, Joshua M (2015) Allogeneic cell therapy: A new paradigm in therapeutics. Circ Res 116: 12-15.
- Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA (2010) Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med 5: 933-946.





