In the present study, the perinatal oral exposure of pregnant mice to 15 and 30 mg/kg lithium (lithium chloride) via their drinking water resulted in a significant and dose-dependent reduction in postnatal body weight gain, delays in opening of the eyes and appearance of the body hair fuzz, and deficits in the sensory motor reflexes of the mice pups during weaning period (from the day of birth to postnatal day 21). At adolescent and adult ages of the male offspring, a significant and dose-dependent deficit was also observed in their learning capability (at post-natal day (PD)25), and cognitive behavior (at PD30-36). Furthermore, a significant and dose-dependent disturbance in the levels of neurotransmitters like dopamine (DA) and serotonin (5-HT); non-enzymatic oxidative stress (OS) indices like thiobarbituric acid-reactive substances (TBARS) and total reduced glutathione (GSH); and enzymatic OS indices like glutathione S-transferase (GST), catalase (CAT), and superoxide dismutase (SOD) were observed in the forebrain region of the offspring at post-natal day (PD)7, PD14, PD21, PD30, and PD36. Thus, perinatal lithium exposure can affect the in utero developing fetus, raising concerns for potential neurotoxic hazards and a longer lasting cognitive dysfunction. Reduced use of lithium during pregnancy is of crucial importance in preventing lithium-induced neurotoxicity in the offspring.
lithium, perinatal, mice offspring, developmental, neurobehavior, oxidative stress
Lithium salt is commonly used worldwide as an important drug for the treatment of bipolar manic disorders and manic depression [1-3]. Prolonged usage of lithium for treatment purposes within therapeutic doses and exposure to its different forms of compounds through various sources like metallurgical processes, pharmaceuticals, air conditioning, dehumidifiers, ceramics and lubricant industries, and from biological and chemical laboratories, induce substantial toxic effects [4]. Lithium is easily absorbed from gut, gets distributed readily in the extracellular fluid and then accumulates throughout in the body tissues and almost entirely excreted through kidneys [5,6]. Lithium salt is known to affect metabolism, and cause disturbances in neuronal communication and cell proliferation [7]. Furthermore, lithium is also reported to induce oxidative stress in rat brain [8].
Long-term developmental effects of lithium exposure have not been studied amply in human populations, although continuous use of lithium throughout the gestation period has been associated with perinatal complications like transient neurodevelopmental deficits, depressed neurological functions in the newborns, teratogenic risk to the developing fetus and toxic effects in the neonatal offspring [9,10]. In contrary, it has also been reported that continuous lithium therapy to the mothers during pregnancy does not have any adverse effect on neonatal behavioral development of their children [11]. However, the possibility of realistic long-term effects on the developing fetal brain due to lithium doses during pregnancy cannot be ruled out completely [12-14]. It is well documented that the brain develops mostly during the first trimester of the pregnancy making it highly vulnerable to the cognitive and neurological impact of various drugs used during pregnancy [15]. Long term effects of lithium exposure during perinatal period have not been studied in human [16].
In animals as well, although some long-term effects of lithium exposure have been studied in preadolescent developing rat brains for increases in anxiety – like behavior [17], and in adult rats for some oxidative damages in the kidney, liver and brain [18]. Such studies in offspring have not been undertaken in details except for the study of Abu-Taweel [19] that observes perinatal exposure effects to lithium on some sensory reflexes, locomotory behavior, and some biochemical enzymes in brain and liver tissues of the mice offspring.
The fact that maternal stress during pregnancy can have serious negative effects on the cognitive dysfunctions of the offspring [20,21], the present study was undertaken to explore the effects of perinatal oral lithium-administration to pregnant mice in the offspring for cognitive effects to assess for a possibility of longer lasting effect of lithium toxicity in the offspring data on maternal effects, however, has not been included herein and shall form a part of a separate communication.
Animals
Three females to one male Swiss–Webster strain mice (10–12 weeks old) were used and each were maintained in opaque plastic cages measuring 30 × 12 × 11 cm, under reversed lighting conditions (with white lights on from 22.30 to 10.30 h local time) and at an ambient temperature (regulated between 18 and 22°C). On day one of pregnancy (appearance of vaginal plug was considered as day one of pregnancy), the males were removed from the cages and the females were subjected to experimental treatments. Food (Pilsbury's Diet) and water were available ad libitum, unless otherwise indicated. All study protocols and animal handling procedures were approved by the Research and Ethics Committee of King Saud University, Riyadh, Saudi Arabia and all precautions were taken to minimize animal stress and pain in the animals.
Lithium treatment and experimental design
The pregnant mice were divided into 3 groups of 10 pregnant mice each. The first group (Group I) serving as the control group received plain tap water only. The second and third groups (Group II and III respectively) were treated with lithium 15 and 30 mg/kg body weight per day, respectively, in the form of lithium chloride (LiCl2), dissolved in plain tap water, through oral administration. Our pilot studies have shown that the normal and/or pregnant mice on an average consume 30 ml water per day and thus the lithium doses were prepared accordingly. These lithium doses formed the sole drinking fluid source for the experimental group of pregnant mice during the period of the experiment and are expressed as Low and High Lithium doses in all figures and tables of the present study. Fresh lithium doses were placed in the drinking bottle every day. All pregnant mice were housed individually. Treatment started from day one of pregnancy and was continued until post-natal day 15 (PD 15) and thereafter the mothers were switched to plain tap water. The pups of each experimental group were culled to only eight per dam on post-natal day 1 (PD 1) after birth and were left with their mothers until PD 22. During this weaning period, three male pups of each litter were color marked, and were subjected to various behavioral tests (described below) under dim lighting (ca 8 lux). In total, 21 pups belonging to seven litters from each treatment category were considered. All observations were recorded on PD 1 and repeated every other day until PD 21 in the same three color marked male pups from each litter. These observations were used to measure the early development of sensory motor coordination reflexes together with morphological development in the pups. For statistical analysis, the mean of all three cohorts (color marked pups) per litter was considered as a single score. Thus, seven replicates from each treatment category were considered for the following observations.
Physical assessment during weaning period
Physical developmental landmarks like body weight, opening of the eyes and appearance of body hair fuzz, were evaluated in the developing offspring starting from day 1 after birth (PD 1) through the entire weaning period until PD 21.
Body weight: Weight is a useful indicator of development. Thus, the pups were weighed every alternate day from PD 1 until PD 21.
Eye opening and hair appearance: The day at which the body hair fuzz appeared, and the eyes opened was also recorded. These two parameters are also useful morphological indicators of development.
Neuromotor maturation assessment during weaning period
The neuromotor maturation of the developing reflexes in the developing offspring were measured on every alternate day starting from day 1 after birth (PD 1) through the entire weaning period until PD 21.
Righting reflex: The time taken by a pup placed on its back to turn over and place all four paws on the substrate was recorded. An upper limit of 2 min was set for this test.
Cliff avoidance: Pups were placed on the edge of a table top with the forepaws and face over the edge. The time taken by the pup to back away and turn from the “cliff” was recorded. Again, an upper limit of 2 min was chosen. A latency of 2 min was attributed when the animal fell from the “cliff”.
Rotating reflex: The surface used to measure the rotating reflex was the same as that used for righting reflex, except that it was inclined at an angle of 30°. The pups were placed on this surface with their heads pointed downward. The time elapsed until the pup rotated its body through 180° geonegatively and faced its head upward, was recorded as the rotating time. The upper limit of this test was also set at 2 min.
Cognitive behavioral assessment during post-weaning period
The following tests were evaluated in the same cohort of male offspring (bearing in mind to include representatives of each litter) in all the behavioral observations.
Active avoidance responses: The active avoidance responses were measured in the animals at PD 25, using an automatic reflex conditioner “shuttle box” (Ugo Basile, Comerio, Varese, Italy). The rectangle shaped shuttle-box was divided into two chambers of equal size by a stainless-steel partition with a gate providing access to the adjacent compartment. Before starting the trial sessions, each animal was allowed to adapt and acquaint itself with the shuttle box for 2 min without any stimulus. A 6 s duration light (21 W) and buzzer (670 Hz and 70 dB) were switched on consecutively and used as a conditioned stimulus (CS).
The CS preceded the onset of the unconditioned stimulus (US) by 5 s. The US was an electric scrambler shock (1 mA for 4 s) applied to the grid floor. If the animal avoided the US by running into the other compartment within 5 s after the onset of the CS, the microprocessor recorder unit of the shuttle box recorded an avoidance response and this was considered as conditioned avoidance response to avoid the electric shock. Each animal was given 50 trials with a fixed inter-trial interval of 15 s. During the 50-trial sessions of each individual animal, the total number of avoidances was measured. The total time taken until the animal entered the other compartment to avoid the shock treatment (latency of avoidance response or escape latency in seconds) was also measured for each animal and the results were expressed for each group of animals. The spontaneous migration of the mouse to the other compartment between trials was also assessed by measuring the number of crossings between the chambers when no shock was present during US and CS (inter-trial crossing). The recorder unit of the automated shuttle box continuously recorded these parameters during the whole experimental period (50 trials) of each animal.
Morris water-maze test: The test has been extensively used to assess cognitive functions in rats [22,23] and mice [24,25] models. Starting at the age of PD 30, the mice offspring were tested for visual–spatial memory using a water-maze [26]. The water-maze consisted of a galvanized white circular water tank (90 cm diameter, 50 cm height) filled with clear tap water (22 ± 1°C) to a depth of 15 cm. A 6 × 6 cm size, stainless steel, adjustable, white, escape platform was placed 1 cm below the water level and 13 cm from the rim. The water was made opaque by the addition of 1 l of milk, which prevented visualization of the platform. Four points on the rim of the tank were designated north (N), south (S), east (E) and west (W), thus dividing the pool into four quadrants (NW, NE, SE and SW). On the first day, each offspring (P 30) was allowed to swim freely in the pool for 60 s without the platform present in the pool. This free swim enabled the mice to become habituated to the training environment. On days 2–5, offspring (P 31 to P 35, respectively) were trained for 24 trials (six trials a day, with an inter-trial interval of 30 s) to locate and escape onto the submerged platform. At the start of each trial, the mouse was held facing the perimeter of the water tank and dropped into the pool to ensure immersion. The latency from immersion into the pool to escape onto the hidden platform (maximum duration of trial 120 s) was recorded. If the mouse did not find the platform in 120 s, it was manually guided with the help of a glass rod to mount on the platform and a score of 120 s was recorded for each of such experimenter-terminated trials. The number of such unsuccessful trials was counted and expressed as a percentage of failures on each testing day. On mounting the platform, each mouse was given a 30 s inter-trial interval for rest and for learning and memorizing the spatial cues to reach the platform for escape. To minimize handling, at the end of the trials, the animals were allowed to climb onto a wire mesh grid and transferred to their cage without further handling. On day 6, P 36 mice were subjected to a 120 s probe trial in which the platform was removed from the pool. The time spent in each quadrant (within 120 s probe test time) was recorded on an electronic time recorder. In this part of probe trials in water-maze test, normal animals typically spent more time in the quadrant where the platform had been previously located than in the other quadrants. The testing procedures used during the four days of locating the hidden platform provide a measure of hippocampal-dependent spatial reference memory, while the probe trial is a measure of the strength of spatial learning, the closest parallel to episodic memory in humans [27,28].
Biochemical studies
For biochemical studies, a sub-set of developing offspring (n = 8/group) were sacrificed at different ages (PD 7, 14, 21, 30 and 36) and the level of some neurotransmitters and some enzymatic and non-enzymatic oxidative stress indices were estimated in their fore-brain. The animals were killed by decapitation and the brains were dissected on ice. The fore-brain was isolated (including the cerebral areas with hippocampus and striatum) and frozen in liquid nitrogen and stored at −70°C for determination of neurotransmitters.
Determination of monoamines: The monoamine neurotransmitters dopamine (DA) and serotonin or 5-hydroxytryptamine(5-HT) were estimated using the modified method described in [29]. A 10% homogenate of the fore-brain was prepared by homogenizing the tissues for 10 s in 0.1 M HClO4 containing 0.05% EDTA and centrifuged at 17,000 rpm at 4°C for 5 min. The supernatants were filtered using 0.45 μm pore filters and analyzed by high performance liquid chromatography (HPLC). The mobile phase consisted of 32 mM citric acid monohydrate, 12.5 mM disodium hydrogen orthophosphate, 7% methanol, 1 mM octane sulfonic acid and 0.05 mM EDTA. The mobile phase was filtered through a 0.22 μm filter and degassed under vacuum before use. μBond pak C18 column was used at a flow rate of 1.2 ml/min and the injection volume of the sample was 20 μl. The levels of DA and 5-HT were calculated using a calibration curve and results were expressed as ng/mg tissue weight.
Determination of non-enzymatic oxidative stress indices
Lipid peroxides: Lipid peroxides (LP) in the fore brain tissue were determined spectrophotometrically as thiobarbituric acid-reactive substances (TBARS) according to the method of Ohkawa et al. [30]. Tissue lipid peroxide levels were quantified using extinction coefficient of 1.56 × 105 m−1 cm−1 and expressed as nano moles of TBARS formed per g tissue weight. The results are expressed as nmol/g wet weight.
Glutathione: Reduced glutathione (GSH) level was measured enzymatically in the forebrain tissues by a slightly modified method [31]. The slope of the change in absorbance was used to quantitate total GSH by comparing the slope of the samples with a standard curve prepared with pure glutathione (Sigma). The specific activity is expressed as umol/g tissue weight.
Determination of enzymatic oxidative stress indices
Glutathione-S-transferase: Glutathione S-transferase (GST) was estimated by the method of Habig et al. [32] using 1-chloro-2,4-dinitrochlorobenzene (CDNB) as substrate at 340 nm. The GST activity is expressed as U/g tissue weight.
Catalase: Catalase (CAT) activity was measured by the method of Aebi [33] by tracking the decomposition of hydrogen peroxide by measuring decrease in extinction of H2O2 at 240 nm. The activity of CAT is expressed as rate constant of first order reaction K per g tissue weight.
Superoxide dismutase: Superoxide dismutase (SOD) activity was estimated by the method of Misra and Fridovich [34]. Activity is expressed as the amount of enzyme that inhibits the oxidation of epinephrine by 50% which is equal to U per g tissue weight.
Statistical analysis
All data were analyzed by one-way analysis of variance (ANOVA), between the experimental groups followed by Student-Newman-Keuls multiple comparison test. The levels of significance were defined at P ≤ .05, P ≤ .01, and P ≤ .001.
Physical assessment during weaning period
Physical developmental landmarks were evaluated in the developing offspring starting from the day 1 after birth (PD1) through entire weaning period unto PD21. The lithium-exposed offspring at both doses, showed a significant decline (p<0.05) in their body weight gain as compared to the controls, from PD2 onwards up to the weaning period (PD21). The lithium-exposed offspring remained lagging behind the controls in body weight very significantly (p<0.001) in a dose-dependent manner (Figure 1).
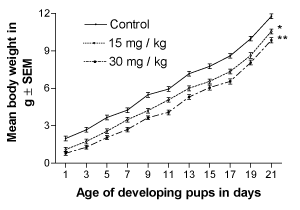
Figure 1. Effect of perinatal lithium dose (15 and 30 mg/kg body weight) exposure on the dose-dependent body weight gain of mouse pups during the weaning (lactation) period. * and **represent statistically significant (P<0.05, P<0.01 and P<0.001, respectively) from the control group; see text
Other morphological developments such as the opening of the eyes and appearance of body hair fuzz were also significantly (p<0.01) delayed in the lithium-exposed offspring in a dose-dependent manner (Figure 2).
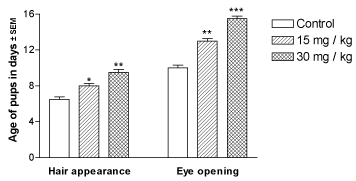
Figure 2. Effect of perinatal lithium dose (15 and 30 mg/kg body weight) exposure on the dose-dependent body hair appearance and eye opening in the mouse pups. *, ** and ***represent statistically significant (P<0.05, P<0.01 and P<0.001, respectively) from the control group; see text
Neuromotor maturation in the developing offspring
The neuromaturation of reflexes during the weaning period of the developing pups was assessed from the day of birth PD1 until PD21. The righting, rotating, and cliff avoidance reflexes in the lithium-exposed offspring were found to be significantly and dose-dependently suppressed throughout the weaning period (Figures 3A-3C, respectively).
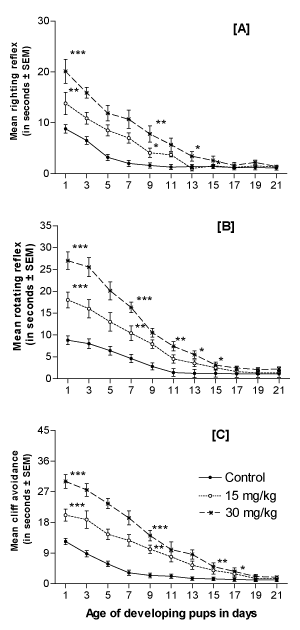
Figure 3. Effect of perinatal lithium dose (15 and 30 mg/kg body weight) exposure on the dose-dependent righting reflex (A), rotating reflex (B) and cliff avoidance (C) of mouse pups during the weaning (lactation) period. *, ** and ***represent statistically significant (P<0.05, P<0.01 and P<0.001, respectively) from the control group; see text
Cognitive behavioral studies
Active avoidance test: In the shuttle-box active avoidance test, the lithium-exposed offspring (PD25), showed a statistically significant and dose-dependent decrease in the number of avoidances during the trial period as compared to the control group (Figure 4A). The spontaneous migration of the mouse to the other compartment during trials measuring the number of crossings between the chambers when no shock was present (inter-trial crossing) showed a significant and dose-dependent decrease as compared to the controls (Figure 4B). The total time taken during the entire trials by the animals to enter the other compartment to avoid the shock treatment (latency of avoidance response in seconds) was also measured for each animal. The results showed that the animals exposed to lithium were poor in learning shock treatment in a dose-dependent manner and took significant time in avoiding the shock treatment as compared to the controls (Figure 4C).
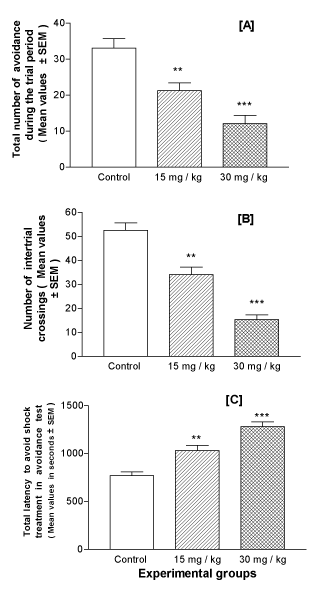
Figure 4. Effect of perinatal lithium dose (15 and 30 mg/kg body weight) exposure on the dose-dependent mean performance value of the mice offspring at the age of 25 days postnatal in the active avoidance task. Mice were given a 50-trial session and the total number of times they avoided the shock by moving to the other compartment of the shuttle box (A), the number of inter-trial crossings between the chambers in the absence of current shock (B) and the total time taken by the animals (latency) to avoid the shock (C) were measured. **and ***represent statistically significant (P<0.01 and P<0.001, respectively) from the control group; see text.
Morris water-maze task: Mice offspring that underwent lithium treatment, exhibited longer escape latencies to reach the platform as compared with the control group (p<0.001; Figure 5A); however, all groups displayed a gradual improvement in performance over the 4 days of testing (training) period. The number of unsuccessful trials (failures) to reach the platform was also significantly higher in lithium-treated offspring as compared to the control group on all testing days (p<0.001; Figure 5B).
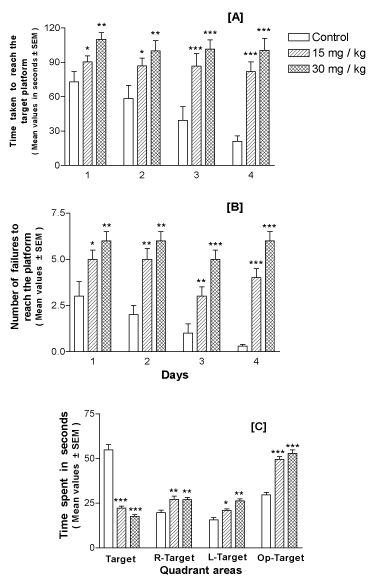
Figure 5. Performance in Morris water-maze of mice offspring at the age of 30 days postnatal and exposed perinatally to lithium doses of 15 and 30 mg/kg body weight in a dose-dependent manner. A – shows the mean latency to reach the hidden platform (y-axis) on each testing day (x-axis). Animals subjected to lithium exposure were slower in finding the platform than the controls on all testing days. B – shows the number of failures or unsuccessful trials (y-axis) on each testing day (x-axis). Lithium exposed offspring showed maximum number of failures in finding the platform as compared to controls. C – shows the outcome of probe test performance. Lithium-exposed offspring spent less time in the target quadrant than the control group. R-Target denotes quadrant on the right side of the target quadrant, L-Target denotes the quadrant on the left side of the target quadrant and O-Target represents quadrant opposite to the target quadrant. *, ** and ***represent statistically significant (P<0.05, P<0.01 and P<0.001, respectively) from the control group; see text
The probe trial studies showed that lithium-exposed offspring spent more time in other three quadrants than the target (platform) quadrant as compared to the control group (p<0.001; Figure 5C), in search of the platform.
Biochemical studies
Levels of monoamines in forebrain: There was a significant (p<0.001) and dose-dependent inhibition of DA as well as 5HT levels in the forebrain of mice offspring treated with lithium as compared to the control group at the ages PD7, PD14, PD21, PD30 and PD36 (Figures 6A and 6B, respectively).
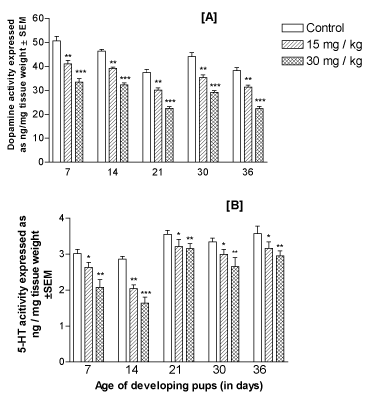
Figure 6. Effect of perinatal lithium dose (15 and 30 mg/kg body weight) exposure on the dose-dependent mean levels of dopamine (A) and 5-HT (5-hydroxy-tryptamine or serotonin) (B), in the forebrain of the offspring at various postnatal developing ages (x-axis). *, ** and ***represent statistically significant (P<0.05, P<0.01 and P<0.001, respectively) from the control group; see text
Levels of non-enzymatic oxidative stress indices: LP determined as TBARS were found to be elevated significantly (p<0.001) due to perinatal lithium exposure in the developing forebrain of the offspring throughout the postnatal development period (PD7, PD14 and PD21) and even at adolescent ages PD30 and PD36 in a dose-dependent manner (Figure 7A). In contrast, reduced glutathione (GSH) level remained depleted significantly (p<0.001) at all developing ages in a dose-dependent manner (Figure 7B).
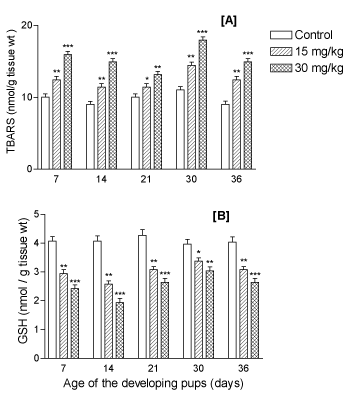
Figure 7. Effect of perinatal lithium dose (15 and 30 mg/kg body weight) exposure on dose-dependent mean levels of non-enzymatic oxidative stress indices like (A) lipid peroxidation content (TBARS), and (B) total glutathione (GSH) level, in the forebrain of the offspring at various postnatal developing ages (x-axis). *, ** and ***represent statistically significant (P<0.05, P<0.01 and P<0.001, respectively) from the control group; see text
Levels of enzymatic oxidative stress indices: The levels of enzymatic OS indices GST, CAT, and SOD remained significantly (p<0.001) depleted due to lithium in a dose-dependent manner in the fore-brain of the developing offspring at PD7, PD14, PD21, PD30 and PD36 (ages) (Figures 8A-8C, respectively).
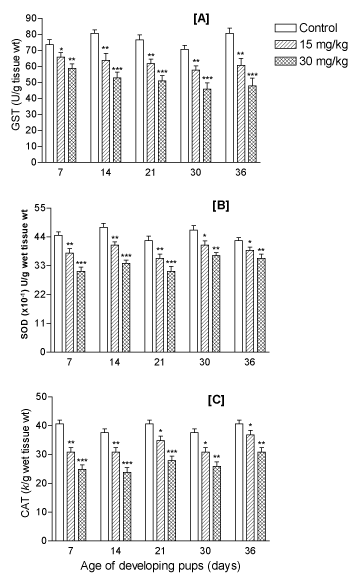
Figure 8. Effect of perinatal lithium dose (15 and 30 mg/kg body weight) exposure on dose-dependent mean levels of enzymatic oxidative stress indices like (A) glutathione S-transferase (GST) level, (B) catalase (CAT) activity, and (C) superoxide dismutase (SOD) activity in the forebrain of the offspring at various postnatal developing ages (x-axis). *, ** and ***represent statistically significant (P<0.05, P<0.01 and P<0.001, respectively) from the control group; see text
In this study, female mice exposed to lithium during pregnancy produced pups that markedly differed from their controls in the rate of physical maturation, motor reflex development at weaning age and furthermore, these pups at early post weaning and adolescent ages showed dysfunctions in active avoidance and cognitive responses and in the levels of neurotransmitters and non-enzymatic as well as enzymatic oxidative stress indices in their forebrain region. The postnatal suppression of body weight gain and the delay in opening of eyes and the appearance of body hair fuzz in the lithium-exposed pups, probably indicate a lasting effect of the prenatal lithium exposure on general growth retardation in mice offspring. Perinatal lithium administration also affected the preweaning reflexes including righting, rotating and avoiding of the cliff in the developing pups significantly as compared to the controls and the results are in agreement with an earlier study [19]. This clearly suggests a direct lithium intervention with the developing pups in utero because lithium ions are water-soluble and are distributed throughout all of the water in the body in a manner that is similar to the distribution of sodium ions (Na+) and crosses the blood-brain barrier (BBB) easily [35]. Furthermore, since lithium completely equilibrates across the placenta and its passage to the newborns is also evidenced [36], our results demonstrate that mice newborns are potentially exposed to the lithium in utero through placenta. Thus, lithium exposure during fetal life does retard motor development and physical maturation, as has been suggested in earlier studies for lithium [19], and for other drugs [37] and compounds [38-40].
Although, the precise mechanism of lithium toxicity still remains incompletely understood, the clinical use of lithium needs careful and cautious attention. In spite of complications related with the level of lithium exposure around delivery period, studies with infant follow-up are needed to have information on longer lasting effects of perinatal exposure to lithium in offspring. In this study, cognitive behavioral assessment in the offspring at post-weaning ages clearly demonstrate that perinatal exposure to lithium causes a significant longer lasting effect by inflicting cognitive dysfunction in them as observed as poor learners in a dose-dependent manner taking significant longer time in avoiding the shock treatment (shuttle-box) and exhibiting longer time as escape latencies to reach the platform (water maze). Clinically, safe use of lithium during prenatal period and its use during lactation has been widely discouraged [41], and its use during late pregnancy has been associated with numerous reports of neonatal complications, like cardiac dysfunction, diabetes insipidus, hypothyroidism, low muscle tone, lethargy, hepatic abnormalities, and respiratory difficulties [36].
For cognitive functions, a number of 5-HT receptor subtypes have been reported to have different roles in the functions of serotonergic neurotransmission, including the functions connected with learning and memory processes [42]. Recently, a growing body of research has focused on the participation of serotonin (5-HT) in the neurochemical mechanisms of cognition and especially of learning and memory. As an important target organ of neurotoxicity, the hippocampus (located in the forebrain region of rodents) is a crucial area for the neurotoxicity of higher cognitive functions [23,43]. It is suggested in the present study that the inhibition of neurotransmitters DA and 5HT in the forebrain tissues might be the most susceptible organ for lithium toxicity in a dose-dependent manner at PD7, PD14, PD21, PD30 and PD36 ages showing a longer lasting effect of lithium.
The present study was also designed to assess the antioxidative defense system in the forebrain tissue of lithium-exposed offspring at PD7, PD14, PD21, PD30 and PD36 ages. Perinatal lithium exposure resulted in a dose-dependent and significant disturbance in the enzymatic (GST, SOD, and CAT) and non-enzymatic (TBARS and GSH) oxidative stress indices in the forebrain tissues. Similar lithium-induced disturbances in the antioxidant barrier (involving glutathione peroxidase and superoxide dismutase) have been shown in the rat serum and tissues [44]. There is ample evidence to suggest that brain tissues are highly vulnerable to oxidative stress [45] and oxidative stress has also been related with cognitive impairment [46-49]. Thus, the cognitive dysfunction observed in the post-weaning offspring suggest a possible strong correlation with the antioxidative defense system of the brain. Furthermore, it reflects in a way for a longer lasting effect expressed as oxidative stress in fore brain of the offspring in perinatally exposed to lithium.
Thus, it is concluded from the present study that females treated with lithium-containing drugs during pregnancy are always at a considerable risk for several complications for their fetus. Carrying out a safe treatment plan during pregnancy and/or post pregnancy (lactation) period has been a formidable challenge to the clinicians since not much experimental data is available on the perinatal risks to the newborns on direct neonatal toxicity and on long-term cognitive dysfunction and oxidative stress in brain tissue due to lithium exposure. The present preliminary results indicate the need for further advanced studies along these lines and the clinical use of lithium needs a careful and rational approach.
The authors are thankful to the Deanship of Scientific Research, College of Nursing, Research Center at King Saud University for funding this research.
The authors declare that there is no conflict of interests regarding the publication of this paper.
- Lenox RH, Hahn CG (2000) Overview of the mechanism of action of lithium in the brain: fifty-year update. J Clin Psych 9: 5. [Crossref]
- Grimes CA, Jope RS (2001) CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium. J Neurochem 78: 1219. [Crossref]
- Chuang DM, Chen RW, Chalecka-Franaszek E, Ren M, Hashimoto R, et al. (2002) Neuroprotective effects of lithium in cultured cells and animal models of diseases. Bipolar Disord 4: 129. [Crossref]
- Zarnescu O, Zamfirescu G (2006) Effects of lithium carbonate on rat seminiferous tubules: an ultrastructural study. Int J Androl 29: 576. [Crossref]
- Rosenthal ME, Goodwim FK (1982) The role of the lithium ion in medicine. Annu Rev Med 33: 555. [Crossref]
- Dhawan D, Mehta J, Mehta M, Kumar R, Chopra JS, et al. (1987) Effect of lithium ingestion on digestive and absorptive function of rat intestine. Digestion 36: 84. [Crossref]
- Phiel CJ, Klein PS (2001) Molecular targets of lithium action. Annu Rev Pharmacol Toxicol 41: 789. [Crossref]
- Bhalla P, Chadha VD, Dhar R, Dhawan DK (2007) Neuroprotective effects of zinc on antioxidant defense system in lithium treated rat brain. Indian J Exp Biol 45: 954. [Crossref]
- Kozma C (2005) Neonatal toxicity and transient neurodevelopmental deficits following prenatal exposure to lithium: Another clinical report and a review of the literature. Am J Med Genet A 132A: 441. [Crossref]
- Gentile S (2012) Lithium in pregnancy: the need to treat, the duty to ensure safety. Expert Opin Drug Saf 11: 425. [Crossref]
- Lugt NM, Maat JS, Kamp IL, Klein EAMK, Hovens JGFM, et al. (2012) Fetal, neonatal and developmental outcomes of lithium-exposed pregnancies. Early Hum Dev 88: 375. [Crossref]
- Simone C, Derewlany LO, Koren G (1994) Drug transfer across the placenta. Considerations in treatment and research. Clin Perinatol 21: 463. [Crossref]
- Iqbal MM, Sohhan T, Mahmud SZ (2001) The effects of lithium, valproic acid, and carbamazepine during pregnancy and lactation. J Toxicol Clin Toxicol 39: 381. [Crossref]
- Carlezon Jr WA, Konradi C (2004) Understanding the neurobiological consequences of early exposure to psychotropic drugs: linking behavior with molecules. Neuropharmacology 47: 47. [Crossref]
- Tueth MJ, Murphy TK, Evans DL (1998) Special considerations: use of lithium in children, adolescents, and elderly populations. J Clin Psychiat 59: 66-73. [Crossref]
- Gentile S (2010) Neurodevelopmental effects of prenatal exposure to psychotropic medications. Depress Anxiety 27: 675-686. [Crossref]
- Youngs RM, Chu MS, Meloni EG, Naydenov A, Carlezon Jr WA (2006) Lithium administration to preadolescent rats causes long-lasting increases in anxiety-like behavior and has molecular consequences. J Neurosci 26: 6031-6039. [Crossref]
- Toplan S, Ozdemir S, Tanriverdi G, Akyolcu MC, Ozeelik D, et al. (2016) The effects of lithium administration on oxidant/antioxidant status in rats: biochemical and histomorphological evaluations. Biol Trace Elem Res 169: 279-284. [Crossref]
- Abu-Taweel MG (2012) Effects of perinatal exposure of lithium on neuro-behaviour of developing mice offspring. Indian J Exp Biol 50: 696. [Crossref]
- Nishio H, Kasunga S, Ushijima M, Harada Y (2001) Prenatal stress and postnatal development of neonatal rats--sex-dependent effects on emotional behavior and learning ability of neonatal rats. Int J Dev Neurosci 19: 37-45. [Crossref]
- Sternberg WF, Ridgway CG (2008) Effects of gestational stress and neonatal handling on pain, analgesia, and stress behavior of adult mice. Physiol Behav 78: 375-383. [Crossref]
- Rutten A, Van Albada M, Silveira DC, Cha BH, Liu X, et al. (2002) Memory impairment following status epilepticus in immature rats: time-course and environmental effects. Eur J Neurosci 16: 501-503. [Crossref]
- Tariq M, Ahmad M, Moutaery KA, Deeb SA (2008) Pentoxifylline ameliorates lithium-pilocarpine induced status epilepticus in young rats. Epilepsy Behav 12: 354-365. [Crossref]
- Lamberty Y, Gower AJ (1991a) Simplifying environmental cues in a Morris-type water maze improves place learning in old NMRI mice. Behav Neural Biol 56: 89-100. [Crossref]
- Lamberty Y, Gower AJ (1991b) Cholinergic modulation of spatial learning in mice in a Morris-type water maze. Arch Int Pharmacodyn Ther 309: 5-19. [Crossref]
- Morris RGM (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11: 47-60. [Crossref]
- Jeltsch H, Bertrand F, Lazarus C, Cassel JC (2001) Cognitive performances and locomotor activity following dentate granule cell damage in rats: role of lesion extent and type of memory tested. Neurobiol Learn Mem 76: 81-105. [Crossref]
- Spiers HJ, Burgess N, Hartley T, Vargha-Khadem F, O’Keefe J (2001) Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus 11: 715-725. [Crossref]
- Patrick OE, Hirohisa M, Masahira K, Koreaki M (1991) Central nervous system bioaminergic responses to mechanic trauma. Surgical Neurol 35: 273.
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351-358. [Crossref]
- Mangino MJ, Murphy MK, Grabau GG, Anderson CB (1991) Protective effects of glycine during hypothermic renal ischemia-reperfusion injury. Am J Physiol 261: F841. [Crossref]
- Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249: 7130-7139. [Crossref]
- Aebi H (1972) Catalase in vitro. Academic Press, New York, USA.
- Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247: 3170. [Crossref]
- Forester BP, Streeter CC, Berlow YA, Tian H, Wardrop M, et al. (2009) Brain lithium levels and effects on cognition and mood in geriatric bipolar disorder: a lithium-7 magnetic resonance spectroscopy study. Am J Ger Psychiatry 17: 13-23. [Crossref]
- Newport DJ, Veguera AC, Beach AJ, Ritchie JC, Cohen LS, et al. (2005) Lithium placental passage and obstetrical outcome: implications for clinical management during late pregnancy. Am J Psychiatry 162: 2162-2170. [Crossref]
- Brain PF, Kurishingal H, Whiting CJ, Restall CJ (1994) Ethology and psychopharmacology. Cooper SJ, Hendrie CA, John Wiley & Sons Ltd, New York, 224.
- Ajarem JS, Ahmad M (1991) Prenatal nicotine exposure modifies behavior of mice through early development. Pharmacol Biochem Behav 59: 313-318. [Crossref]
- Ajarem JS, Ahmad M (1998) Behavioral and biochemical consequences of perinatal exposure of mice to instant coffee: a correlative evaluation. Pharmacol Biochem Behav 40: 847-852. [Crossref]
- Abu-Taweel MG, Ajarem JS, Ahmad M (2012) Neurobehavioral toxic effects of perinatal oral exposure to aluminum on the developmental motor reflexes, learning, memory and brain neurotransmitters of mice offspring. Pharmacol Biochem Behav 101: 49-56. [Crossref]
- American academy of pediatrics committee on drugs (2001) Transfer of drugs and other chemicals into human milk. Pediatrics 108: 776-789. [Crossref]
- Petkov VD, Belcheva S, Konstantinova E, Kehayov R (1995) Participation of different 5-HT receptors in the memory process in rats and its modulation by the serotonin depletor p-chlorophenylalanine. Acta Neurobiol Exp 55: 243-252. [Crossref]
- Savage LM, Buzzetti RA, Ramirez DR (2004) The effects of hippocampal lesions on learning, memory, and reward expectancies. Neurobiol Learn Memory 82: 109. [Crossref]
- Kielczykowska M, Pasternak K, Musik I, Wroniska J (2004) The effect of lithium administration in a diet on the chosen parameters of the antioxidant barrier in rats. Annal Universit Mar Curie-Skłod Medi 59: 140-145. [Crossref]
- Freitas RM (2009) Investigation of oxidative stress involvement in hippocampus in epilepsy model induced by pilocarpine. Neurosci Lett 462: 225-229. [Crossref]
- Reeta KH, Mehla J, Gupta YK (2009) Curcumin is protective against phenytoin-induced cognitive impairment and oxidative stress in rats. Brain Res 1301: 52-60. [Crossref]
- Reeta KH, Mehla J, Gupta YK (2010) Curcumin ameliorates cognitive dysfunction and oxidative damage in phenobarbitone and carbamazepine administered rats. Eur J Pharmacol 644: 106-112. [Crossref]
- Reeta KH, Mehla J, Pahuja M, Gupta YK (2011) Pharmacokinetic and pharmacodynamic interactions of valproate, phenytoin, phenobarbitone and carbamazepine with curcumin in experimental models of epilepsy in rats. Pharmacol Biochem Behav 99: 399-407. [Crossref]
- Ataie A, Sabetkasaei M, Haghparast A, Moghaddam AH, Kazeminejad B (2010) Neuroprotective effects of the polyphenolic antioxidant agent, Curcumin, against homocysteine-induced cognitive impairment and oxidative stress in the rat. Pharmacol Biochem Behav 96: 378-385. [Crossref]








