Some patterns of uterine adenomyosis (AM) are more common in China than Europe or USA. In this new classification derived from clinical observations in Shanghai, there are four patterns of adenomyosis, and, three out of the four have neurologic abnormalities associated with specific injuries to uterotubal nerves (“Shanghai system” (SS), types 1-4, AM). Typically, European or US women present with “painful” uterine bleeding in their 30-40’s that results in hysterectomy at reduced uterine weights (80-150g). Histology shows aberrant reinnervation throughout the body of the uterus and uterosacral ligaments, as a result of intrapartum injuries, or, excessive traction applied to the cervix, or, over-vigorous curettage (SS, type 2, AM).
In Shanghai there are two common variations that have very different clinical presentations. The first is diffuse, symmetric adenomyosis (SS, type 1, AM) where women present with “painless”, pelvic masses weighing 250-1250g. Laparoscopy shows complete avulsion of their uterosacral-cardinal ligament complexes, and, histologic examination shows there are no nerves in their uteri or Fallopian tubes. The second variation is a focal adenomyoma occupying the upper half of the uterus that may also be come very large over the course of a reproductive career (SS, type 3, AM). These women have torn nerve fibers in their uterosacral ligaments associated with paracervical infiltration of local anesthesia and subsequent traction to the cervix during outpatient evacuation of the first trimester uterus. The result is a focal adenomyoma, typically, in the postero-superior surface of the uterus. Embryologic, or, pedunculated, or, polypoid patterns may contribute a fourth, non-neurologic pattern of adenomyosis (SS, type 4, AM).
This account provides clinical, laparoscopic, radiologic and histologic evidence for each of these neurologic injuries. Many are preventable by simple amendments to clinical policies or surgical techniques.
In 1908, TS Cullen classified adenomyosis into three distinctive subtypes of adenomyosis based on morphologic appearances [1]. He described:
- smooth enlargement of the uterus without disturbing its contour,
(SS, type 1 or type 3, AM)
- subperitoneal or intraligamentary adenomyomata, (SS, type 4, AM)
- submucous adenomyomata.(SS, type 3, AM)
It is now clear to us in Shanghai, that there are two, common patterns of adenomyosis in China that are relatively unusual in Western countries though TS Cullen illustrated both types in his book under the category “smooth enlargement of the uterus without disturbing its contour” [2]. The most distinctive is diffuse, symmetric, adenomyosis that usually presents as a painless mass (250-1250g) with symptoms of “pressure” on adjacent organs. (“Shanghai system” (SS), Type 1, AM,). Histologic examination shows there is no evidence of nerves anywhere in either the uterus, or Fallopian tubes [2] (Figure 1A-F). In a second, common, clinical presentation in Shanghai, there may be extreme enlargement of a focal posterior wall adenomyoma that produces marked distortion of the uterine cavity, asymmetry of the uterus, and often, uterine retroversion (Figure 3E-F, type 3, AM, SS). The clinical presentation is also usually painless, with some asymmetry of the uterosacral ligaments, and, on histologic examination there are no nerves in the myoma. Both are relatively rare presentations in UK or US gynecology.
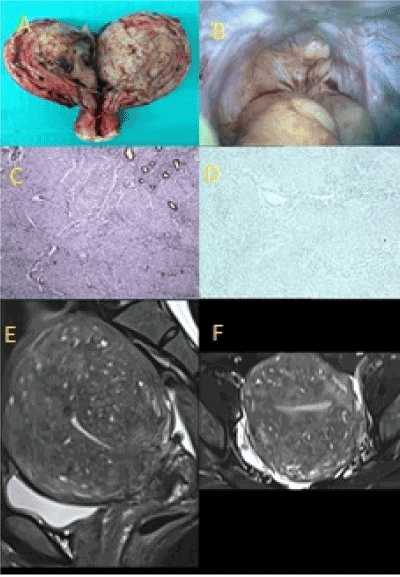
Figure 1(A-F). Type 1, diffuse, symmetric, painless adenomyosis. (A) Diffuse, symmetric adenomyosis at uterine weight of 260g. (B) Complete absence of uterosacral ligaments at laparoscopy. (C) Complete loss of the endometrial-myometrial nerve plexus (anti-S100, x100). (D) Complete loss of uterotubal nerves throughout the resected specimen. (E) Diffuse, symmetric adenomyosis (T2 weighted, MR scan, LS). (F) Diffuse, symmetric adenomyosis (T2 weighted, MR scan, TS).
The best evidence in the US literature for the etiology of adenomyosis derives from the observation that vigorous curettage of one horn of the uterus of a pregnant rabbit produces adenomyosis, whilst allowing the pregnancy to continue in the opposite horn [3]. This is the “trauma” hypothesis which is reinforced by radiologic interest in the width of the endometrial- myometrial interface (or, junctional zone (JZ) of the uterus). Twelve millimeter thickness of the JZ guarantees a histologic diagnosis of adenomyosis though others discuss different degrees of sensitivity and specificity at different thicknesses [4,5]. These radiologic colleagues, however, make no mention of the endometrial-myometrial nerve plexus described by Krantz [6] that postdates many of the classic descriptions of adenomyosis in the US literature [3,7], and is not mentioned in more recent updates [8,9]. If there is interruption of this nerve plexus either by direct trauma at curettage, or, through uterine tachysystole, or, through injuries to the uterotubal nerves that pass through the uterosacral ligaments to supply it, then each may contribute to loss of innervation in different parts of the uterus and Fallopian tubes. Tubal ligation and Cesarean section have both been discussed in the context of the etiology of adenomyosis. Tubal ligation may increase intrauterine pressures at different times of the menstrual cycle [10] whereas a myometrial incision (above the lower segment of the uterus) may injure endometrial-myometrial nerves, or, tearing one, or both, angles of the uterine incision may do the same ? [11] Some series of US patients refer to uterine weights of greater than 250g with the highest recorded weight of 705 g but they are rare [3]. Typically, US women present with painful periods that require hysterectomy at uterine weights of 80-150g (type 2, AM, SS) that are common in Western clinical practice, though not mentioned in Cullen’s original account. They is no published evidence for significant numbers of cases of diffuse, painless adenomyosis (SS, type 1, AM) and focal, painless adenomyosis (SS, type 3, AM), that we frequently observe in Shanghai ?
The nerve supply of the uterus
As undergraduates we saw cadaveric material preserved in formalin (that destroys autonomic nerves) whereas Lee used alcohol in his dissections [12,13]. In an alcohol-embalmed cadaver the full morphology of the pelvic plexi is clear [14] as the nerves pass through the fan of the uterosacral-cardinal ligament plexus to converge on the vaginal vault where they are susceptible to injury through difficult first labors, physical efforts during defaecation, and, complications of the management of evacuation of the uterus through surgical and medical techniques [15]. Partial, or complete, injuries to the uterosacral ligaments results in partial, or complete, injuries to the uterotubal nerves with differing degrees of aberrant reinnervation depending on the extent of the injury [15]. The distal third of the Fallopian tubes receives nerve supply from the ovarian plexus through the free edge of the mesosalpinx [16].
In this discussion it is both the intrauterine, and extrauterine, nerve supply that assumes significance in relation to different patterns of adenomyosis. Within the uterus mixed nerve bundles delivered (a) in the uterosacral ligaments, and, (b) along with the uterine artery, form the endometrial-myometrial nerve plexus and subserosal nerve plexus, respectively, that reflects the differing embryologic origins of the endometrium and myometrium [6,17]. Both deliver sparse nerve bundles to the myometrium though normal endometrium does not carry a nerve supply. Intra-uterine injuries to the nerve supply will cause collateral sprouting of nerve bundles and aberrant reinnervation of injured nerves (Figure 2C-D) whereas pre-uterine injuries to nerves in the uterosacral ligaments may demonstrate loss of nerves in adjacent nerve bundles (Figure 3C-D). The two patterns of injury are separate and distinctive.
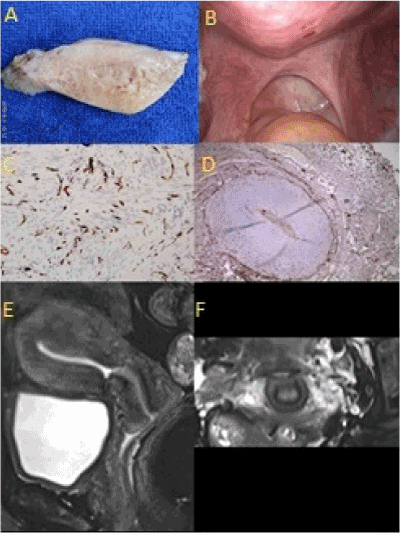
Figure 2(A-F). Type 2, irregular, asymmetric, painful adenomyosis. (A) Irregular, asymmetric, painful in uterus weighing less than 150g. (B) Irregular scarring of uterosacral ligaments at laparoscopy. (C) Aberrant reinnervation of endometrial-myometrial interface (anti-S100, x 100). (D) Aberrant reinnervation in the uterosacral ligaments (anti-S100, x100). (E) Irregular, asymmetric adenomyosis with enlarged cervix and Cesarean scar on the anterior uterine wall (T2, weighted MR scan, LS). (F) High signal intensity of injured uterosacral ligaments on either side of the cervix (T2 weighted, MR scan, coronal section)
Previous classifications of adenomyosis
A number of classifications of the varying clinical phenotypes of adenomyosis have been set out. KF von Rokitansky identified the condition in 1860 with one of the original, light microscopes [18] though it was TS Cullen (1908) who presented the first classification of adenomyosis based largely on macroscopic and microscopic studies of the condition [1]. John Sampson (1921) discussed a similar classification to that of TS Cullen [19]. More recently, there have been two clinical contributions to attempt to improve the classification of adenomyosis; Dr Kishi (KS) in Takanohara Hospital, Nara, Japan discussed four subtypes of adenomyosis based on magnetic resonance imaging [20], and, Dr. Bazot (BZ), at Tenon Hospital, Paris, who described three different patterns of adenomyosis using vaginal sonography and MR imaging [21]. There are some minor differences between the two classifications but both are based on specific, radiologic appearances (Table 1).
Table 1A. The “Kishi” classification of adenomyosis. It is based on stepwise, logistic regression analysis of 152, surgically-treated cases of adenomyosis and published as Kishi Y, Suginami H, Kuramori R et al. “Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification.” Am J Obstet Gynecol 2012; 207: 114 e1-7
- Intrinsic adenomyosis that develops in direct connection with a thickened junctional zone (JZ) (KS, type 1, AM)
|
- Extrinsic adenomyosis that develops in the outer shell of the myometrium and the junctional zone was intact (KS, type 2, AM)
|
- Intramural adenomyosis which develops without relationship to the JZ or the subserosal layers (KS, type 3, AM)
|
- Indeterminate adenomyosis that develops outside of the three prior subtypes (KS type 4, AM)
|
The “Kishi” classification, 2012 (Table 1A)
In 152 women undergoing MRI, Kishi [20] described four subtypes of adenomyosis (Table 1A). In their stepwise regression analysis, Kishi et al. noted that a significant proportion of type 1 adenomyosis (KS 1) had prior terminations of pregnancy whereas type 2 (KS 2) did not (32.2% v 7.8%), they proposed that a “barrier” exists at the endometrial-myometrial interface which prevents adenomyosis, and, this barrier may be injured by pregnancy or curettage. In type 3 adenomyosis (KS 3), clinical features included menorrhagia (18/22, 81.8%) and dysmenorrhea (18/22, 81.8%). They suggested that this is a “de novo product” of embryologic or metaplastic origin. In type 4 adenomyosis (KS 4) this was a heterogeneous group where the adenomyosis was so “widely extended and expanded that the relationship with uterine structural components could not be clearly evaluated”. The authors conclude that this subgroup is a heterogeneous mix of types 1-3 adenomyosis (KS 1-3). In their discussion, they draw attention to the fact that type 3 adenomyosis (KS 3), was the youngest group of women (34 v 38 years), and, to the paradox that some women in each group were symptomatic, though others were asymptomatic.
Table 1B. The “Bazot” classification of adenomyosis. It is based on a descriptive evaluation of transvaginal sonography and magnetic resonance imaging descriptions of adenomyosis resulted in three categories of adenomyosis with eleven anatomic, MRI descriptions, published as M. Bazot, E Darai. “Role of transvaginal sonography and magnetic resoanance imaging in the diagnosis of uterine adenomyosis” Fertil Steril 2018; 109(3) 389-397
- Internal adenomyosis (BZ, type 1, AM)
Diffuse, symmetric or asymmetric widening of myometrium secondary to myometrial hypertrophy with thickening of the junctional zone, often with concurrent leiomyomas
|
- External adenomyosis (BZ, type 2, AM)
Arises in the outer part of the uterus disrupting the subserosa but not affecting the JZ, often associated with “deep” endometriosis. It can be found with internal adenomyosis or adenomyomas.
|
- Adenomyomas (BZ, type 3, AM)
An adenomyoma is an ill-defined, myometrial mass containing high-intensity, central cystic areas. Neither JZ or subserosa are affected by the presence of isolated adenomyoma
|
The “Bazot” classification, 2018 (Table 1B)
Bazot recognizes three types of adenomyosis [21]. He draws attention to the classification by John Sampson (1921) which he replicates with this MRI classification [19]. He draws distinctions between what ultrasound examination can do, and, how MR imaging adds to the ultrasound observations. Interestingly, Bazot distinguishes 11 patterns of adenomyosis on MR imaging, most are recognizable variations of his three main groups (BZ 1-3) but two relate to adjacent injuries to the subserosal uterine plexus, and, bladder or rectum. These imply significant intrapartum injuries to adjacent organs though these are not discussed in detail.
The “Shanghai system” of classification (Table 2)
For a variety of reasons, we offer a third classification of adenomyosis based on clinical, laparoscopic, histologic and radiologic appearances that define the clinical anatomy and histologic injuries carefully (SS types 1-4, AM; Table 2).
Table 2. The “Shanghai system” of classification of adenomyosis. This system relies on clinical, laparoscopic, histologic and radiologic features of adenomyosis to produce a neuro-aetiologic classification. Each pattern of adenomyosis arises from an injury to the uterine supply that varies with the site, nature and extent of the injury; (A) complete avulsion of the uterosacral ligaments leads to diffuse, symmetric, painless adenomyosis (type 1), (B) excessive traction to the cervix (less than that necessary to produce avulsion) combined with overvigorous curettage of the endometrium, or, excessive uterine activity, leads to irregular, asymmetric, painful adenomyosis (type 2, AM), (C) partial, pre-uterine injury to a few nerves in either uterosacral ligament leads to endometrial or myometrial hyperplasia and development of focal, painless, myoma
|
Type 1 Diffuse, symmetric, painless adenomyosis (SS, type 1, AM; Figure 2)
This pattern of adenomyosis typically presents as a painless pelvic mass in a parous woman in her mid-forties with a prior history of one, or more, previous first or second trimester abortions. At laparoscopy there is no evidence of uterosacral ligaments. Histologically there are no nerves in the uterus or Fallopian tubes, particularly there are no nerves at the site of the endometrial-myometrial nerve plexus (JZ). MR imaging shows symmetrical enlargement of the uterus, with accompanying enlargement of the cervix.
|
|
Type 2 Irregular, asymmetric, painful adenomyosis (SS, type 2, AM; Figure 3)
This pattern of adenomyosis typically presents with heavy painful, menstrual bleeding in a parous woman in her fifth decade. Typically, there is evidence of injuries to the uterosacral ligaments with scarring of their uterine insertions. Histologically there is aberrant reinnervation in the body of the uterus associated with the endometrial-myometrial interface (JZ). MR imaging shows an irregular, asymmetric pattern of adenomyosis centred on the endometrial-myometrial interface. The uterine cervix may be enlarged owing to accompanying injuries to its nerve supply.
|
|
Type 3 Focal painless adenomyoma (SS type 3, AM; Figure 4)
This pattern of adenomyosis is typically asymptomatic unless associated with other forms of “endometriosis”. Typically, its “maps” to the two main nerve plexi in the uteri i.e. the endometrial-myometrial nerve plexus, or, the subserosal nerve plexus, or, their myometrial branches. MR imaging shows a focal, high signal intensity, “adenomyoma”. The uterine cervix is usually not enlarged as there has been no injury to its nerve supply. These adenomyomas can be very large and appear irregular because they are “compressed” between intact, subserosal and endometrial-myometrial nerve plexi.
|
|
Type 4 Painless intraligamentary adenomyosis (SS type 4, AM)
There are a group of other sources of adenomyosis – some are of embryological origin, others are pedunculated, others form atypical adenomyomatoid polyps, that require a separate category. They are rare, and, usually distinguishable from more traditional patterns of adenomyosis.
|
- we noticed clear differences in incidence of some patterns of adenomyosis in China compared to Europe.
- we observed some unusual neurologic injuries to the uterine nerve supply in different patterns of adenomyosis
- we noted limited awareness of the nerve supply of the uterus, and, different lesions of adenomyosis appears to “map” very precisely to the anatomic sites of the two key, uterine nerve plexi (endometrial-myometrial interface and subserosa)
- in this classification there are four types of adenomyosis (SS, types 1-4, AM) with types 1-3 associated with precise neurologic injuries.
Type 1 AM, Diffuse, symmetric, painless adenomyosis (SS type 1, AM Figure 2A-F)
In our initial series of 34/34 women with diffuse, symmetric, painless adenomyosis, these women had no uterosacral ligaments (P<0.001), no nerves at the endometrial-myometrial nerve plexus (P<0.001), and, no nerves in their uteri or Fallopian tubes (P<0.001) compared to controls [2]. Either the injury to uterotubal nerves came first, or, the enlargement of the uterus destroyed the nerves during the enlarging process. We prefer the former explanation since:
- all these women had, at least one, second trimester abortion, (which can avulse both uterosacral ligaments in nulliparous women)
- there are no nerves of any kind at the endometrial-myometrial nerve plexus which is continuous with the nerves in the uterosacral ligaments
- all uteri in this series had similar histology of uterus, uterosacral ligaments and Fallopian tubes, and weighed between 260g and 1160g,
- no woman complained of pain; the worst symptoms being “heaviness” with “pressure” on bladder and bowel leading to frequency passing urine, and, constipation.
We believe this pattern of adenomyosis results from complete loss of the nerve supply at the endometrial-myometrial interface owing to significant tractino being applied to the cervix (and uterosacral ligaments) during second trimester abortions that results in complete avulsions of the uterosacral ligaments. Second trimester abortions are relatively common in China, though unusual in UK clinical practice where most procedures take place during the first trimester of pregnancy, often using medical treatment.
Type 2 AM, asymmetric, painful adenomyosis (SS type 2, AM; Figure 2A-F)
In this second pattern of adenomyosis, there is aberrant reinnervation in the myometrium in association with asymmetric, painful, adenomyosis associated with the endometrial-myometrial interface (SS 2, type 2 adenomyosis, Figure 2A-F). These often occur in association with collateral sprouting of nerve bundles that is pathognomonic of prior traumatic injury (Figure 2C-D). In many cases we find evidence of over-vigorous uterine curettage, difficult vaginal delivery, or, excessive uterine activity associated with administration of oxytocin, prostaglandins or misoprostol – drugs that were not available to TS Cullen in 1908 or JR Sampson in 1921. The injury is largely confined to the body of the uterus and the clinical presentation often includes irregular, painful bleeding at reduced uterine weights (80-150g) that regularly results in hysterectomy.
There are two patterns of neural injury caused by, at least, two different patterns of trauma, in type 2, AM. Firstly, there is a degree of traction to the cervix that is not sufficient to completely avulse the uterosacral ligaments, but, allows them to reattach to the lower uterus and cervix as scarring (Figure 2B) that enables a “bridge” for reinnervation of the lower uterus (Figure 2D). Over-vigorous curettage at the same procedure causes a direct injury to the endometrial-myometrial interface creating aberrant reinnervation at the endometrial-myometrial interface (Figure 2C) with subsequent, marked dysmenorrhea that often necessitates an early hysterectomy at low uterine weights (80-150g) [3]. Both of these specific injuries may also result from trauma during vaginal delivery; typically uterine tachsystole replicates the partial injury to the endometrial-myometrial interface whereas big babies (>4000g), malpresentations, and, operative vaginal deliveries may all contribute to injuries to the insertions of the uterosacral ligaments and their, contained uterotubal nerves [22].
Type 3 AM, painful or painless, adenomyomas, (SS type 3, AM; Figure 3A-F)
In the third pattern of adenomyosis, there is a circumscribed tumor that often “maps” to either the anatomic position of the endometrial-myometrial or subserosal nerve plexi, or, most commonly the posterosuperior myometrium of the uterus. Histologically, there is loss of nerve fibers in myometrial nerve bundles adjacent to painless adenomyomas and leiomyomas (SS 3, Type 3 AM), often with large numbers of narrowed arterioles adjacent to the tumor. We believe these tumors arise from pre-uterine neural injuries because there is no evidence of collateral sprouting in these nerve bundles implying that a focal, pre-uterine injury to the nerve bundle had taken place resulting in loss of specific, nerve fibers (Figure 3C-D). Sometimes it is possible to discern an injury to the uterosacral ligament, or, there is a deposit of ectopic endometrium to mark the underlying injury (Figure 3B).
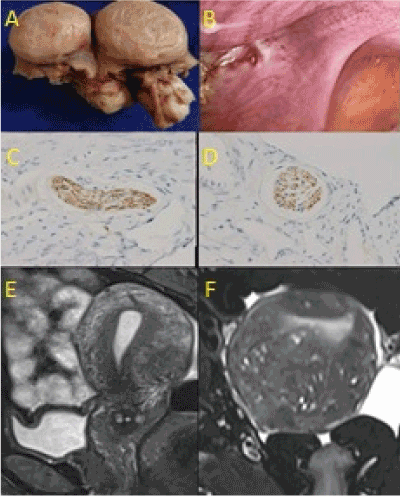
Figure 3(A-F). Type 3, focal, painless adenomyoma. (A) Small, (1-2 cm diameter) leiomyoma does not compress adjacent histologic features. (B) Focal injury, marked by ectopic endometrium, at anterior border of uterosacral ligament. (C) Loss of nerve fibers in a myometrial nerve bundle beneath the myoma (LS, anti-S100, x100). (D) Loss of nerve fibers in a myometrial nerve bundle beneath the myoma (TS, anti-S100, x100). (E) Focal, low intensity signal of a myoma on posterior wall of uterus (T2 weighted, MR scan, LS). (F) Focal, low intensity signal of a myoma on posterior wall of uterus (T2 weighted, MR scan, TS)
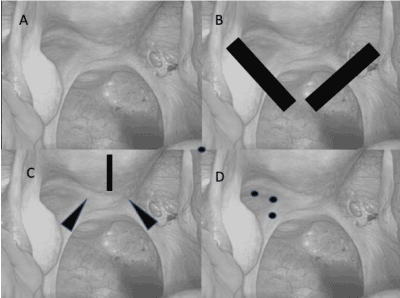
Figure 4(A-D). Patterns of injury to the uterosacral ligaments in different patterns of adenomyosis. (A) Normal uterosacral ligaments with peritoneal window associated with right, uterosacral ligament have nerves running through them that converge on the cervix. (B) Bilateral avulsions of uterosacral ligaments cause diffuse, symmetric adenomyosis (SS, type 1, AM, Fig. 1a-f). (C) Bilateral scarring of uterosacral ligaments at uterine insertions & over-vigorous curettage of the endometrial cavity cause irregular painful, adenomyosis (SS, type 2, AM, Fig. 2a-f). (D) Discrete injuries to the nerves running through the uterosacral ligaments associated with paracervical infiltration of anesthetic agents result in focal, painless adenomyoma (SS, type 3, AM, Fig 3a-f).
This injury takes place as part of a first trimester abortion procedure when paracervical infiltration of local anesthesia creates the conditions for injuries to individual nerve fibers, particularly those in the superior quadrants of paracervical tissue. Injuries to individual nerve fibers in the uterosacral ligaments create neurologic deficits in the myometrium leading to focal hyperplasia, and, formation of an adenomyoma or leiomyoma. We do not understand the difference between the etiology of the two tumors as yet?
Type 4, painless, intraligamentary adenomyoma (SS type 4, AM)
TS Cullen was aware of this least common pattern of adenomyosis though we are aware there are also adenomyomatous patterns of uterine polyp, and, pedunculated forms of adenomyosis that appear to have a non-neurologic origin.
The “Shanghai system” of classification puts together clinical, laparoscopic, histologic and radiologic features to produce explanations for three of the four types of adenomyosis. This appears to be a robust system given that we have looked at 25-40 cases of each type of adenomyosis – some prospectively, some retrospectively – to form this classification. It also appears to “map” to specific injuries to uterotubal nerves in, and around, the insertion of the uterosacral ligaments (and the endometrial-myometrial interface) caused by specific clinical procedures with geographical variations in incidence between Eastern and Western clinical practice. In that respect it has similarities to the “trauma” hypothesis to explain the effects of over-vigorous curettage in pregnant rabbits [3], though we extend that hypothesis to include subtle, and less subtle, injuries to the uterosacral ligaments at different anatomic sites.
The radiologic classifications discussed in this account, are broadly similar to the “Shanghai system” though vary because they do not include reference to injuries to uterine nerves. Both Kishi and Bazot’s classifications [20, 22] “map” to the neuroanatomy of the uterus without either author openly identifying the relationship to the two important nerve plexi [6,17]. The two key, neurologic plexi being the nerve plexus at the endometrial-myometrial junction (Krantz’s plexus) that originates in the uterosacral ligaments, and the subserosal nerve plexus that enters the uterus with the uterine artery [6,17]. Leyendecker has been a proponent of the importance of prior tissue injury in the “endometriosis-adenomyosis” spectrum and has emphasized his observation of 11 cases of “cornual” adenomyotic cysts as a rare but distinctive form of adenomyosis [23,24]. In the “Shanghai system”, these result from an unusual injury to the uterine nerve supply at the anterior border of the uterosacral ligament (SS, type 3, AM; Figure 2B). In this account, these injuries to the anterior border of the uteosacral ligament are likely to result from paracervical infiltration with lignocaine followed by traction to the cervix, that contributes to focal tearing of pre-uterine, nerve fibers with subsequent development of a myoma. There may be other reasons.
The increased frequency of the diffuse, symmetric, “painless” pattern of adenomyosis in China raises a number of important aetiologic questions. Firstly, it appears that 1000g of “ectopic” endometrium can occupy a woman’s pelvis without any discomfort to her. Secondly, excessive traction to the cervix during any gynecologic procedure may avulse one, or more, nerve bundles creating the conditions for all three types of adenomyosis (SS, types 1-3, AM). Thirdly, the apparent absence of SS, type 2, AM in the Cullen and Sampson classifications raises the concern that the injury to the endometrial-myometrial interface, or, “junctional zone” is often a contemporary, iatrogenic injury associated with uterotonic agents such as oxytocin, prostaglandins or misoprostol, leading to injury to the endometrial-myometrial nerve plexus [1,19]. Some patterns of adenomyosis may be preventable by improved management of labour, and, evacuation of the uterus. Active measures to prevent excessive uterine activity with oxytocin, prostaglandins and misoprostol need more emphasis. There is no reason for excessive traction to the cervix if the surgeon understands there are large numbers of delicate nerve bundles associated with its posterior surface.
All three, benign, gynecologic pathologies (endometriosis, adenomyosis, leiomyoma) carry injuries to the uterine nerve supply [26-32]. In this classification we propose that three patterns of adenomyosis carry different neuro-anatomic injuries based on simple anatomic observations. If the neuro-etiology of adenomyosis and adenomyoma is confirmed then it raises the question as to the origins of “endometriosis” in which increasing numbers of authors are finding neural injuries in association with deposits of ectopic endometrium [32-34]. If it becomes clear that injured nerves are the source of persistent, and progressive, symptoms of pelvic pain, bleeding and infertility then clearly, we may have to amend our approach to different patterns of “endometriosis”, as well as adenomyosis?
- TS Cullen (1908) Adenomyoma of the uterus. Philadelphia: WB Saunders.
- Xia WT, Cai YY, Yang, SM, Zhang SS, Wu XQ, et al. (2016) Injuries to uterotubal nerves in advanced painless adenomyosis. International Society for the Study of Reproductive Surgery and the Fallopian Tube, RCOG.
- Benson RC, Sneeden VC (1958) Adenomyosis; a reappraisal of symptomatology. Am J Obstet Gynecol 76: 1044. [Crossref]
- Reinhold C, McCarthy S, Bret PM, Mehio A, Zakarian R, et al. (1996) Diffuse adenomyosis: comparison of endovaginal and MR imaging with histopathologic correlation. Radiology 199: 151-158. [Crossref]
- Brown HK, Stoll BS, Nicosia SV, Fiorica JV, Hambley PS, et al. (1991) Utrine junctional zone: correlation between histologic findings and MR imaging. Radiology 179: 409-413. [Crossref]
- Krantz KE (1959) Innervation of the human uterus. Ann N Y Acad Sci 75: 770-784.
- Israel SL, Woutersz T (1959) Adenomyosis: a neglected diagnosis. Obstet Gynecol 13: 168. [Crossref]
- Bird CC, McElin TW, Manalo-Estrella P (1972) The elusive adenomyosis of the uterus revisited. Am J Obstet Gynecol 112: 582-593. [Crossref]
- Molitor JJ (1971) Adenomyosis: a clinical and pathologic appraisal. Am J Obstet Gynecol 110: 275. [Crossref]
- Siegler AM, Camillieri L (1994) Adenomyosis. J Reprod Med 39: 841-853.
- Harris JW, DAniell JF, Baxter JW (1985) Prior Cesarean section: a risk factor for adenomyosis. J Reprod Med 30: 173-175. [Crossref]
- R Lee (1841) The anatomy of the nerves of the uterus. London: Hippolyte Bailliere.
- R Lee (1849) Memoirs on the ganglia and nerves of the uterus. London: John Churchill.
- Spackman R, Wrigley B, Roberts A, Quinn M (2007) The inferior hypogastric plexus: a different view. J Obstet Gynaecol 27: 130-133. [Crossref]
- Atwal G, du Plessis D, Armstrong G, Slade R, Quinn M (2005) Uterine innervation after hysterectomy for chronic pelvic pain with, and without, endometriosis. Am J Obstet Gynecol 193: 1650-1655. [Crossref]
- Mitchell GA (1938) The innervation of the ovary, uterine tube, testis and epididymis. J Anat 72: 508-517. [Crossref]
- Quinn M (2003) The endometrial-myometrial interface. AJOG 188:857-858.
- Rokitansky KF (1860) Ueber uterus-neubildung. Z Gesellshaft Wien. 16: 577.
- Sampson JA (1921) Perforating hemorrhagic (“chocolate”) cysts of the ovary. Their importance and especially their relation to pelvic adenomas of endometrial type. Adenomyoma of the uterus, rectovaginal septum, sigmoid, etc. Arch Surg 3: 245-323.
- Kishi Y, Suginami H, Kuramori R, Yabuta M, Suginami R, et al. (2012) Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am J Obstet Gynecol 207: 114.e1-7. [Crossref]
- Bazot M, Daraï E (2018) Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril 109: 389-397. [Crossref]
- Wu XQ, Cai YY, Xia WT, Quinn MJ (2016) Excessive uterine activity is a maternal neurological emergency. BJOG 123: 2130.
- Leyendecker G, Wildt L, Mall G (2009) The pathophysiology of endometriosis and adenomyosis: tissue injury and repair. Arch Gynecol Obstet 280: 529-538. [Crosrsef]
- Leyendecker G, Bilgicyildirim A, Inacker M, Stalf T, Huppert P, et al. (2015) Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch Gynecol Obstet 291: 917-932. [Crossref]
- Quinn M (2007) Uterine innervation in adenomyosis. J Obstet Gynaecol 27: 287-291.
- Ibrahim MG, Chiantera V, Frangini S, Younes S, Köhler C, et al. (2015) Ultramicro-trauma in the endometrial-myometrial junctional zone and pale cell migration in adenomyosis. Fertil Steril 104: 1475-1483.e1-3. [Crossref]
- Barcena de Arellano ML, Oldeweme J, Arnold J, Schneider A, Mechsner S (2013) Remodeling of estrogen-dependent sympathetic nerve fibers seems to be disturbed in adenomyosis. Fertil Steril 100: 801-809. [Crossref]
- Lu BC, Huang XF, Zhou CY, Xu H, Lin J, et al. (2009) Distribution of nerve fibers in endometrium and its clinical significance in adenomyosis. Zhonghua Fu Chan Ke Za Zhi 44: 324-327. [Crossref]
- Savitskiĭ GA, Morozov VV, Svechnikova FA (1980) Tumor factor" in uterine myoma (uterine myoma as an active component of the tumor-uterus system). Akush Ginekol (Mosk) 1: 35-37. Russian.
- Savitskiĭ GA, Morozov VV, Svechnikova FA, Ivanova RD (1981) Pathogenesis of uterine myoma development]. Akush Ginekol (Mosk) 4: 13-15.
- Savitskiĭ GA, Skopichev VG, Rakitskaia VV (1986) Denervation" of the tumor node as one of the elements of the pathogenesis of uterine myoma. Akush Ginekol (Mosk) 2: 24-27. Russian.
- Wang G, Tokushige N, Fraser IS (2011) Nerve fibers and menstrual cycle in peritoneal endometriosis. Fertil Steril 95: 2772-2774. [Crossref]
- Wang G, Tokushige N, Russell P, Dubinovsky S, Markham R, et al. (2009) Hyperinnervation in intestinal deep infiltrating endometriosis. J Minim Invasive Gynecol 16: 713-719. [Crossref]
- Al-Jefout M, Andreadis N, Tokushige N, Markham R, Fraser I (2007) A pilot study to evaluate the relative efficacy of endometrial biopsy and full curettage in making a diagnosis of endometriosis by the detection of endometrial nerve fibers. Am J Obstet Gynecol 197: 578.e1-4. [Crossref]




