Abstract
Neonatal hydrocephalus is a neurologic congenital condition where excess CSF accumulates in the brain’s ventricles and aqueducts, causing increased intracranial pressure. Global rising rates of neonatal hydrocephalus call for a better understanding of the etiology and advancement of more modernized therapeutic approaches. Currently, causes of neonatal hydrocephalus are believed to be genetic or from acquired injuries. Socioeconomic factors and maternal comorbidities also affect the health outcomes of neonatal hydrocephalus patients. Evaluation of development, presence, and recovery from neonatal hydrocephalus are through brain imaging, physical examination, and history taking. Infants with the condition most experience little loss of neurological function, but a significant fraction also faces worsening morbidities and sudden fatality. The two major treatments for the condition are ventriculo-subgaleal shunt (VSGS) and endoscopic third ventriculostomy (ETV), and both carry high risks of perioperative complications. Nevertheless, the chosen therapy for each patient requires consideration of their health status, and more importantly, their informed decision. The patient should also be informed about postoperative management and the importance of regular follow-ups with their neurosurgeon and neurologist.
Keywords
hyperbaric oxygen; hyperbaric medicine; Carbon Monoxide; Central retinal artery occlusion; Idiopathic sudden sensorineural hearing loss.
Introduction
Neonatal hydrocephalus, or congenital hydrocephalus, is the accumulation of excessive cerebrospinal fluid (CSF) in enlarged brain ventricles caused by cerebral malformation or defective development of CSF pathways. The resulting impaired CSF circulation for defective flow currents, fluid overaccumulation, and fluid imbalance is a major indication for neurosurgical shunting [1]. At 0.3 to 2.5 cases per 1,000 live births, hydrocephalus is one of the most common congenital abnormalities occurring in the nervous system [2]. Studies claim that up to 78% of patients with congenital hydrocephalus show residual neurological deficits which can reach disability rates of up to 28% of patients [3]. Neonatal hydrocephalus is considered a multifactorial disorder [3], typically stems from CNS perinatal or neonatal infection [4], aqueductal stenosis, Dandy-Walker Malformation (DWM), and holoprosencephaly [5]. The physiological mechanisms leading to the disorder can be separated into primary and secondary. Mechanical compression and stretching of brain parenchyma leads to mechanisms involving neuron alternation and axonal degeneration on a cellular level [3]. Furthermore, hydrocephalus can be classified as communicating and non-communicating hydrocephalus. Communicating hydrocephalus is characterized by CSF being able to flow after blocking the ventricles due to passages remaining open. Non-communicating hydrocephalus ceases the communication between the ventricles and no CSF is able to flow. The significance of categorizing hydrocephalus as either communicating or non-communicating lies in the choice of treatment determined by current guidelines [6].
Treatment involves neurosurgical CSF diversion, in most cases, which has high morbidity and failure rates [7]. Neonatal hydrocephalus’ high morbidity and failure rates are explained by its heterogeneous etiology including neurodevelopment alterations that challenge therapy options [8,9]. CSF diversion is accomplished with shunting, which drains CSF. Post-surgical complications include poor neurodevelopmental outcomes and persistent ventriculomegaly. Currently, the peritoneal-ventricular shunt is the best surgery treatment, but the alternative endoscopic third ventriculostomy (ETV} is indicated in some treatments and presents fewer complications [10]. Guidelines suggest that peritoneal-ventricular shunt should be indicated as the first option for neonates with a greater weight than 2000 grams [11]. However, neonates of low body weight (<2000 grams) are suggested to undergo external ventricular drain as an alternative to low weight until the 2000 grams goal is reached for surgical intervention [9]. The alternative endoscopic third ventriculostomy is conditionally limited to the type of non-communicating hydrocephalus and in patients of at least one year of age [6]. Current treatment options for neonatal hydrocephalus have shown in studies to reverse neurological deterioration by 10-20% through surgical procedures [12]. The importance of this review is to show a clinical presentation of congenital hydrocephalus, guidelines for its treatment, and alternative therapies with the goal of improving neurological outcomes.
Incidence and prevalence of neonatal hydrocephalus The World Health Organization-affiliated International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR) estimates the incidence of neonatal hydrocephalus secondary to congenital anomalies and hydrocephalus associated with spina bifida [13]. The ICBDSR reports the incidence of congenital hydrocephalus to be approximately 50 cases per 100,000 births annually [14]. However, the overall incidence of hydrocephalus related to spina bifida rises to 81 cases per 100,000 births annually [14]. When taking into account congenital and acquired etiologies including posthemorrhagic and postinfectious hydrocephalus, the registry states the incidence of neonatal hydrocephalus to be as high as 270 cases per 100,000 births annually which would represent over 380,000 global new cases of hydrocephalus every year [15]. Based on the data collected, hydrocephalus can be associated with a congenital source in 44% of cases, spina bifida in 12% of cases, infection in 42% of cases, and only 2% caused by intraventricular hemorrhage of prematurity [15]. Socioeconomic factors play a significant factor in terms of the incidence of neonatal hydrocephalus [16]. When comparing high-income countries to low and middle-income countries, the annual incidence was reported for congenital and spina bifida-associated hydrocephalus to be 78 cases per 100,000 births and 106 cases per 100,000 births, respectively [14]. Moreover, when observing the incidence of all congenital, spina bifida-associated, and acquired cases of hydrocephalus, there is a greater difference between high-income countries (79 cases per 100,000 births) and low and middle-income countries (123 cases per 100,000 births) [14]. We can propose higher birth rates along with the higher incidence of postinfectious and spina bifida-related hydrocephalus in low and middle-income countries to be mechanisms of the observed differences [16].
The prevalence of congenital hydrocephalus is expected to increase as the survival rate for patients is reaching higher percentages due to new treatment options and early surgical interventions [17]. Studies report that the global prevalence of neonatal hydrocephalus is 72 cases per 100,000 population group [14]. When introducing spina bifida-associated prevalence, an increase to 88 cases per 100,000 population group is observed [14]. Similar to incidence, the prevalence in low and middle-income countries is shown to be higher due to proposed socioeconomic effects compared to those in high-income countries [16].
Pathophysiology of neonatal hydrocephalus Besides the prognosis of patients with neonatal hydrocephalus being poorly optimal, the pathophysiology is not well understood. In the ventricular system, an obstructive malformation that causes partial or complete physical blockage of the CSF flow may result in hydrocephalus [18]. The path that CSF takes, known as the “bulk flow” model, is characterized by a slow and unidirectional movement through the ventricular system [19]. The CSF exits the ventricular system through the fourth ventricle into the subarachnoid space where arachnoid granulations absorb the CSF and dump it into the systemic circulation [19]. Once outside of the ventricular system, the CSF flow into systemic circulation can be impaired by inflammation or scarring of the subarachnoid space [20]. Congenital hydrocephalus can be a result of primary genetic abnormalities that develop the injury as a direct mechanism or secondary mechanisms that are caused by the consequence of the expansion of the ventricles or alteration of CSF physiology [21]. Ongoing studies are attempting to establish the congenital mechanisms that contribute to ventriculomegaly in humans in addition to gross malformations like Chiari II and Dandy-Walker [3]. McAllister explains that the main congenital mechanisms in hydrocephalus are aqueductal stenosis or obstruction, ependymal denudation, and modifications in the subcommissural organ.
The multifactorial nature of congenital hydrocephalus forces the concept that all ventriculomegaly can be obstructive by blocking the absorption of CSF with a physical wall or reduced transport to the subarachnoid space, arachnoid granulations, and ultimately the systemic circulation [22]. Other factors to consider based on the disease’s multifactorial nature are the overlapping injury mechanisms [23]. In the timeline of the first few hours to a few days after the onset of ventriculomegaly, the most critical mechanisms are increased CSF pulsatility in the cerebral aqueduct, compressions and stretch of periventricular tissue, and ischemia and hypoxia [24]. Once, the timeline of chronic ventriculomegaly is reached, additional mechanisms are contributing to a more severe form of hydrocephalus: gliosis, periventricular edema, demyelination, axonal degeneration, dendritic and synaptic deterioration, altered blood-brain barrier transportation, impaired CSF flow, and cell death [25]. Neurodegeneration can be attributed to the presence of apoptosis and necrosis of cortical neurons in the pathophysiology of prolonged hydrocephalus [26]. Another addition to mechanisms of neurological injury is the observed apoptosis of oligodendrocytes. In the early stages of hydrocephalus, oligodendrocytes undergo apoptosis in the periventricular white matter resulting in a potential impediment in the formation of myelin [26]. Ongoing efforts are being made to understand the multifactorial nature of hydrocephalus and how injury mechanisms contribute to the neurological outcome.
Classification of neonatal hydrocephalus
Hydrocephalus in infants can be categorized into congenital or acquired forms of the disease. Congenital or neonatal hydrocephalus occurs by an intrinsic mechanism that is present at birth and can present at birth or later develop such as genetics [27]. In the case of an extrinsic prenatal cause such as a complication of a hemorrhage, infection, or neoplasm, can result in the same form of neonatal hydrocephalus, however, it is denominated as acquired hydrocephalus [28]. The recently introduced multi-categorical hydrocephalus classification (Mc HC) takes into consideration onset, cause, underlying lesion, symptomatology, pathophysiology, and CSF flow, and divides obstructive from communicating cases [29]. Lastly, an additional classification can be portrayed when a specific syndrome or genetic basis is attributed [30]. This is categorized as syndromic or non-syndromic as hydrocephalus being associated with a syndrome or not, respectively. Studies choose to distinguish hydrocephalus as characterized by a clinical phenotype and brain findings, or characterized by major physical abnormalities or clinical signs [30].
Causes of hydrocephalus in neonates
Aqueductal Stenosis: The most common site of intraventricular blockage is the Sylvius aqueduct which is known to be the narrowest section CSF flows through to reach systemic circulation [31]. Stenosis, or narrowing of the aqueduct, is approximated to be responsible for 6-66% of cases of neonatal hydrocephalus [32]. The Sylvius aqueduct can become stenotic as a result of compression from mass lesions or by a mechanism of intrinsic pathologies such as non-tumoral aqueduct stenosis [33]. Intrinsic aqueductal stenosis has been classified as either congenital or acquired, idiopathic, or secondary to a known disease [34]. The lumen of the Sylvius aqueduct is lined with a single layer of ependymal cells [34]. Russell classifies histopathologically non-tumoral aqueduct stenosis into four causes: stenosis, forking, septum formation, and gliosis [35].
Stenosis is observed as the narrowing of the aqueduct and presentation of the ependymal lines in the lumen with the absence of gliosis [36]. Stenosis can occur through a mechanism of simple stenosis, where an abnormally sized aqueduct with normal cells is observed, or congenital atresia. In congenital atresia, abnormal infolding of the neural plate is suspected to be the cause of the narrowing of the neural tube [37]. Forking is a condition that occurs through the incomplete fusion of the median fissure resulting in the decrease in size of the lumen for CSF to flow [38]. The partial fusion ramifies into two or more individual channels that communicate with each other, however, communicate with the ventricles separately [38]. A gliotic membrane can form from glial overgrowth at the lower end of the aqueduct. This overgrowth formation restricts the space for CSF to flow at the lower end of the aqueduct and can reach to totally obstruct the aqueduct [39]. Similarly, to the overgrowth of glial cells, gliotic stenosis is observed. The proliferation of glial cells and overproduction of glial fibers or gliosis may be associated with a reaction to irritant agents such as infection, or hemorrhage, and is responsible for the obstruction of the aqueduct [40] .
Intraventricular Hemorrhage and Germinal Matrix Hemorrhage
Intraventricular hemorrhage (IVH) is characterized by blood leakage into the ventricular space. Pathogenesis of IVH is attributed to vascular fragility and fluctuations of CSF flow which may be observed in 20% of preterm newborns and further develop into neonatal hydrocephalus [41]. The area’s high demand for oxygen places it in a hypoxic state which upregulates the synthesis of vascular endothelial growth factor (VEGF) and in addition to its high vascularity, this increases the likelihood of hemorrhage [42]. Germinal matrix hemorrhage may originate in the capillary vessels of the subependymal germinal matrix (GM) [43]. This is clinically significant due to the association of the GM with developing neural networks which can potentially lead to neurodevelopmental disability [44]. The pathogenesis of GM hemorrhage might be contributed by a combination of the fragile GM vasculature, abnormal flow of blood in the brain due to low mean arterial pressure, and impaired cerebral autoregulation [45]. This multifactorial component makes a preterm neonate susceptible to vascular rupture and hemorrhage [46]. Germinal matrix hemorrhage is graded (Figure 2) such that grade 1 depicts the hemorrhage limited to the GM; grade 2 describes the hemorrhage partially extending to the ventricular system; grade 3 connects GM and IVH due to complete expansion to the ventricular system; and grade 4 shows hemorrhage fully extended to brain tissue [47]. If hemorrhage is left untreated, hydrocephalus may contribute to seizures, cognitive impairment, and white matter injury [48]. In order to treat IVH that may lead to hydrocephalus, CSF flow must be diversified to a temporary external drain, access reservoir, permanently implanted shunt device, or endoscopic third ventriculostomy [49].
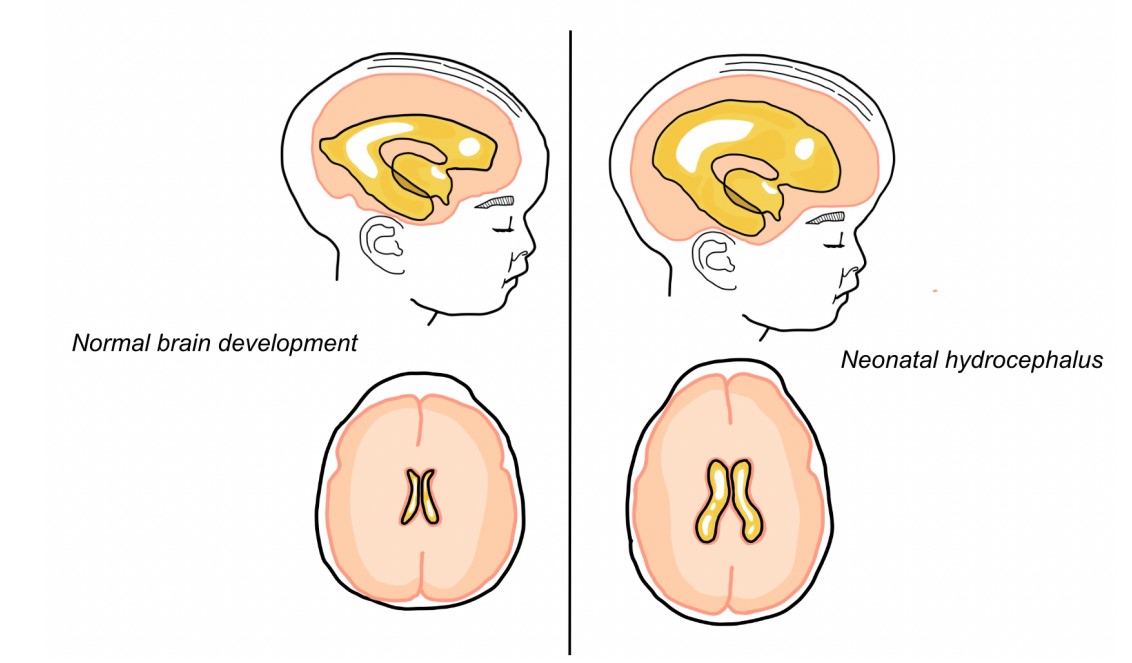
Figure 1: Macrocephaly and ventriculomegaly in neonatal hydrocephalus.
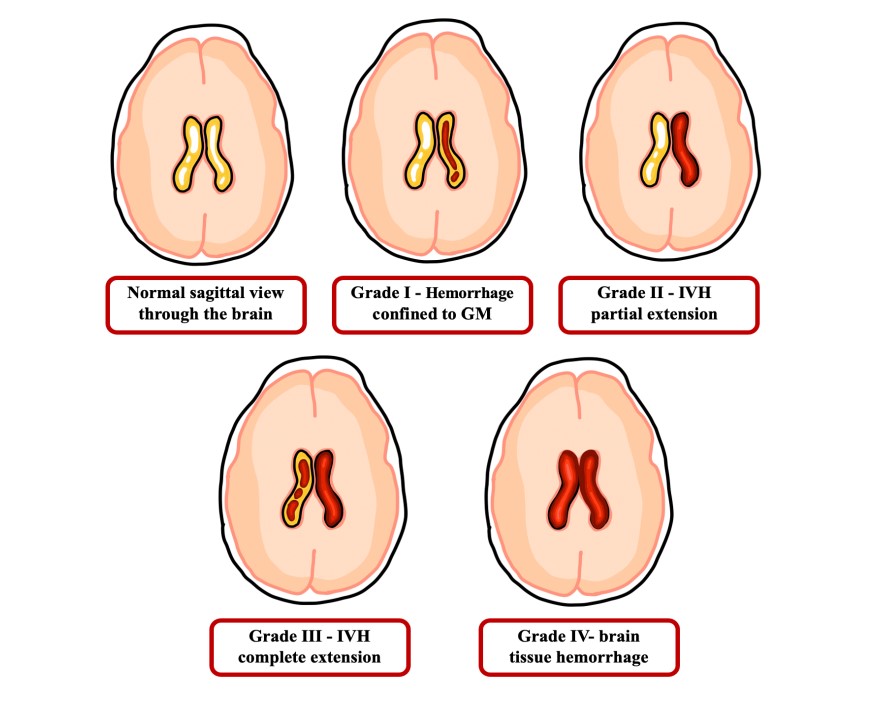
Figure 2: Germinal Matrix-Intraventricular Hemorrhage Grading.
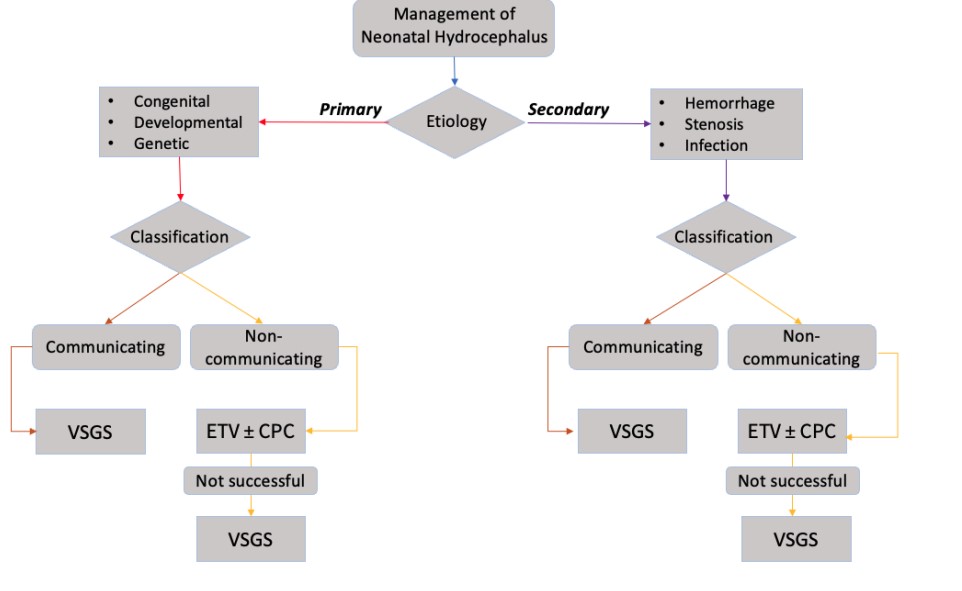
Figure 3: Current Procedures for Management of Neonatal Hydrocephalus based on Classification.
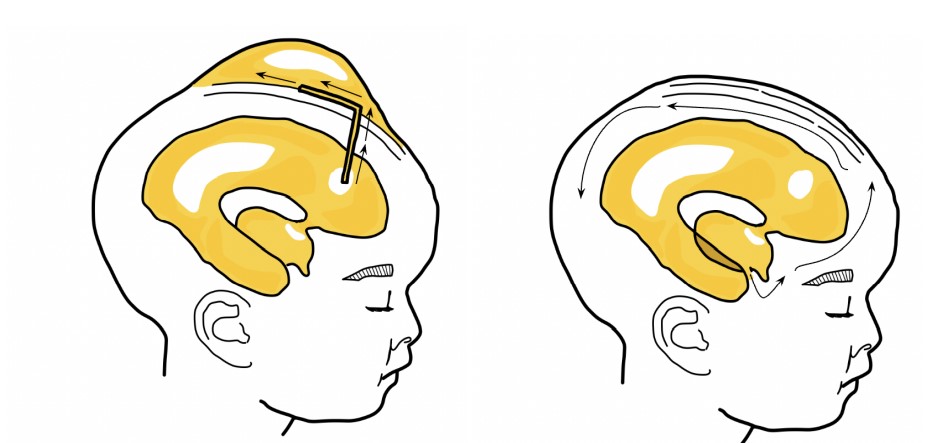
Figure 4: Ventriculosubgaleal shunt (VSGS) & Endoscopic Third Ventriculostomy (ETV).
Diagnosis of neonatal hydrocephalus
A diagnosis of neonatal hydrocephalus is made through a medical history, neurological exam, and imaging. Since there is a genetic component to neonatal hydrocephalus, some may find it helpful to assess the risk of a fetus developing hydrocephalus [50]. Hydrocephalus can begin prenatally before skull closure and full development of the brain [51]. Therefore, prenatal brain imaging [52] is the most effective approach in tracking the development of the fetus’ brain and hydrocephalus [53]. Ventricular dilatation is an important indicator for the currently developing or the future development of hydrocephalus [54]. Imaging techniques such as ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI) are decided based on the potential benefits of the test, cost, and risk of harm from radiation [55]. Detection often occurs between 15 and 35 weeks of gestation [56], and most cases arise during the third trimester [56]. The current most common method of diagnosing and monitoring ventricular dilatation is via cranial ultrasound [57], and confirmation is made with more defined, comprehensive images from an MRI [58]. An ultrasound can check for fluid accumulation in the ventricles through the fontanelle of the fetus’ skull [59]. The anterior fontanelle can also be used for the US postnatally before it closes. Generally, the risks and radiation effects of ultrasounds are low [60]. After birth, an MRI can visualize surrounding brain tissue, ventriculomegaly, and assess CSF flow [61]. In the case of IVH-cased hydrocephalus, a cranial US is performed right after birth, as the highest risk of bleeding is found within a week of birth [62]. A CT scan can also check for ventricular dilatation and CSF accumulation [63]. These tests are also used to distinguish communicating or non-communicating and compensated or arrested hydrocephalus [64]. After birth and during surgical follow-ups, neuropsychological evaluations would be performed to assess for cognitive impairment [65]. Physical examination [18] involves measuring the head circumference, looking for firmness or elevation of the anterior fontanelle, and splitting or splaying or cranial sutures to assess for macrocephaly caused by CSF accumulation [66].
Treatment options for neonatal hydrocephalus
The goal of treatment is to reduce intracranial pressure by draining CSF from the ventricles and restoring CSF circulation [10]. Considering the many possible causes and multifactorial nature of neonatal hydrocephalus [67], the mechanism is difficult to understand, and subsequently, the development of novel therapeutic strategies is slow. The aforementioned “bulk flow” model is considered too simplistic since CSF uses perivascular networks [68] to deposit itself into different brain cavities [69]. Shunting has been the go-to treatment since the 1950s [70], and the procedure itself has evolved over the past seven decades, but such improvements have not been able to eliminate the significant rates of surgical complications and shunt failure [68].
Treatments for neonatal hydrocephalus are still largely debated, as each of the few available options carry high morbidity and risks [71]. Most would agree that the best treatment options produce the best health outcomes and induce the least risk for impaired brain growth and development [72]. There are currently three surgical options that can treat neonatal hydrocephalus: ventriculo-subgaleal shunt (VSGS), endoscopic third ventriculostomy (ETV), and choroid plexus cauterization (CPC) [73]. Surgical decision-making depends on patient age, etiology, neuroanatomy, imaging findings, and existing comorbidities [66]. All three procedures are performed under general anesthesia and can be completed within a few hours [74]. Overall, receiving any of the surgical treatments reduces deaths by neonatal hydrocephalus by 50% [75]. Trends show that the earlier the diagnosis and treatment improves the prognosis of neonatal hydrocephalus, and most can lead functional lives with few limitations [76] .
Ventriculo-subgaleal Shunt (VSGS)
Most cases of neonatal hydrocephalus, regardless of cause, can be treated with a ventriculoperitoneal shunt [77], but complications can significantly impact brain function during development [78]. A burr hole is drilled through the frontal bone and a synthetic shunt tube is inserted to drain the CSF-filled ventricles into a pocket under the skin [79]. There are three parts of a shunt: one end of the shunt stays in the ventricle, the body tunnels under the skin, while the other end drains into another body cavity [80]. At times, a valve within the shunt can control the rate of drainage to prevent rapid flushing [81]. However, the slit valve can increase the occurrence of an intraparenchymal hemorrhage [82].
Around 40-50% of neonatal hydrocephalus cases in premature infants are caused by germinal matrix hemorrhage (GMH). These ruptures in the germinal matrix vessels lead to intraventricular hemorrhage (IVH) [83]. IVH subsequently leads to disruption of CSF circulation and ventricular dilation as seen in neonatal hydrocephalus. In such cases, ventriculo-subgaleal shunt (VSGS) is typically chosen for neonates because it drains CSF into other areas of the brain cavity to reduce intracranial pressure [83]. Other options such as ventriculo-peritoneal shunt (VPS) and ventriculo-atrial shunt (VAS) are more common in more developed children, whereas VSGS procedures are performed soon after birth [84]. Corrective procedures are always performed postnatally, even in the case of a prenatal diagnosis of neonatal hydrocephalus. Guidelines for the timing of VSGS procedures are still being debated, but most would agree that VSGS should be performed within a month following birth. Statistically, VSGS is typically performed 35.04 days after birth [84], with delays resulting from poor vital signs and/or respiratory function. After management with a CSF drain, the infant may graduate to a ventriculo-peritoneal or -atrial shunt or consider an ETV. As opposed to ventriculo-peritoneal shunt (VPS), it does not require a mature immune system, developed abdomen absorption capacity, elimination of blood products from CSF, and sufficient thickness. If a neonate receives a VPS without sufficient development, they would need external drainage and frequent aspiration of CSF [82]. VSGS can be a temporary means of managing the hydrocephalus before the neonate matures enough to tolerate the VPS. Other temporary means of managing progressive post-hemorrhagic hydrocephalus include intermittent lumbar punctures, placement of a tapping reservoir for CSF withdrawal, or the placement of an external drain [85]. In neonates with post-hemorrhagic hydrocephalus, VSGS produces lower infection and complication rates. VSGS is comparatively simpler and faster than alternative procedures, avoids repetitive aspiration for CSF evacuation, and most importantly, permanently decompresses the ventricles without electrolyte and nutritional loss [84].
However, shunt failures caused by infection and malfunction could be potentially fatal in the future. Shunts can stop draining and poorly regulate circulation because of blockages, infections, or unintended shifts. Shunt failures in older patients can often go unnoticed as there may be life-threatening increases in intracranial pressure without detectable increases in ventricular volume [51]. Moreover, risks are elevated if the VSGS procedure is performed too early on premature infants. Shunt dysfunction in pediatric hydrocephalus can affect 440% of cases within 1 year and 50% of cases within 2 years [78]. Even so, any newly-placed shunt requires an average of 2 to 4 revision surgeries within a decade of implantation [86]. Should the shunts become blocked for infection, shunt repair surgery is necessary to correct the complication. As a result, new treatment procedures are being developed, as VSGS complication rates are high in low birth weight (LBW) and premature infants receiving VSGS procedures [84]. The first way to reduce the risk of infection, post-surgical complications, and shunt dependence is to avoid long-term insertion of a foreign synthetic object.
Endoscopic third ventriculostomy (ETV)
Neuroendoscopic third ventriculostomy for noncommunicating hydrocephalus is now being explored as an alternative for VSGS, with lower risks of surgical perioperative hypersensitivity reactions and lower long-term risks after an early high-risk period [87]. With the rise of ETV, the use of shunting has declined [51]. Like in VSGS, a hole is drilled through the skull, but an endoscope enters the hole and punctures a hole into the floor of the third ventricle, creating an alternate route for CSF circulation [88]. Next, a balloon is inserted into the hole and inflated, then deflated and removed, to stretch the opening. Similar to the valve in the shunt, the size of the ETV would control the rate of CSF flow [89]. Unlike VSGS, the ETV procedure leaves no foreign hardware in the body, which lowers the risk of hypersensitivity reactions and post-surgical infection [90]. Another procedure combines the ETV with choroid plexus cauterization (CPC). After the endoscope punctures through the ventricle, choroid plexus tissue is cauterized. By trimming the choroid plexus, the procedure reduces the overall CSF production [91]. The resulting ETV allows fluid to pass from the ventricles into the subarachnoid space surrounding the brain. This procedure is preferred over ventriculo-peritoneal shunts for pediatric patients under the age of 2 [92]. Despite initial success with the procedure, the ETV can close during development or during adulthood, which may lead to an acute emergency situation [93]. Currently, the ETV is the standard option for children over 2 in cases of obstructive hydrocephalus where a blockage is present [94]. Infants receiving an ETV under 1 year of age have a much higher failure rate than those receiving it who were older than 1 year of age [95].
Novel preclinical studies for treatments
Novel therapies aim to avoid shunting in neonatal cases by opting for exogenous fibrinolytic agents [78]. A study found that fibrinolytic therapy, CSF drainage, and irrigation on posthemorrhagic hydrocephalus can reduce the fatality rate [96]. Possible biomarkers of TGF-β1 and TGF-β2 [97] have the potential to show where brain cells are being damaged, and where therapeutic interventions are needed. Such treatments are still being tested on animal models and require larger sample sizes and application to human models to prove safety and efficacy. Since these studies are still in the early stages of development, shunts and ETVs remain the two primary interventions for treating neonatal hydrocephalus.
Follow-up regimen
Proper management of neonatal hydrocephalus requires early identification, follow-ups during development, and monitoring of shunts and ETVs [98]. Long-term management of lasting complications includes frequent visits with pediatric specialists, OTs, mental health professionals, and social workers [99]. The first visit should be within two to four weeks of the surgery, and sooner if symptoms or complications arise. Post-surgical visits should involve neuroimaging studies [100]. ETV cases, for instance, require an MRI to assess CSF flow through the ventriculostomy [101]. Patients with ventriculoatrial shunt, which drain into the thoracic cavity, need echocardiograms to avoid cardiac vegetations and/or cor pulmonale after surgery [102]. Follow-up visits to neurologists and neurosurgeons are then spaced out with time intervals dependending on their condition and recovery rate [2]. Neuroimaging during subsequent follow-ups checks for infections, bleeding, and ventricular size, and is done once every few years. Imaging can be more frequent with a subscription to shunt malfunction [2]. Neurodevelopmental monitoring through neurologic exams assesses behavior and cognitive abnormalities during development. Fatality, epilepsy, and loss of function are possible complications of surgery, but risks can be reduced with regular follow-ups [2].
Conclusion
Neonatal hydrocephalus is the most common congenital abnormality, which requires surgical treatment to relieve CSF accumulation that causes enlarged brain ventricles and aqueducts. CSF accumulation is often caused by a physical blockage rom abnormal formation of the ventricular system. The pathophysiology of hydrocephalus can be caused by primary mechanisms such as ventriculomegaly, and secondary mechanisms such as infection, inflammation, and scarring of the subarachnoid space. Potential causes of neonatal hydrocephalus include aqueductal stenosis, intraventricular hemorrhage, germinal matrix hemorrhage, and stroke. Current classifications of neonatal hydrocephalus help identify which treatment option is most suitable for the patient. First, the presence and characteristics of the hydrocephalus are found through brain imaging techniques — most commonly using ultrasonography. Postnatal assessments involve medical and genetic family history, neurologic exams, and brain imaging, as well. The two main types of surgical procedures for neonatal hydrocephalus patients are the ventriculo-subgaleal shunt (VSGS) and endoscopic third ventriculostomy (ETV). Surgical decision-making depends on the patient’s age, preterm or full-term delivery, etiology, neuroanatomy, and comorbidities. The ideal treatment for neonatal hydrocephalus is still being debated as each option carries an increased risk of morbidity during development and infant mortality. After the surgical treatment, a follow-up is typically scheduled within the first month to check for recovery progress. Then, visits are spaced out and often involve brain imaging, physical examination, and history-taking to manage any complications. Out of 100,000 births, there are an estimated 50 cases of neonatal hydrocephalus annually. Increased rates of neonatal hydrocephalus occur in low- to middle-income countries due to higher birth rates and incidence of postinfectious or spina bifida-related hydrocephalus. These trends of neonatal hydrocephalus are expected to expand globally, and therefore, it is imperative to create more definitive classifications of hydrocephalus and establish safer and more effective treatments.
References
- Duy PQ, Weise SC, Marini C, Li X, Liang D, et al. (2022) Impaired neurogenesis alters brain biomechanics in a neuroprogenitor-based genetic subtype of congenital hydrocephalus. Nat Neurosci 25: 458-473. [Crossref]
- Flanders TM, Billinghurst L, Flibotte J, Heuer GG (2018) Neonatal hydrocephalus. NeoReviews 19: e467–e477.
- McAllister JP 2nd. (2012) Pathophysiology of congenital and neonatal hydrocephalus. Semin Fetal Neonatal Med 17: 285-294. [Crossref]
- Garg K, Gupta D (2021) Post-Infective Hydrocephalus. Neurol India 69: S320-S329. [Crossref]
- Verma R, Srivastava C, Ojha BK, Chandra A, Garg RK, et al. (2021) Complications Encountered with ETV in Infants with Congenital Hydrocephalus. Neurol India 69: S520-S525. [Crossref]
- Nishiyama K, Mori H, Tanaka R (2003) Changes in cerebrospinal fluid hydrodynamics following endoscopic third ventriculostomy for shunt-dependent noncommunicating hydrocephalus. J Neurosurg 98: 1027-1031. [Crossref]
- Jin SC, Dong W, Kundishora AJ, Panchagnula S, Moreno-De-Luca A, et al. (2020) Exome sequencing implicates genetic disruption of prenatal neuro-gliogenesis in sporadic congenital hydrocephalus. Nat Med 26: 1754-1765. [Crossref]
- Uparela-Reyes MJ, Narváez-Rojas AR, Quintana-Pájaro L, Ramos-Villegas Y, Moscote-Salazar LR (2018) Congenital-neonatal hydrocephalus: therapeutic alternatives to derivation. A look at cell therapy. Cir Cir 86: 575-582.
- Oi S, Inagaki T, Shinoda M, Takahashi S, Ono S, et al. (2011) Guideline for management and treatment of fetal and congenital hydrocephalus: Center of Excellence-Fetal and Congenital Hydrocephalus Top 10 Japan Guideline 2011. Childs Nerv Syst 27: 1563-1570. [Crossref]
- Malagón-Valdez J (2006) Congenital hydrocephalus. Rev Neurol 42: S39-S44.
- Rekate HL (2004) Selecting patients for endoscopic third ventriculostomy. Neurosurg Clin N Am 15: 39-49. [Crossref]
- Guerra M, Blázquez JL, Rodríguez EM (2017) Blood-brain barrier and fetal-onset hydrocephalus, with a view on potential novel treatments beyond managing CSF flow. Fluids Barriers CNS 14: 19. [Crossref]
- Tamber MS (2021) Insights into the epidemiology of infant hydrocephalus. Childs Nerv Syst 37: 3305-3311. [Crossref]
- Isaacs AM, Riva-Cambrin J, Yavin D, Hockley A, Pringsheim TM, et al. (2018) Age-specific global epidemiology of hydrocephalus: Systematic review, metanalysis and global birth surveillance PLoS One 13: e0204926. [Crossref]
- Dewan MC, Rattani A, Mekary R, Glancz LJ, Yunusa I, et al. (2018) Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. J Neurosurg 1-15. [Crossref]
- Reynolds RA, Dixon M, Gannon S, Zhao S, Bonfield CM, et al. (2020) The interaction between parental concern and socioeconomic status in pediatric hydrocephalus management. J Neurosurg Pediatr 27: 16-22. [Crossref]
- Li M, Wang H, Ouyang Y, Yin M, Yin X (2017) Efficacy and safety of programmable shunt valves for hydrocephalus: A meta-analysis. Int J Surg 44:139-146. [Crossref]
- Tully HM, Dobyns WB (2014) Infantile hydrocephalus: a review of epidemiology, classification and causes. Eur J Med Genet 57: 359-368. [Crossref]
- Kahle KT, Kulkarni AV, Limbrick DD Jr, Warf BC (2016) Hydrocephalus in children. Lancet 387: 788-799. [Crossref]
- Filis AK, Aghayev K, Vrionis FD (2017) Cerebrospinal Fluid and Hydrocephalus: Physiology, Diagnosis, and Treatment. Cancer Control 24: 6-8. [Crossref]
- Toyota Y, Shishido H, Ye F, Koch LG, Britton SL, et al. (2021) Hydrocephalus Following Experimental Subarachnoid Hemorrhage in Rats with Different Aerobic Capacity. Int J Mol Sci 22: 4489. [Crossref]
- Rekate HL (2008) The definition and classification of hydrocephalus: a personal recommendation to stimulate debate. Cerebrospinal Fluid Res 5:2. [Crossref]
- Del Bigio MR (2004) Cellular damage and prevention in childhood hydrocephalus. Brain Pathol 14: 317-324. [Crossref]
- Del Bigio MR (2001) Pathophysiologic consequences of hydrocephalus. Neurosurg Clin N Am 12: 639-649. [Crossref]
- McAllister JP 2nd, Chovan P (1998) Neonatal hydrocephalus. Mechanisms and consequences. Neurosurg Clin N Am 9: 73-93. [Crossref]
- Del Bigio MR, da Silva MC, Drake JM, Tuor UI (1994) Acute and chronic cerebral white matter damage in neonatal hydrocephalus. Can J Neurol Sci 21: 299-305. [Crossref]
- Garcia-Bonilla M, McAllister JP, Limbrick DD (2021) Genetics and Molecular Pathogenesis of Human Hydrocephalus. Neurol India 69: S268-S274. [Crossref]
- Dorner RA, Burton VJ, Allen MC, Robinson S, Soares BP (2018) Preterm neuroimaging and neurodevelopmental outcome: a focus on intraventricular hemorrhage, post-hemorrhagic hydrocephalus, and associated brain injury. J Perinatol 38: 1431-1443. [Crossref]
- Oi S (2011) Classification of hydrocephalus: critical analysis of classification categories and advantages of "Multi-categorical Hydrocephalus Classification" (Mc HC). Childs Nerv Syst 27: 1523-1533. [Crossref]
- Verhagen JMA, Schrander-Stumpel CTRM, Krapels IPC, de Die-Smulders CEM, van Lint FHM, et al. (2011) Congenital hydrocephalus in clinical practice: a genetic diagnostic approach. Eur J Med Genet 54: e542-e547. [Crossref]
- Robertson IJ, Leggate JR, Miller JD, Steers AJ (1990) Aqueduct stenosis--presentation and prognosis. Br J Neurosurg 4: 101-106. [Crossref]
- Hirsch JF, Hirsch E, Sainte Rose C, Renier D, Pierre-Khan A (1986) Stenosis of the aqueduct of Sylvius. Etiology and treatment. J Neurosurg Sci 30: 29-39. [Crossref]
- Viola L, Graziussi G, Granata F, Schisano G (1979) Non-tumoral aqueduct stenosis. Acta Neurol (Napoli) 1: 123-132.
- Cinalli G, Spennato P, Nastro A, Aliberti F, Trischitta V, et al. (2011) Hydrocephalus in aqueductal stenosis. Childs Nerv Syst 27: 1621-1642. [Crossref]
- Aronyk KE (1993) The history and classification of hydrocephalus. Neurosurg Clin N Am 4: 599-609. [Crossref]
- Gullotta F (1977) Anatomo-pathological aspects of nontumoral hydrocephalus. Pathologica 69: 555-568.
- Jellinger G (1986) Anatomopathology of non-tumoral aqueductal stenosis. J Neurosurg Sci 30: 1-16. [Crossref]
- Feletti A, Fiorindi A, Longatti P (2016) Split cerebral aqueduct: a neuroendoscopic illustration. Childs Nerv Syst 32: 199-203. [Crossref]
- Gökalp HZ, Tascioglu AO (1977) Membranous occulusion of the aqueduct of Sylvius. Surg Neurol 8: 103-105. [Crossref]
- Jacobson EE, Fletcher DF, Morgan MK, Johnston IH (1999) Computer modelling of the cerebrospinal fluid flow dynamics of aqueduct stenosis. Med Biol Eng Comput 37: 59-63. [Crossref]
- Garvey AA, Walsh BH, Inder TE (2022) Pathogenesis and prevention of intraventricular hemorrhage. Semin Perinatol 46: 151592. [Crossref]
- Ballabh P, Xu H, Hu F, Braun A, Smith K, et al. (2007) Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med 13: 477-485. [Crossref]
- Ballabh P (2010) Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res 67: 1-8. [Crossref]
- Bolisetty S, Dhawan A, Abdel-Latif M, Bajuk B, Stack J, et al. (2014) Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics 133: 55-62. [Crossref]
- Lin PY, Hagan K, Fenoglio A, Grant PE, Franceschini MA (2016) Reduced cerebral blood flow and oxygen metabolism in extremely preterm neonates with low-grade germinal matrix- intraventricular hemorrhage. Sci Rep 6: 25903. [Crossref]
- Tsuji M, Saul JP, du Plessis A, Eichenwald E, Sobh J, et al. (2000) Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 106: 625-632. [Crossref]
- Egesa WI, Odoch S, Odong RJ, Nakalema G, Asiimwe D, et al. (2021) Germinal Matrix-Intraventricular Hemorrhage: A Tale of Preterm Infants. Int J Pediatr 2021: 6622598. [Crossref]
- Strahle JM, Mahaney KB, Morales DM, Buddhala C, Shannon CN, et al. Longitudinal CSF Iron Pathway Proteins in Posthemorrhagic Hydrocephalus: Associations with Ventricle Size and Neurodevelopmental Outcomes. Ann Neurol 90: 217-226. [Crossref]
- Holste KG, Xia F, Ye F, Keep RF, Xi G (2022) Mechanisms of neuroinflammation in hydrocephalus after intraventricular hemorrhage: a review. Fluids Barriers CNS 19: 28.
- Habib Z (1981) Genetics and genetic counselling in neonatal hydrocephalus. Obstet Gynecol Surv 36: 529-534. [Crossref]
- Rekate HL (2020) Hydrocephalus in infants: the unique biomechanics and why they matter. Childs Nerv Syst 36: 1713-1728. [Crossref]
- Zarutskie A, Guimaraes C, Yepez M, Torres P, Shetty A, et al. (2019) Prenatal brain imaging for predicting need for postnatal hydrocephalus treatment in fetuses that had neural tube defect repair in utero. Ultrasound Obstet Gynecol 53: 324-334. [Crossref]
- Iacopino DG, Zaccone C, Molina D, Todaro C, Tomasello F, et al. (1995) Intraoperative monitoring of cerebral blood flow during ventricular shunting in hydrocephalic pediatric patients. Childs Nerv Syst 11: 483-486. [Crossref]
- Gupta P, Sodhi KS, Saxena AK, Khandelwal N, Singhi P (2016) Neonatal cranial sonography: A concise review for clinicians. J Pediatr Neurosci 11: 7-13. [Crossref]
- Krishnan P, Raybaud C, Palasamudram S, Shroff M (2019) Neuroimaging in Pediatric Hydrocephalus. Indian J Pediatr 86: 952-960. [Crossref]
- SSM Health (2023) Fetal hydrocephalus: Causes, diagnosis & treatment. Causes, Diagnosis & Treatment | SSM Health.
- Brouwer AJ, Groenendaal F, Han KS, de Vries LS (2015) Treatment of neonatal progressive ventricular dilatation: a single-centre experience. J Matern Fetal Neonatal Med 28: 2273-2279. [Crossref]
- Kartal MG, Algin O (2014) Evaluation of hydrocephalus and other cerebrospinal fluid disorders with MRI: An update. Insights Imaging 5: 531-541. [Crossref]
- Marchie TT, Ayara CO (2013) Investigation of Infant Brain with or without Hydrocephalous in Our Environment Using Anterior Transfontanelle Ultrasound Scan. Niger J Surg 19: 7-12. [Crossref]
- Rajaraman P, Simpson J, Neta G, de Gonzalez AM, Ansell P, et al. (2011) Early life exposure to diagnostic radiation and ultrasound scans and risk of childhood cancer: case-control study. BMJ 342: d472. [Crossref]
- Korbecki A, Zimny A, Podgórski P, Sąsiadek M, Bladowska J (2019) Imaging of cerebrospinal fluid flow: fundamentals, techniques, and clinical applications of phase-contrast magnetic resonance imaging. Pol J Radiol 84: e240-e250. [Crossref]
- Volpe JJ (2009) Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 8: 110-124. [Crossref]
- Zhang T, Zhou Y, Su G, Shi D, Gopinath SCB, et al. (2019) Hydrocephaly Analysis Supported by Computerized Tomography and Nuclear Magnetic Resonance. J Anal Methods Chem 2019: 5872347. [Crossref]
- Infantile hydrocephalus (1969) Br Med J 2: 265.
- Venkataramana NK, Mukundan CR (2011) Evaluation of functional outcomes in congenital hydrocephalus. J Pediatr Neurosci 6: 4-12. [Crossref]
- Pindrik J, Schulz L, Drapeau A (2022) Diagnosis and Surgical Management of Neonatal Hydrocephalus. Semin Pediatr Neurol 42: 100969. [Crossref]
- Maduemem K, Khalid S, Hariharan M, Siddique A (2018) Intraventricular Haemorrhage Complicated by Hydrocephalus in an Acutely Encephalopathic Preterm Infant. Cureus 10: e2193. [Crossref]
- Verghese JP, Terry A, de Natale ER, Politis M (2022) Research Evidence of the Role of the Glymphatic System and Its Potential Pharmacological Modulation in Neurodegenerative Diseases. J Clin Med 11: 6964.
- Bramall AN, Anton ES, Kahle KT, Fecci PE (2022) Navigating the ventricles: Novel insights into the pathogenesis of hydrocephalus. EBioMedicine 78: 103931.
- Weisenberg SH, TerMaath SC, Seaver CE, Killeffer JA (2016) Ventricular catheter development: past, present, and future. J Neurosurg 125: 1504-1512. [Crossref]
- Cherian S, Whitelaw A, Thoresen M, Love S (2004) The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol 14: 305-311. [Crossref]
- Di Rocco C, Massimi L, Tamburrini G (2006) Shunts vs endoscopic third ventriculostomy in infants: are there different types and/or rates of complications? A review. Childs Nerv Syst 22: 1573-1589. [Crossref]
- Krause M, Panteliadis C, Hagel C, Hirsch F, Thome U, et al. (2018) Surgical Treatment for Neonatal Hydrocephalus: Catheter-Based Cerebrospinal Fluid Diversion or Endoscopic Intervention? Open J Modern Neurosurg 8: 30-45.
- Krovvidi H, Flint G, Williams AV (2018) Perioperative management of hydrocephalus. BJA Educ 18:140-146. [Crossref]
- Tully HM, Doherty D, Wainwright M (2022) Mortality in pediatric hydrocephalus. Dev Med Child Neurol 64: 112-117. [Crossref]
- McCullough DC, Balzer-Martin LA (1982) Current prognosis in overt neonatal hydrocephalus. J Neurosurg 57: 378-383. [Crossref]
- Valle D, Villarreal XP, Lunny C, Chalamgari A, Wajid M, et al. (2022) Surgical Management of Neurotrauma: When to Intervene. J Clin Trials Regul 4: 41-55. [Crossref]
- Park YS (2022) Treatment Strategies and Challenges to Avoid Cerebrospinal Fluid Shunting for Pediatric Hydrocephalus. Neurol Med Chir (Tokyo) 62: 416-430. [Crossref]
- Hsu CH, Chou SC, Yang SH, Shih MC, Kuo MF (2019) Using a burr hole valve prevents proximal shunt failure in infants and toddlers. J Neurosurg Pediatr 1-8. [Crossref]
- Eid S, Iwanaga J, Oskouian RJ, Loukas M, Jerry Oakes W, et al. (2018) Ventriculosubgaleal shunting-a comprehensive review and over two-decade surgical experience. Childs Nerv Syst 34: 1639-1642. [Crossref]
- Sil K, Ghosh SK, Chatterjee S (2021) Ventriculo-subgaleal shunts-broadening the horizons: an institutional experience. Childs Nerv Syst 37: 1113-1119. [Crossref]
- Fulmer BB, Grabb PA, Oakes WJ, Mapstone TB (2000) Neonatal ventriculosubgaleal shunts. Neurosurgery 47: 80-84. [Crossref]
- Robinson S (2012) Neonatal posthemorrhagic hydrocephalus from prematurity: pathophysiology and current treatment concepts. J Neurosurg Pediatr 9: 242-258. [Crossref ]
- Köksal V, Öktem S (2010) Ventriculosubgaleal shunt procedure and its long-term outcomes in premature infants with post-hemorrhagic hydrocephalus. Childs Nerv Syst 26: 1505-1515. [Crossref]
- Badhiwala JH, Hong CJ, Nassiri F, Hong BY, Riva-Cambrin J, et al. (2015) Treatment of posthemorrhagic ventricular dilation in preterm infants: a systematic review and meta-analysis of outcomes and complications. J Neurosurg Pediatr 16: 545-555. [Crossref]
- Piatt JH Jr, Cosgriff M (2007) Monte Carlo simulation of cerebrospinal fluid shunt failure and definition of instability among shunt-treated patients with hydrocephalus. J Neurosurg 107: 474-478. [Crossref]
- Kulkarni AV, Drake JM, Kestle JR, Mallucci CL, Sgouros S, et al. (2010) Endoscopic third ventriculostomy vs cerebrospinal fluid shunt in the treatment of hydrocephalus in children: a propensity score-adjusted analysis. Neurosurgery 67: 588-593. [Crossref]
- Yadav YR, Parihar V, Pande S, Namdev H, Agarwal M (2012) Endoscopic third ventriculostomy. J Neurosci Rural Pract 3: 163-173. [Crossref]
- Guzman R, Pendharkar AV, Zerah M, Sainte-Rose C (2013) Use of the NeuroBalloon catheter for endoscopic third ventriculostomy. J Neurosurg Pediatr 11: 302-306. [Crossref]
- Fountain DM, Chari A, Allen D, James G (2016) Comparison of the use of ventricular access devices and ventriculosubgaleal shunts in posthaemorrhagic hydrocephalus: systematic review and meta-analysis. Childs Nerv Syst 32: 259-267. [Crossref]
- Coulter IC, Dewan MC, Tailor J, Ibrahim GM, Kulkarni AV (2021) Endoscopic third ventriculostomy and choroid plexus cauterization (ETV/CPC) for hydrocephalus of infancy: a technical review. Childs Nerv Syst 37: 3509-3519. [Crossref]
- El Damaty A, Marx S, Cohrs G, Vollmer M, Eltanahy A, et al. (2020) ETV in infancy and childhood below 2 years of age for treatment of hydrocephalus. Childs Nerv Syst 36: 2725-2731. [Crossref]
- Mahapatra A, Mehr S, Singh D, Tandon M, Ganjoo P, et al. (2011) Ostomy closure and the role of repeat endoscopic third ventriculostomy (re-ETV) in failed ETV procedures. Neurol India 59: 867-873. [Crossref]
- Rahman MM, Khan SIMKN, Khan RA, Islam R, Sarker MH (2021) Endoscopic third ventriculostomy in children: problems and surgical outcome: analysis of 34 cases. Chin Neurosurg J 7: 3. [Crossref]
- Koch D, Wagner W (2004) Endoscopic third ventriculostomy in infants of less than 1 year of age: which factors influence the outcome?. Childs Nerv Syst 20: 405-411. [Crossref]
- Whitelaw A, Jary S, Kmita G, Wroblewska J, Musialik-Swietlinska E, et al. (2010) Randomized trial of drainage, irrigation and fibrinolytic therapy for premature infants with posthemorrhagic ventricular dilatation: developmental outcome at 2 years. Pediatrics 125: e852-e858. [Crossref]
- Li X, Miyajima M, Jiang C, Arai H (2007) Expression of TGF-betas and TGF-beta type II receptor in cerebrospinal fluid of patients with idiopathic normal pressure hydrocephalus. Neurosci Lett 413: 141-144. [Crossref]
- Özek E, Kersin SG (2020) Intraventricular hemorrhage in preterm babies. Turk Pediatri Ars 55: 215-221. [Crossref]
- Koleva M, De Jesus O (2022) Hydrocephalus. In: StatPearls. Treasure Island (FL): StatPearls Publishing. [Crossref]
- Morimoto K, Nishikuni K, Hirano S, Takemoto O, Futagi Y (2003) Quantitative follow-up analysis by computed tomographic imaging in neonatal hydrocephalus. Pediatr Neurol 29: 435-439. [Crossref]
- Lucic MA, Koprivsek K, Kozic D, Spero M, Spirovski M, et al. (2014) Dynamic magnetic resonance imaging of endoscopic third ventriculostomy patency with differently acquired fast imaging with steady-state precession sequences. Bosn J Basic Med Sci 14: 165-170. [Crossref]
- Magrassi L, Mezzini G, Moramarco LP, Cionfoli N, Shepetowsky D, et al. (2021) Minimally invasive procedure for removal of infected ventriculoatrial shunts. Acta Neurochir (Wien) 163: 455-462. [Crossref]




