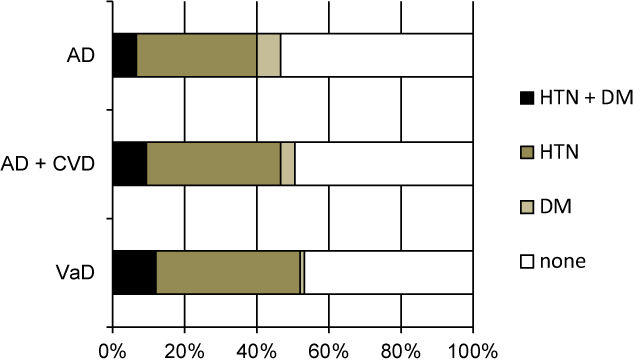Objective: There have been quite a few reports on the positive relationship between diabetes mellitus (DM) and the onset of dementia. However, we have clinical impression that well-controlled DM patients do not always manifest cognitive impairment.
Methods: We retrospectively analyzed the relationship between DM and dementia using the database from the prevalence study in Kurihara (n = 592), and that of consecutive outpatients in the Tajiri SKIP Center (n = 730). All subjects underwent evaluation for the Clinical Dementia Rating (CDR) and 1.5T-MRI to assess cerebrovascular diseases.
Results: There were no differences in the prevalence of DM among the CDR 0, CDR 0.5 and CDR 1+ patients. Only 3 patients exhibited Alzheimer onset from among the 94 consecutive outpatients with DM.
Discussion: Taking into consideration that our participants/patients with DM were well-controlled, these results suggest that DM might not be simply attributed to dementia risk. Uncontrolled DM might be more likely of a dementia risk.
diabetes mellitus, alzheimer disease, clinical dementia rating, cerebrovascular disease, vascular dementia
Abbreviations
AD: Alzheimer Disease; DM: Diabetes Mellitus; CVD: Cerebrovascular Disease; VaD: Vascular Dementia; CDR: Clinical Dementia Rating; MRI: Magnetic Resonance Imaging; iNPH: Idiopathic Normal Pressure Hydrocephalus; DLB: Dementia with Lewy Bodies; FTLD: Frontotemporal Lobar Degeneration; HTN: Hypertension.
For countries with increasingly large populations of older adults, knowledge of the risk factors of dementia onset, especially that of Alzheimer disease (AD), is essential for health policy planning. As one of the life style associated diseases, there have been quite a few reports indicating that diabetes mellitus (DM) is not only one of the main risk factors related to cognitive disturbance and dementia onset [1], but also cognitive decline in patients with AD [2]. Beyond the clinical and epidemiologic reports, pathological investigations have revealed common phenomenon between the two diseases, such as beta-amyloid deposition or tau phosphorylation [3,4] as well as insulin resistance [5]. Although these findings are not as simple as earlier concept proposed by Dr. de la Monte et al. [6], DM is currently known as “brain disease” and AD is called as “type 3 DM” [7].
Clinically we know that uncontrolled DM or other vascular risk factors easily leads to cerebrovascular disease (CVD), thus resulting in cognitive impairment or subsequently meeting the criteria of vascular dementia (VaD) [8,9]. Hypoglycemia due to mal-control of insulin often causes consciousness disturbances, which if they occur repeatedly, may result in cognitive disturbance [10] defined as metabolic dementia or specifically as “diabetic dementia”. However, the authors also have the clinical impression that well-controlled DM does not always lead to cognitive disturbance or even dementia onset after following patients for several years, although the conversion from mild cognitive impairment to AD is also promoted by uncontrolled situation [11]. This impression matches a recent population-based case-control study from epidemiological aspect [12].
To clarify this important topic, an integrated institute for possible investigation of epidemiologic as well as clinical databases is necessary. As one of such research institutes in Japan, we have been working on community-based programs for stroke, dementia, and prevention of bed-confinement since 1988: the Osaki-Tajiri [13] and Kurihara Projects [14]. We have also continued to perform as physicians in the Osaki-Tajiri SKIP Center clinic, where patients with DM were well-controlled by diet, exercise, and drug therapy, since 1997 [13].
Herein, we re-analyzed all of the databases associated with DM and dementia. This finding showed a lack of association between DM and dementia, and is therefore a negative report; however, even negative reports can add information for systematic review in the future. Therefore, herein, we would like to report the current results.
We retrospectively analyzed the relationship between DM and dementia using the database of the prevalence study in Kurihara (the Kurihara Project), and that of consecutive outpatients at the clinic in the Osaki-Tajiri SKIP Center. We also followed consecutive outpatients with DM for 5 years, in order to examine for the onset of dementia. The cities of Kurihara and Osaki are closely located in the northern part of Miyagi Prefecture, northern Japan. They are typical agricultural towns.
Epidemiologic database
The database of the Kurihara Project in 2008 was used. A detailed methodology has been described previously [14]. Briefly, in the project, the prevalence of dementia and dementing diseases were surveyed in 2008-2010. The total population in the city was about 76,708 and the population of the old-old was about 14,579 (17.9%; November 2010). From among 255 communities in the city, 19 were selected by the officials, and asked to participate in the project (target population of 1,254). These populations underwent 1) Clinical Dementia Rating (CDR) [15,16] assessment (see below), 2) neuropsychological tests (see below), 3) blood and urine tests, and 4) magnetic resonance imaging (MRI) scans.
Ultimately, 590 people agreed to participate (47.0%), and 73 (12.4%) were diagnosed with dementia using the DSM-IV criteria after rating a CDR 1+ status. MRI images of 576 people were analyzed successfully, and an intensive evaluation of CVD was performed (see below). For the blood tests, vitamin B1, B6, and B12, thyroid hormones, and HbA1c levels were determined. The subjects showed no abnormal findings by either blood tests or routine urine tests.
Using the database, the relationships between DM and three CDR groups, i.e., the CDR 0 group (healthy), the CDR 0.5 group (questionable dementia), and the CDR 1+ group (dementia) were analyzed. For the dementia group, we focused on only three types of dementia, i.e., AD, AD with CVD, and vascular dementia (VaD) (see below the diagnostic criteria), since these three types represent the major dementing diseases.
Outpatients database
This database was a prospective data collection in the Osaki-Tajiri SKIP Center clinic. This was performed at the Tajiri Clinic, an integrated institute for stroke and dementia, which is situated in Osaki-Tajiri, Miyagi Prefecture, northern Japan. All patients suspected of having dementia underwent routine medical checkups, including head MRI, electrocardiogram, chest X ray, and laboratory urine and blood tests, as part of the differential diagnoses.
Consecutive first-visit outpatients who were seen at the Tajiri Clinic from 2001 to 2013 were enrolled. Similar to the Kurihara Project database, we focused only on the three types of dementia, i.e., AD, AD with CVD, and vascular dementia (VaD) (see below the diagnostic criteria). Ultimately, 730 patients with three types of dementia, i.e., AD, AD with CVD, and VaD, in 2001-2013 were enrolled for the analyses.
Following consecutive outpatients with DM
In addition to the aforementioned groups, we also followed 94 consecutive outpatients with DM for 5 years to examine for the onset of dementia.
Diagnosis of DM
Type 2 DM was defined by a blood HbA1c level > 6.5%, according to the Guideline of Japan Diabetes Society 2013. The condition of “well-controlled DM” was defined as an HbA1c < 6.5%. The participants were classified into five categories, i.e., 0. Normal, 1. Not treated, 2. Diet therapy, 3. Medication, and 4. Insulin therapy, and when they were considered to be in category 1, they were referred to physicians including our SKIP center to treat their diseases.
CDR assessments
A clinical team, comprised of medical doctors and public health nurses, determined the CDR, blinded to the results of the cognitive tests, as follows: before being interviewed by the doctors, the public health nurses visited the participants’ homes to evaluate their daily activities. Observations by the family with respect the participants’ lives were described in a semi-structured questionnaire; for participants who lived alone, the public health nurses visited them frequently to evaluate their daily lives. The participants were interviewed by doctors to assess episodic memory, orientation, etc. Finally, with reference to the information provided by family, the participants’ CDR stages were decided at a joint meeting. A reliable Japanese version of the CDR Work Sheet [16] was established, and dementia was diagnosed based on the DSM-IV criteria. One of the authors (K.M) was certified as CDR rater at the Alzheimer’s Disease Research Center Memory & Aging Project, Washington University School of Medicine.
MRI
All participants underwent MRI (Achiva 1.5T, Philips Electronics Japan) at the hospital. Combined axial T1-weighted, T2-weighted and FLAIR images were used to evaluate atrophy and CVD. Lesions were considered to be CVD when they exhibited low intensity on the T1-weighted or FLAIR images and high intensity on the T2-weighted image at the same location, with diameters ≥ 4 mm [17]. MRI examinations were used to assess brain atrophy and vascular changes, to allow the diagnosis of the dementing diseases (see below).
Dementing diseases
After dementia was diagnosed using the DSM-IV criteria after rating a CDR 1+ status as described above, the physician’s team comprised of two neurologists, a psychiatrist, and a physicist diagnosed the following “probable” dementing diseases. The following diagnosis was performed after a meeting of two neurologists, a psychiatrist, and a physician.
- AD: People who met the following criteria were considered to have AD, i.e., pure AD without CVD; the NINCDS-ADRDA criteria for probable AD [18], and no CVD, as shown by MRI. On MRI images, the signal changes were considered to be CVD, which showed low signal intensity on T1-weighted images, high signal intensity on T2-weighted images, and high signal intensity surrounding the low signal intensity areas on FLAIR images.
- AD with CVD: People who met the following criteria were considered to have AD with CVD: the NINCDS-ADRDA criteria for probable AD, and the presence of CVD as shown by MRI; however, the CVD was considered to be a concomitant lesion, and the CVD areas were not responsible for the cognitive deterioration.
- VaD: People were considered to have VaD if they met the probable VaD criteria, as per the NINDS-AIREN [19].
- Others: Idiopathic normal pressure hydrocephalus (iNPH), dementia with Lewy Bodies (DLB) and frontotemporal lobar degeneration (FTLD) were diagnosed according to each of the consensus criteria.
Ethics
Written informed consent was obtained from all of the participants, patients, and the family. The study was approved by the Ethical Committees of Tohoku University Graduate School of Medicine, Kurihara City, and the Osaki-Tajiri SKIP Center.
All participants/patients with DM were previously diagnosed, as per the criteria, but all of them exhibited well-controlled conditions soon after diagnosis.
Epidemiologic database
Figure 1 shows the prevalence of DM and hypertension for each CDR group in the Kurihara Project database (n = 592). There were no differences in the prevalence of DM among the CDR 0 (healthy), CDR 0.5 (questionable dementia) and CDR 1+ (dementia) patients.
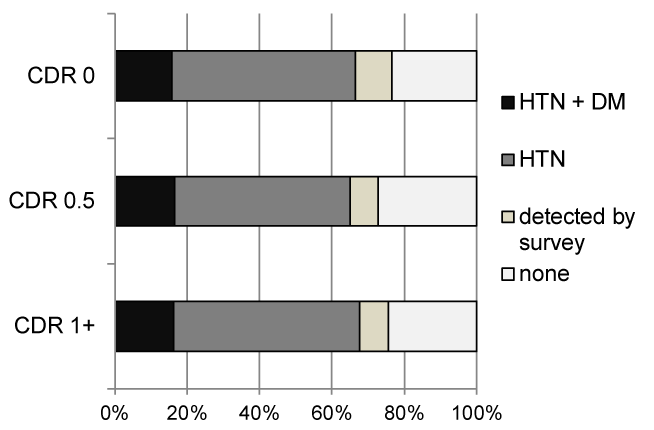
Figure 1. Prevalence of hypertension and diabetes mellitus for each CDR group in the Kurihara Project database (n = 592)
CDR: Clinical Dementia Rating; HTN: Hypertension; DM: Diabetes Mellitus
For the three main dementing diseases, i.e., AD, AD with CVD, and VaD, there were no differences in DM prevalence (the Chi-square test) (Figure 2).
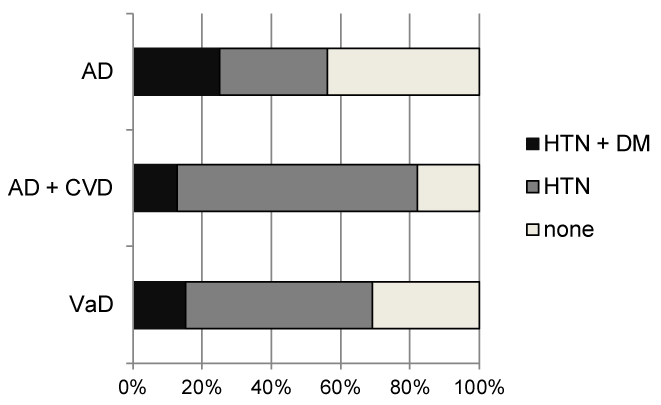
Figure 2. Prevalence of hypertension and diabetes mellitus for each of the dementing diseases in the Kurihara Project database (n = 592)
AD: Alzheimer’s Disease; CVD: Cerebrovascular Disease; VaD: Vascular Dementia; HTN: Hypertension; DM: Diabetes Mellitus
Outpatient database
Figure 3 presents the ratios of accompanying hypertension and DM mellitus for each dementia group in the consecutive outpatient database in Tajiri (2001-2013, n = 730). No group differences were noted (the Chi-square test).
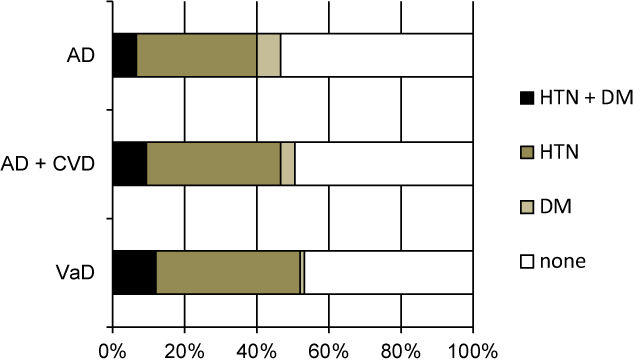
Figure 3. Ratios of accompanying hypertension and diabetes mellitus for each dementia group in the consecutive outpatient database in Tajiri (2001-2013, n = 730)
AD: Alzheimer’s Disease; CVD: Cerebrovascular Disease; VaD: Vascular Dementia; HTN: Hypertension; DM: Diabetes Mellitus
Following consecutive outpatients with DM
We followed 94 consecutive outpatients with DM for 5 years in Tajiri (2009-2013, n = 94), and only 3 patients exhibited the onset of AD. The demographics, other risk factors, and the number of patients exhibiting the onset of AD are shown in table 1.
Table 1. Onset of Alzheimer disease in the 5-year follow up period in the consecutive outpatient’s database in Tajiri (2009-2013, n = 94)
| |
n |
Mean age (SD) |
HTN |
DL |
IHD |
CKD |
AD onset |
Men |
40 |
68.5 (9.4) |
31 |
26 |
9 |
7 |
0 |
Women |
54 |
71.9 (11.5) |
37 |
43 |
11 |
15 |
3 |
All |
94 |
70.5 (10.8) |
68 |
69 |
20 |
22 |
3 |
HTN: Hypertension; DL: Dyslipidemia; IHD: Ischemic Heart Disease; CKD: Chronic Kidney Disease; AD: Alzheimer Disease
Taking into consideration that our participants/patients with DM were well-controlled, the current results suggest that DM itself might not be simply attributed to dementia “risk”. Uncontrolled DM might be more likely of a dementia risk.
Although the identification of “risk factors” can be utilized strategically for medical screening, they are “correlational” and not necessarily “causal”, because correlation does not prove causation. For example, being young cannot be said to cause measles, but young people have a higher rate of measles because they are less likely to have developed immunity during a previous epidemic. However, statistical methods are frequently used to assess the strength of an association and to provide causal evidence, such as the link between smoking and lung cancer. Generally, the term risk factor refers to causal determinants of increased rates of disease.
In this regard, DM may be an aging-related disorder that is not necessarily associated with the pathological condition of AD or dementia. Two extreme hypotheses are possible for the DM-dementia link. One is that DM patients have difficulty in controlling their daily lives, e.g. diet, and that CVD or other brain damage would occur, as described earlier. The other hypothesis is that cognitive functions do not merely represent “cognitive” but rather global “brain” function, or even “human” function. Both DM and dementia are affected by decreasing “energy” levels, thus leading to seemingly “causative” correlations. This is similar to the concept that the brain functions as a regulator of the psychological and physiological milieu of the body.
Since we previously reported that chronic kidney disease could be a risk factor for dementia, independent of DM or other vascular risk factors [20], further investigation is necessary.
Data collection: Satoshi Yamaguchi, Kenichi Meguro, Masahiro Nakatsuka, Kei Nakamura.
Data analysis and writing the manuscript: Kenichi Meguro.
The authors thank all the staff at the Geriatric Behavioral Neurology of Tohoku University, and that of the Osaki-Tajiri-SKIP Center.
The abstract was previously presented as a poster session at the AAIC conference in 2016.
There is no potential conflict of interests to be disclosed.
- Zhang J, Chen C, Hua S, Liao H, Wang M, et al. (2017) An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res Clin Pract 124: 41-47. [Crossref]
- Li J, Cesari M, Liu F, Dong B, Vellas B (2017) Effects of diabetes mellitus on cognitive decline in patients with Alzheimer disease: A systematic review. Can J Diabetes 41: 114-119. [Crossref]
- Baglietto-Vargas D, Shi J, Yaeger DM, Ager R, LaFerla FM (2016) Diabetes and Alzheimer’s disease crosstalk. Neurosci Biobehav Rev 64: 272-287. [Crossref]
- Pugazhenthi S, Qin L, Reddy PH (2017) Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis 1863: 1037-1045. [Crossref]
- Rani V, Deshmukh R, Jaswal P, Kumar P, Bariwal J (2016) Alzheimer’s disease: Is this a brain specific diabetic condition? Physiol Behav 164: 259-267. [Crossref]
- de la Monte SM, Wands JR (2008) Alzheimer’s disease is type 3 diabetes: Evidence reviewed. J Diabetes Sci Technol 2: 1101-1113. [Crossref]
- Leszek J, Trypka E, Tarasov VV, Ashraf GM, Aliev G (2017) Type 3 diabetes mellitus: A novel implications of Alzheimers disease. Curr Top Med Chem 17: 1331-1335. [Crossref]
- Meguro K, Akanuma K, Meguro M, Kasai M, Ishii H, et al. (2012) Prognosis of vascular MCI includes vascular dementia onset and death by cardiovascular diseases: Reanalysis from the Osaki-Tajiri Project. J Stroke Cerebrovasc Dis 21: 607-611. [Crossref]
- Jacquin A, Binquet C, Rouaud O, Graule-petot A, Daubail B, et al. (2014) Post-stroke cognitive impairment: High prevalence and determining in a cohort of mild stroke. J Alzheimers Dis 40: 1029-1038. [Crossref]
- Wang F, Zhao M, Han Z, Li D, Zhang S, et al. (2017) Long-Term Subclinical Hyperglycemia and Hypoglycemia as Independent Risk Factors for Mild Cognitive Impairment in Elderly People. Tohoku J Exp Med 242: 121-128. [Crossref]
- Li J, Wang YJ, Zhang M, Xu ZQ, Gao CY, et al. (2011) Chongqing Ageing Study Group. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology 76: 1485-1491. [Crossref]
- Kadohara K, Sato I, Kawakami K (2017) Diabetes mellitus and risk of early-onset Alzheimer’s disease: A population-based control study. Eur J Neurol 24: 944-949. [Crossref]
- Meguro K, Ishii H, Yamaguchi S, Ishizaki J, Shimada M, et al. (2002) Prevalence of dementia and dementing diseases in Japan: The Tajiri Project. Arch Neurol 59: 1109-1114. [Crossref]
- Meguro K, Tanaka N, Kasai M, Nakamura K, Ishikawa H, et al. (2012) Prevalence of dementia and dementing diseases in the old-old population in Japan: The Kurihara Project. Implications for Long-Term Care Insurance Data. Psychogeriatrics 12: 226-234. [Crossref]
- Morris JC (1993) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43: 2412-2414. [Crossref]
- Meguro K (2004) A clinical approach of dementia: An instruction of CDR worksheet (in Japanese). Tokyo, Igaku-Shoin pp: 75-88. [Japanese].
- Ishii H, Meguro K, Yamaguchi S, Ishikawa H, Yamadori A (2007) Prevalence and cognitive performances of vascular cognitive impairment no dementia in Japan: the Osaki-Tajiri Project. Eur J Neurol 14: 609-616. [Crossref]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, et al. (1984) Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34: 939-944. [Crossref]
- Róman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, et al. (1993) Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 43: 250-260. [Crossref]
- Sasaki Y, Marioni R, Kasai M, Ishii H, Yamaguchi S, et al. (2011) Chronic kidney disease is a risk factor for dementia onset: A population-based study. The Osaki-Tajiri Project. J Am Geriatr Soc 59: 1175-1181. [Crossref]