A 63-years old male was diagnosed for HIV (HIV-RNA undetectable and CD4 T cell count 191/mmc) and HCV active infection (HCV-RNA: 101027 cp/ml, genotype 1a) in May 2018. In September 2018, the patient presented a bacterial pneumonia with fever, severe leukopenia and thrombocytopenia. HIV-RNA persisted lower than 30 cp/ml and CD4 T cell count was 101/mmc. The severe leukopenia was associated with a general low growth capability of bone marrow derived hematopoietic progenitors and with a low frequency of multipotent lymphoid precursors, suggesting a possible impairment of leukocyte replenishment. T cells were few and dramatically skewed toward an effector profile, suggesting a strongly engaged immune system. Finally, a very high frequency of HIV-specific T cells (3.5 %) showing a polyfunctional profile was found: 70% of HIV specific T cells are able to simultaneously mediate 4 different functions (IFN-γ, TNF-α MIP-1β, CD107a).
In conclusion, we presented a case of one HIV-HCV co-infected patient who, despite an effective immune response able to control HIV replication, showed a progressive disease. The high frequency of polyfunctional CD8 T cells together with a threadbare immune system and with an impaired hematopoiesis may explain the disease progression in the absence of HIV replication.
HIV controller, leukopenic HIV/HCV patient, T cell response, lymphoid progenitors
HIV infection presents different clinical profiles regarding disease progression and immunodeficiency clinical course. A small group of patients (HIV Controllers) is able to control spontaneously HIV replication without experiencing clinical symptoms [1] and maintaining a normal CD4 T cell count [2]. In this study, we describe a clinical case of natural HIV control in one patient presenting HCV co-infection and a severe leukopenia. We aimed to define the hematopoietic progenitor cells features and viro-immunological properties in order to identify his protective immune profile.
HIV-RNA and HIV-DNA quantification: This work has been carried out in accordance with the Declaration of Helsinki and a written informed consent for publication has been obtained. Plasma HIV-1 RNA was assessed by using the Aptima HIV-1 Quant Dx (quantification range of 30–10,000,000 copies/ml). HIV-DNA quantification in PBMC and bone marrow cells was analyzed as previously described [3].
Hematopoietic progenitor phenotype and functional assay for colony forming cells: Bone marrow mononuclear cells (BM-MNC) were isolated from BM aspirate and analyzed by flow cytometry by quantifying: HPC: CD34+Lin-; early lymphoid progenitors (CD38-CD45RA+CD10+ among HPC). Clonogenic and differentiation potential of BM-MNC were tested by Colony-Forming Cell (CFC) Assays (MethoCult, Stem cell Technologies) after 15 days of culture.
T cell phenotype and function: T cell phenotype was investigated with eight-colour flow-cytometry assay (BD LyoTube 8-color, BD Biosciences). T cell functionality was assessed by ELISpot assay (AID GmbH, Strabberg) and polychromatic flow cytometry (BD Biosciences).
The patient is a caucasian 63-years old male with a medical history of chronic obstructive pulmonary disease and chronic alcohol-related hepatopathy. In May 2018, he was diagnosed with HIV and he was treated with cotrimoxazole for suspected pulmonary pneumocystosis and antibiotic therapy for interstitial pneumonia. At the time of HIV diagnosis, CD4 T cell count was 191/mmc (34%) and HIV-RNA was undetectable. The patient refused antiretroviral treatment. Concurrently, he was diagnosed with HCV infection: liver stiffness was 36.4 KPa at hepatic elastography, HCV RNA was 101027 cp/ml and HCV genotype was 1a.
In September 2018, the patient was admitted to hospital because of fever, leukopenia, asthenia and dyspnea. CT scan documented apical pulmonary nodular lesions and pulmonary emphysema. Tuberculosis was ruled out performing molecular, direct and cultural assays for Mycobacteria in sputum. No mycobacterial and leishmania infections and no malignancies were found in bone marrow specimens. The patient received antibiotics for bacterial pneumonia with clinical improvement. Blood test showed persisting leukopenia (Leukocytes: 990/mmc, Neutrophils: 500/mmc, Lymphocytes: 320/mmc) and thrombocytopenia (Platelets: 33000/mmc). HIV-1 RNA persisted lower than 30 cp/ml, and HIV-DNA was 6061 HIV-1 DNA copies/106 in PBMC (with a wild type HIV genotype) and was not detected in bone marrow cells (BM). Phenotypic analysis of T cells showed a normal frequency of CD4 (43%), CD8 (35%) and CD4/CD8 T cell ratio (1.2), suggesting that the low CD4 T cell count observed in the patient was due to lymmphopoenia rather than to HIV-induced CD4 loss.
In HIV infection, leukopenia may be associated with impaired hematopoiesis. The analysis of BM mononuclear cells (BM-MNCs) showed a very low frequency of early progenitors (17% of CD34+Lin-) and a quite absence of multipotent lymphoid progenitors (defined as CD34+Lin-CD38-CD10+CD45RA+, Figure 1A). Moreover, clonogenic potential of BM-MNCs, measured after 15 days of culture, was strongly impaired (112 colonies in the patient vs. 781 of healthy controls) and frequency of immature progenitors (GEMM) was higher respect to healthy controls (14% vs 1%). The absence of HIV-DNA in BM cells suggested that other mechanisms than HIV infection/replication may be involved in the hematopoietic differentiation impairment observed in the patient.
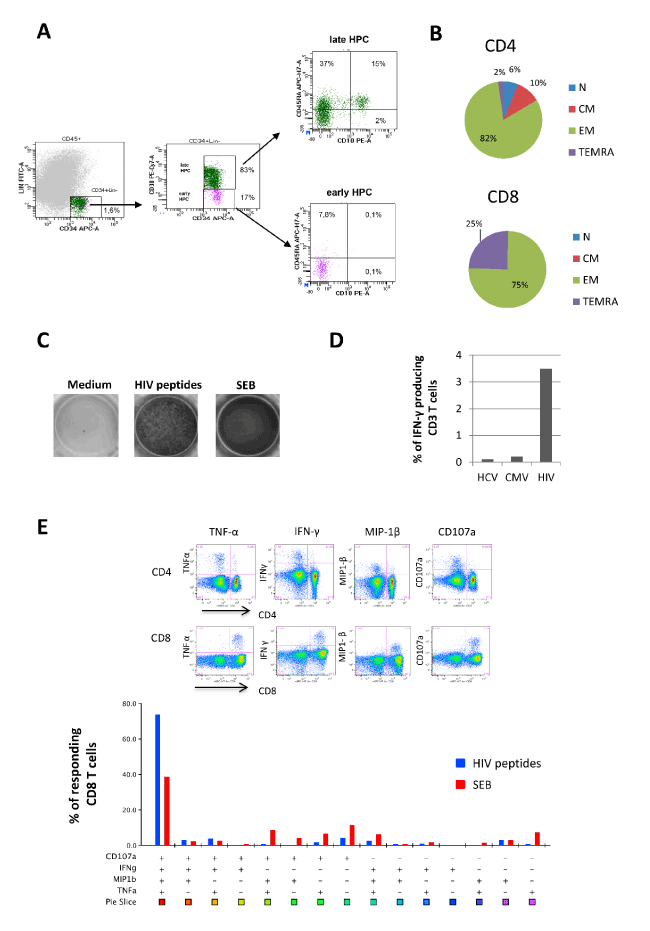
Figure 1. Hematopoietic and immunological features (A). The frequency of hematopoietic progenitors cells (HPC, CD34+Lin- gated on CD45low cells), early (CD38-) and late (CD38+) HPC and lymphoid HPC (gated on early HPC, CD45RA+CD10+) was performed by flow cytometry. (B) The frequency of Naïve (N: CD45RA+CD27+), Central Memory (CM: CD45RA-CD27+), Effector Memory (EM: CD45RA-CD27-) and Terminally Differentiated (TEMRA: CD45RA+CD27-) CD8 and CD4 T cells was performed by flow cytometry. (C) The HIV specific T cells response was performed by Elispot assay after 16 hours of stimulation with a pool of gag, nef and tat-derived peptides (1 ug/mL for each peptide) in the presence of 2 μg/ml αCD28 and αCD49d. Staphylococcal Enterotoxin B (SEB, 200 ng/mL) was used as positive control. (D) HCV-, CMV- and HIV-specific T cell responses was performed by intracellular staining and flow cytometry after 16 hours of stimulation with HCV, CMV and HIV derived peptides (1 ug/mL for each peptide) in the presence of of 2 μg/ml αCD28 and αCD49d and Brefeldin A. (E) Poly-functional profile of HIV-specific T cells was analyzed by polychromatic flow cytometry. Cytometric panels show the ability of CD4 and CD8 T cells to produce TNF-α, IFN-γ, CCL-4 and CD107a in response to HIV peptides. The bottom graph shows CD8 unique subsets response against HIV peptides (blue bars) and SEB antigen (red bars). Analysis was performed by using FlowJo 9.3.2 software (TreeStar, Olten, Switzerland) that, through the Boolean gate platform, allowed for creating the full array of possible combination response patterns for 4 functions, equating to 15 (24-1) excluding the fully negative subset. IL-2 was not considered in the polyfunctional response because it was not produced. The colored graph shows the percentage of multiple CD8 cell subpopulations, among CD8 responding cells, expressing the particular combinations of functions. Each + denotes CD107a, IFN-γ, CCL-4 and/or TNF-α positivity. Pie chart representing response across each stimulus. Each sector of the pie chart represents the unique subset expressing the particular combination of functions matched to the colored squares show below the bars.
Unexpectedly, this patient can control HIV replication, despite severe leukopenia and concurrent active HCV infection. The immunological profiling revealed a deep skewing of T cell differentiation toward effector cells (Figure 1B): more than 80% of CD4 T cells and 100% of CD8 T cells expressed an effector phenotype, suggesting a strongly engaged immune system in fighting infections. Moreover, a significant clonal expansion of HIV-specific T cells was observed, as a very high frequency of HIV-specific T cells (3.5%) was observed when compared to other antigen specific response (against CMV or HCV) that were lower than 0.5% as generally reported [4,5] (Figure 1C). Notably, HIV-specific T cells expressed CD8 profile and presented a polyfunctional signature (Figure 1D and 1E): more than 70% of HIV-specific CD8 T cells produced simultaneously several cytokines (TNF-α and IFN-γ), chemokine (MIP-1β) and were prone to degranulate (CD107a).
In this paper, we describe a patient who, despite his ability to control HIV replication, showed an advanced HIV/HCV co-infection, a pulmonary disease and a severe leukopenia. These observations highlight that the absence of HIV replication, a common feature of elite controller patients, may coexist with an advanced disease, suggesting that other factors than viral replication may drive disease progression.
His severe leukopenia was associated with a general low growth capability of hematopoietic progenitors and with a low frequency of multipotent lymphoid precursors, suggesting a possible impairment of hematopoiesis, still described in HIV infection [6-8]. Both direct and indirect mechanisms might contribute to the HIV-induced hematopoietic defects and lymphopoiesis exhaustion. The absence of HIV-DNA in BM cells suggested that other mechanisms than HIV infection/replication may be involved in the hematopoietic impairment observed in the patient. We can speculate that residual inflammation and immune activation associated to chronic HIV and HCV infections [8-10] could drive limited immune recovery regenerative capability, that may contribute to develop peripheral blood cell count abnormalities [11].
The analysis of the differentiation profile of T cells showed a strongly skewed profile toward effector cells, with a dramatic naïve T cell reduction. The mechanisms underlying the ability to control HIV replication are only partially understood. Genetic factors (such as CCR5-∆32 deletion), cytotoxic CD8+ T cell killing capacity, and several protective HLA alleles have been identified as possible contributors in defining controller patients [12,13]. Functional analysis showed a very strong CD8 T cell response against HIV (more than 3% of T cells respond to HIV epitopes) with a polyfunctional profile, suggesting the maintenance of a large pool of HIV specific effector cells. These data are in line with several studies in HIV natural controller patients, which clearly demonstrated a main role of polyfunctional CD8 T cells in the control of HIV replication [14,15]. Unfortunately, we did not perform HLA typing to evaluate the impact of HLA alleles in shaping the effective anti-HIV immune response.
In conclusion, we presented a case of one HIV-HCV co-infected patient who, despite an effective immune response able to control HIV replication, showed a progressive disease. The differentiation homeostatasis of both CD4 and CD8 T cells was completely subverted with an apparently loss of naïve T cells, an aged immune profile and a general decline of T cell renewal capacity. The high frequency of polyfunctional CD8 T cells together with a threadbare immune system and with an impaired hematopoiesis may explain the disease progression in the absence of HIV replication.
Not applicable
Written consent for publication was obtained from the patient
No conflict of interests to declare
This work was supported by grants from Ministero della Salute (Ricerca Corrente) to the National Institute for Infectious Diseases Lazzaro Spallanzani-IRCCS. The funding body was not involved in the design of the study and collection, analysis, and interpretation of data or in writing the manuscript.
- Migueles SA, Connors M (2010) Long-term nonprogressive disease among untreated HIV-infected individuals: clinical implications of understanding immune control of HIV. JAMA 304:194-201.
- Kamya P, Tsoukas CM, Boulet S, Routy JP, Thomas R, et al. (2011) T cell Activation does not drive CD4 decline in longitudinally followed HIV-infected Elite Controllers. AIDS Res Ther 8: 20.
- Viard JP, Burgard M, Hubert J.B, Aaron L, Rabian C, et al. (2004) Impact of 5 years of maximally successfulhighly active antiretroviral therapy on CD4 cell count and HIV-1 DNA level. AIDS 18: 45-49.
- Calarota SA, Chiesa A, Scaramuzzi L, Adzasehoun Kodjo M.G, Comollia G, et al. (2014) Normalizing ELISPOT responses to T-cell counts: A novel approach for quantification of HCMV-specific CD4+ and CD8+ T-cell responses in kidney transplant recipients. J of Clin Virol 61: 65-73.
- Rallon NI, Soriano V, Restrepo C, Garcia-Samaniego J, Labarga P, et al. (2011) HCV-Specific T-Cell Responses in HIV/Hepatitis C Virus-Coinfected Patients on Highly Active Antiretroviral Therapy Are Comparable to Those Observed in Hepatitis C Virus-Monoinfected Individuals. J Acquir Immune Defic Syndr 57: 1-8.
- Gaardbo JC, Hartling HJ, Gerstoft J, Nielsen SD (2012) Incomplete immune recovery in HIV infection: Mechanisms, relevance for clinical care, and possible solutions. Clin Dev Immunol 670957.
- Alexaki A, Wigdahl B (2008) HIV-1 infection of bone marrow hematopoietic progenitor cells and their role in trafficking and viral dissemination. PLOS Pathogens 4.
- Sauce D, Larsen M, Fastenackels S, Pauchard M, Ait-Mohand H, et al. (2011) HIV disease progression despite suppression of viral replication is associated with exhaustion of lymphopoiesis. Blood 117: 5142-51.
- Bordoni V, Casetti R, Viola D, Abbate I, Rozera G, et al. (2015) Early ART in primary HIV infection may also preserve lymphopoiesis capability in circulating haematopoietic progenitor cells: A case report. J Antimicrob Chemother 70: 1598-1600.
- Bordoni V, Bibas M, Viola D, Sacchi A, Cimini E, et al. (2017) Bone Marrow CD34+ Progenitor Cells from HIV-Infected Patients Show an Impaired T Cell Differentiation Potential Related to Proinflammatory Cytokines. AIDS Res Hum Retroviruses 33: 590-596.
- Pascutti MF, Erkelens MN, Nolte MA (2016) Impact of Viral Infections on Hematopoiesis: From Beneficial to Detrimental Effects on Bone Marrow Output. Front Immunol 7: 364.
- Fellay J, Ge D, Shianna KV, Colombo S, Ledergerber B, et al. Common genetic variation and the control of HIV-1 in humans. PLoS genetics 5(12).
- MacDonald KS, Fowke KR, Kimani J, Dunand VA, Nagelkerke NJ, et al. Influence of HLA supertypes on susceptibility and resistance to human immunodefciency virus type 1 infection. J. Infect. Dis 181: 1581-1589.
- Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, et al. (2008) Lytic granule loading of CD8+T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29:1009-1021.
- Ndhlovu ZM, Chibnik LB, Proudfoot J, Vine S, McMullen A, et al. (2013) High-dimensional immunomonitoring models of HIV-1-specific CD8 T-cell responses accurately identify subjects achieving spontaneous viral control. Blood 121: 801-811.

