New treatment options, such as proteasome inhibitors, have significantly increased survival for patients with multiple myeloma. However, proteasome inhibitors like Bortezomib and Carfilzomib have been associated with cardiotoxicity.
We report the case of a 65-year-old woman with MM who was referred for cardiac evaluation suffering from exertional dyspnea and a significant lack of physical stamina. She was on therapy with Lenalidomide combined with Dexamethasone after having received chemotherapy using BCD regimen and stem cell transplantation. VO2 max, NT-proBNP and global longitudinal strain were assessed and showed a mildly reduced systolic function after treatment with Bortezomib.
To conclude, peak oxygen consumption and global longitudinal strain are important parameters for non-invasive assessment for myocardial impairment related to cardiotoxicity which can easily be obtained in a clinical setting. Health care professionals should promote exercise testing for patients undergoing chemotherapy to identify early stages of myocardial impairment.
proteasome inhibitor, multiple myeloma, bortezomib, heart failure, heart disease, cardiotoxicity
New therapies have improved outcomes among patients with malignant diseases. However, as the number of cancer survivors rises, there has been a new focus on the potential cardiotoxic effects that can result from chemotherapy and oncology treatment. With cancer patients living longer, these cardiotoxic effects are becoming increasingly recognized and therefore early detection and treatment of cardiovascular adverse effects is highly important for the patient’s future outcome and well-being [1]. New treatment options with proteasome inhibitors (PI) have substantially increased survival for patients with multiple myeloma (MM). Bortezomib is a proteasome inhibitor, a dipeptide boronic acid, that inhibits the proteolytic activity of the proteasome chymotrypsin like site [2]. These therapies are generally well tolerated but may cause serious cardiovascular toxicity [3].
The aim of this case report is to discuss various options for early detection of cardiovascular toxicity in a patient treated with Bortezomib.
A 65-year-old woman who suffered from multiple myeloma was referred to our department of cardiology for cardiac evaluation of dyspnea at exertion and fatigue. The patient had been treated with 6 cycles of chemotherapy using a BCD regimen (subcutaneous Bortezomib, Cyclophosphamide and Dexamethasone) followed by Melphalan and stem cell transplantation. Thereafter, she had received another 3 cycles of Bortezomib.
At referral she was on treatment with Lenalidomide combined with Dexamethasone.
Transthoracic echocardiography was performed on a GE Vivid E9 System to assess myocardial impairment. Exercise capacity was tested using a cycle ergometer (VYNTUS CPX, Carl Rainer Austria). A ramp protocol, adapted to reach maximum exercise capacity after 8-10 minutes, was chosen to evaluate cardiac limitation during exercise. Ventilation and pulmonary gas exchange, as well as cardiac parameters were measured during cardiopulmonary exercise testing (CPET). Furthermore N-terminal pro-B-natriuretic peptide level was assessed.
Transthoracic echocardiography revealed slightly reduced left ventricular (LV) global longitudinal strain (GLS) and global circumferential strain by 2D speckle-tracking (GLPS ‑18,4% at admission; ‑16.6% at follow up 3 years later). Left ventricular ejection fraction was in normal range (62% at admission; 56% at follow up) with abnormal basal and mid- interventricular septal motion and diastolic impairment (Figure 1).
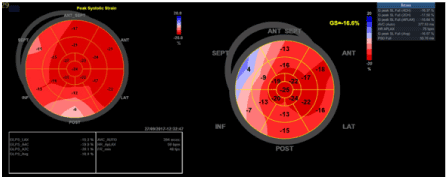
Figure 1. Bull´s eye representation (Strain) at admission and follow up
Cardiopulmonary exercise testing (CPET) showed a reduced aerobic exercise capacity with a VO2 max. of 16.6 ml/kg/min (=1162 ml/min which is 83% of predicted) at admission and VO2max. of 16.3 ml/kg/min (=1144 ml/min which is 85.3% of predicted) at follow up. Heart rate (HR) response to exercise was slightly below reference value (resting HR: 96 b/min, HR end of exercise: 130 b/min) at admission and (resting HR: 98 b/min, HR end of exercise: 146 b/min) follow up (Figures 2 and3).

Figure 2. HR (Heart rate), O2 puls, VO2 (oxygen consumption), VCO2 (carbon dioxide production), Watt – per minute; at admission

Figure 3. HR (Heart rate), O2 puls, VO2 (oxygen consumption), VCO2 (carbon dioxide production), Watt - per minute; at follow up
The patient remained stable during the entire follow up of three years and laboratory assessment showed constant NT-proBNP levels between 242 pg/mL and 224 pg/mL.
Cardiac dysfunction has been reported in 2% to 17.9% of patients taking Bortezomib [4] and cardiovascular complications as a result of the use of proteasome inhibitors (PI) as targeted chemotherapeutics have been described in several case reports [5].
This case is highlighting the importance of cardiac risk assessment after Bortezomib-based chemotherapy.
As cardiotoxicity may be due to the direct effects of antineoplastic treatments on the function of the heart, CPET is useful for early detection of cardiac limitations [6]. In this case CPET provides reliable risk stratification of unexplained dyspnea [7]. Developments in imaging, specifically myocardial deformation imaging (=strain), are sensitive tools in detecting early changes in cardiac function [8]. The assessment of global longitudinal strain (GLS) from speckle-tracking analysis of 2-dimensional echocardiography provides additional prognostic information to LVEF and has been proposed as the test of choice in guidelines for monitoring asymptomatic cardiotoxicity related to chemotherapy [9,10].
VO2 max, GLS and NT-proBNP have been shown to predict outcome in heart failure and have proven useful in this case of cardiotoxicity after allogenic bone marrow transplantation and chemotherapy. These 3 parameters can easily be determined in a clinical setting and appear useful for cardiac follow-up of cancer patients.
For non-invasive assessment of myocardial impairment due to cardiotoxicity global longitudinal strain and peak oxygen consumption are important parameters. Advanced echocardiography and CPET are sensitive tools to monitor cardiac function which are widely available and feasible in various clinical settings. This case report should encourage health care professionals to promote exercise testing for patients undergoing chemotherapy to identify development of early myocardial impairment.
The authors declare that there is no conflict of interest.
- Chang VY, Wang JJ (2018) Pharmacogenetics of chemotherapy-induced cardiotoxicity. Curr Oncol Rep 20: 52. [Crossref]
- https://www.ema.europa.eu/en/documents/overview/velcade-epar-medicine-overview_en.pdf
- Iannaccone A, Bruno G, Ravera A, Gay F, Salvini M, et al. (2018) Evaluation of cardiovascular toxicity associated with treatments containing proteasome inhibitors in multiple myeloma therapy. High Blood Press Cardiovasc Prev 25: 209–218. [Crossref]
- Yeh ETH, Bickford CL (2009) Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 53: 2231–2247. [Crossref]
- Cole DC, Frishman WH (2018) Cardiovascular complications of proteasome inhibitors used in multiple myeloma. Cardiol Rev 26: 122–129. [Crossref]
- Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, et al. (2016) 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 37: 2768–2801. [Crossref]
- Albouaini K, Egred M, Alahmar A, Wrighjt DJ (2007) Cardiopulmonary exercise testing and its application. Postgrad Med J 83: 675–682. [Crossref]
- Ribeiro ML, Jorge AJL, Nacif MS, Martins W de A (2019) Early detection and monitoring of cancer chemotherapy-related left ventricular dysfunction by imaging methods. Arquivos Brasileiros de Cardiologia 112: 309–316. [Crossref]
- Potter E, Marwick TH (2018) Assessment of left ventricular function by echocardiography: The case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging 11: 260–274. [Crossref]
- Triposkiadis F, Butler J, Abboud FM, Armstrong PW, Adamopoulos S, et al. (2019) The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J 40: 2155–2163. [Crossref]

