Thirty-two rabbit embryos at embryonic days (E) 10-29 as well as six rabbits aged 1 & 2 weeks were used to study the developmental changes of the thymus grossly and by light microscope. The rabbit thymus develops from the third pharyngeal pouch very late at E 10 therefore, the characteristic histogenetic changes in it occur relatively late in development where the demarcation of the cortex and the medulla begins at E 26 as well as Hassall's corpuscles appear at the end of gestation (E 29). The lymphocytes invade the thymus from the surrounding mesenchyme to form the bulk of the cortex. The thymus is bilobed. The lobe is surrounded by thin capsule and subdivided into separate lobules. Each lobule has its own cortex and medulla therefore, the interlobular blood vessels enter the lobule from all its aspects to continue its course as intralobular blood vessels. The rabbit thymus consists of two parts; large thoracic and small cervical which are continuous with each other without isthmus. In conclusion, the significant developmental changes of rabbit thymus are similar to those in other species but they keep a delayed time-course.
rabbit, thymus, development, embryos, morphogenesis
The thymus is the first of the lymphoid organs to be formed. It is unique among the lymphoid organs in being an epithelial organ in which the epithelial cells provide a framework containing predominantly T lymphocytes as well as smaller numbers of other lymphoid cells [1]. Because it is predominantly lymphoid in structure and hormone production is questionable, it is described under the lymphoid rather than the endocrine system [2]. The thymus is a central site of formation of T-lymphocytes. It is recognised as the pace-maker of lymphopoiesis as if it failed to develop prenatally, then the immune system cannot be established [3].
The origin of the lymphocytes present in the embryonic thymus is questionable. In rabbit, they are probably derived from the invasion of extrathymic lymphocytic cells into the organ parenchyma [4]. However, in the mouse as well as in chick and hamster, the first lymphocytes appearing in the thymus arise in situ by the direct transformation of undifferentiated epithelial cells [5-8]. Therefore, we aim in this article to study the primordium of the rabbit thymus, its migration and structure as well as the origin of the thymic lymphocytes in order to understand how the thymus develops to form a microenvironment that supports T cell maturation.
Thirty-two normal New Zealand white rabbit embryos at embryonic days (E) 10-29 in addition to six rabbits 1 and 2 weeks old were collected from the Research Farm of Faculty of Agriculture, Assiut University, Egypt (Table 1). Ethical approval was obtained from Animal Care and Use Committee of Assiut University. The pregnant rabbits were sacrificed at various periods of gestation and the embryos were removed shortly after evisceration. The entire embryos were fixed and serially sectioned from the 10th to 20th day while in the older embryos from the 24th to 29th day and in the 1 & 2 weeks old rabbits, the cervical-thoracic region was dissected for complete exposure of the thymus gland to examine its position and relations then the gland was removed to determine its weight, length and width as well as it was examined histologically. The specimens were fixed in 10% neutral buffered formalin or Bouin’s fluid. Both sagittal and transverse paraffin serial 3-5 µm sections were prepared and stained with Harris haematoxylin and eosin [9]. The obtained histological preparations were examined by light microscope. For estimating the length and width of the thymus, digital caliper was employed.
Table 1. Age and number of used animals
Age |
(Day) prenatally |
(Week) postnatally |
10 |
12 |
14 |
15 |
16 |
18 |
20 |
24 |
26 |
29 |
1 |
2 |
Number |
4 |
4 |
3 |
4 |
2 |
4 |
2 |
3 |
3 |
3 |
3 |
3 |
At E 10, the primordium of the thymus could be detected as a bilateral endodermal thickening in the wall of the third pharyngeal pouch. It projects laterally to be surrounded by the mesenchyme between the third and the fourth pharyngeal arches. In sagittal section, it is triangular in outline with its base is medially directed. The cells are numerous and small in size with darkly stained nuclei (Figures 1 and 2).
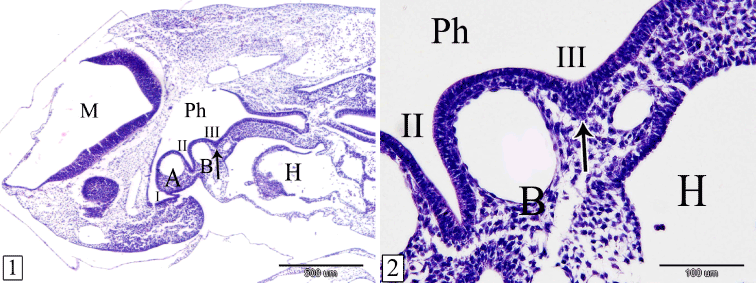
Figures 1&2. Sagittal sections in a rabbit embryo at E 10 showing the primordium of the thymus (arrow). Pharynx (Ph), 1st, 2nd&3rd pharyngeal pouches (I, II, III), 2nd pharyngeal arch (A), 3rd pharyngeal arch (B), mylencephalon (M) and heart (H). (H&E, 1, X 40 & 2, X 200)
At E 12, the thymus primordium becomes enlarged, extended laterally, and remains connected to the third pharyngeal pouch by a stalk. The stalk is located between the small third and the large fourth aortic arches while the gland is located cranial to the fourth aortic arch. It is composed wholly of epithelial cells. Lymphocytes could not be observed within the gland while the surrounding mesenchyme contains some lymphocytes (Figures 3 and 4).
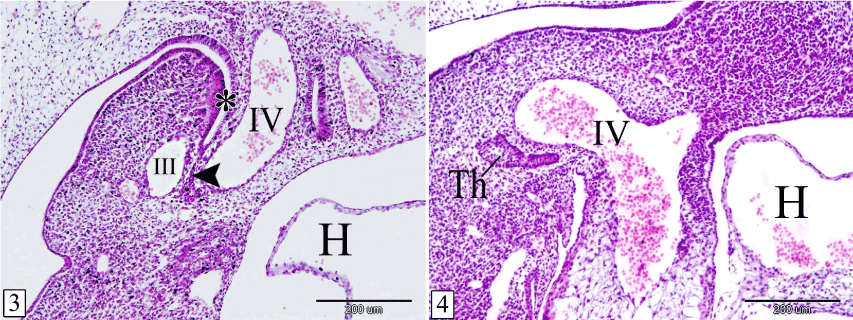
Figures 3&4. Sagittal sections in a rabbit embryo at E 12 showing the thymus remains connected with the pharynx by a stalk. 3rd pharyngeal pouch (*), stalk (arrow head), thymus (Th), 3rd aortic arch (III), 4th aortic arch (IV) and heart (H). (Figure 3) is a more medial level than (Figure 4). (H&E, X 100)
At E 14, the thymus is detached from the pharynx. It lies ventral to the level of the larynx. The gland acquires the lobulated appearance. The lobules are not separated from each other (Figure 5). The lobe of each side converges towards each other ventrally, medially and caudally. Therefore, the long axis of the thymus is obliquely located. The lateral end is bifurcated by the common carotid artery and the surrounding mesenchyme (Figure 6). Lymphocytes are rarely observed within the epithelial parenchyma and different stages of mitotic division could be detected (Figure 7).
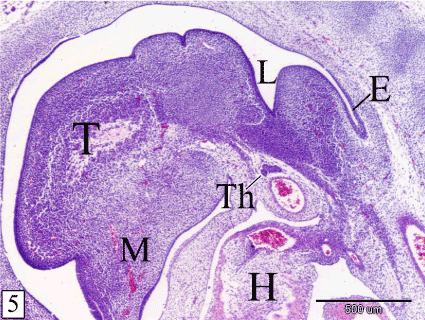
Figure 5. Sagittal section in a rabbit embryo at E 14 showing the thymus (Th) is detached from the pharynx and located ventral to the larynx (L). Notice, the lobulated appearance of the thymus. Tongue (T), mandible (M), esophagus (E) and heart (H). (H&E, X 40)
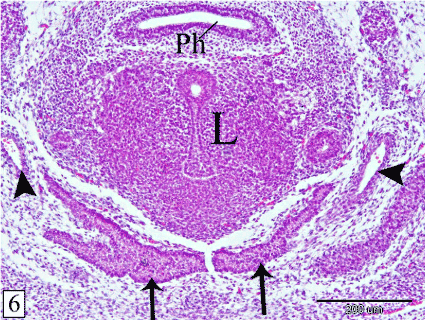
Figure 6. Transverse section in a rabbit embryo at E 14 showing the right and left lobes of the thymus (arrow) converge towards each other ventromedially. Pharynx (Ph), larynx (L) and common carotid artery (arrow head). (H&E, X 100)

Figure 7. Sagittal section in a rabbit embryo at E 14 showing sporadic lymphocytes (arrow head) within the epithelial parenchyma of the thymus (Th) and in the surrounding mesenchyme (M). Notice the different stages of mitotic division (arrow). (H&E, X 400)
At E 15, the thymus increases in length and extension where, it gains the level of the 6th cervical vertebra. The lobules are separated by wide interlobular spaces occupied by the surrounding mesenchyme which contains some lymphocytes (Figure 8). The bulk of the thymus is composed of epithelial cells and sporadic lymphocytes. The epithelial cells are large with rounded or oval nuclei. The lymphocytes are small with rounded darkly stained nuclei and scanty cytoplasm. It is located peripherally, close to the basement membrane (Figure 9).
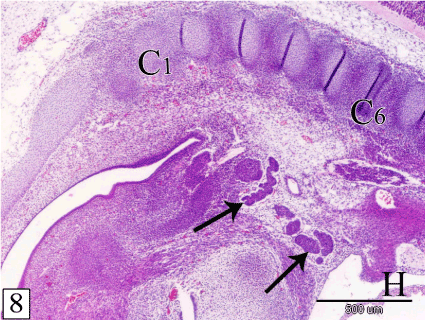
Figure 8. Sagittal section in a rabbit embryo at E 15 showing the thymus increases in length and extension to gain the level of the 6th cervical vertebra (C6). Notice, the lobules (arrow) are separated from each other by wide interlobular spaces. 1st cervical vertebra (C1) and heart (H). (H&E, X 40)
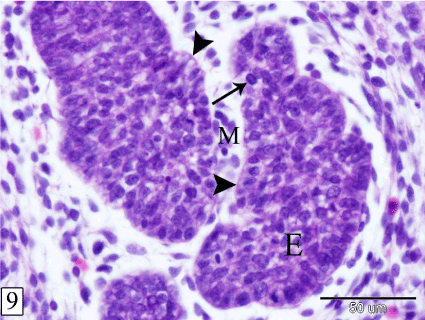
Figure 9. Sagittal section in a rabbit embryo at E 15 showing the thymus consists of epithelial mass (E) with sporadic lymphocytes (arrow). Basement membrane (arrow head) and interlobular mesenchyme (M). (H&E, X 400)
At E 16, the thymus reaches the thoracic inlet where, it is situated in the mesenchyme on either side of the pericardium. The cranial end of each lobe is related cranially to the parathyroid gland, dorsally to the common carotid artery and separated medially from the trachea by the thyroid gland. Along its course within the neck, it is located ventral to the common carotid artery and medial to the vagosympathetic nerve trunk. At the thoracic inlet, the right and left lobes of the thymus are separated by the pericardium and related dorsally to the pulmonary trunk. The caudal end of the left lobe extends slightly more caudal than that of the right one. The gland is narrow cranially and increases in size caudalwards (Figures 10-12).
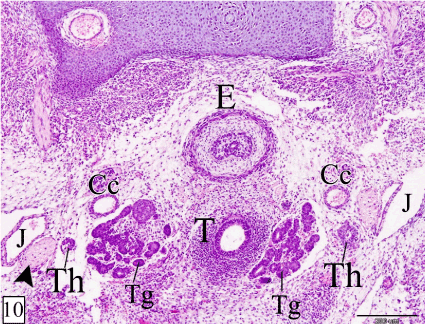
Figures 10-12. Transverse sections in a rabbit embryo at E 16 showing the relation of the thymus to the surrounding structures at its cranial end (Figure 10), along the neck (Figure 11) and at the thoracic inlet (Figure 12). Thymus (Th), vagosympathetic trunk (arrow head), common carotid artery (Cc), thyroid gland (Tg), trachea (T), esophagus (E), jugular vein (J), first rib (R1), pulmonary trunk (P) and pericardium (*). (H&E, X 100)
At E 18, the thymus is located in the cranial mediastinum, cranial to the heart, between the trachea and the sternum with a small narrow cranial part extends in the neck. In sagittal section, the thymus is elongated triangle in outline with its base is caudally directed. It takes the shape of bipinnate compound leaf. The residual cavity of the thymus could be observed in its cranial part (Figure 13). The right and left lobes of the thymus become in contact with each other medially and are separated only by thin layer of mesenchyme. The lobules and the interlobular spaces increase in size caudad (Figure 14).
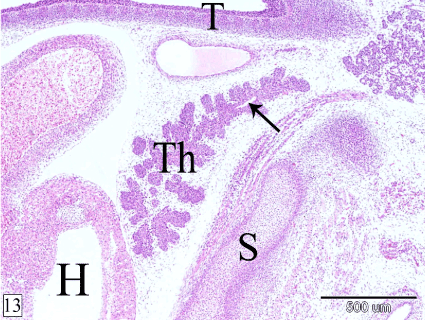
Figure 13. Sagittal section in a rabbit embryo at E 18 showing the thymus (Th) takes the shape of bipinnate compound leaf and is located in the cranial mediastinum. Heart (H), sternum (S), trachea (T) and residual cavity (arrow). (H&E, X 40)
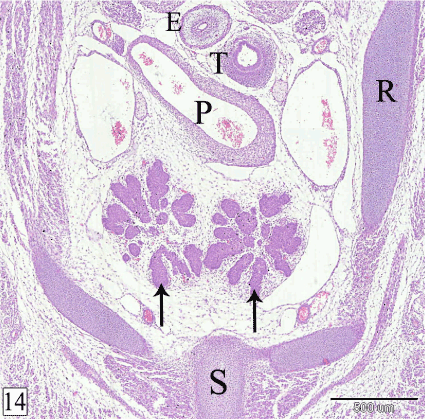
Figure 14. Transverse section in a rabbit embryo at E 18 showing the two lobes of the thymus (arrow) are in contact with each other medially. Esophagus (E), trachea (T), pulmonary trunk (P), rib (R) and sternum (S). (H&E, X 40)
At E 20, the thymus is surrounded by thin capsule. The interlobular mesenchyme becomes highly vascular (Figure 15). The lymphocytes within the gland increase in number (Figure 16).
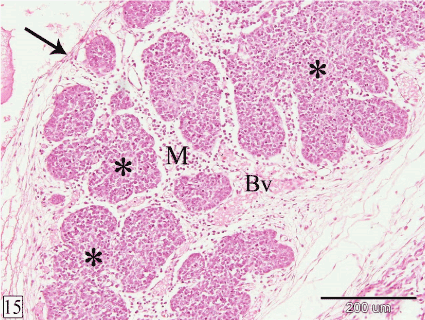
Figure 15. Sagittal section in a rabbit embryo at E 20 showing the thymus is surrounded by thin capsule (arrow) and the interlobular mesenchyme (M) is highly vascular. Lobules of the thymus (*) and blood vessels (Bv). (H&E, X 100)
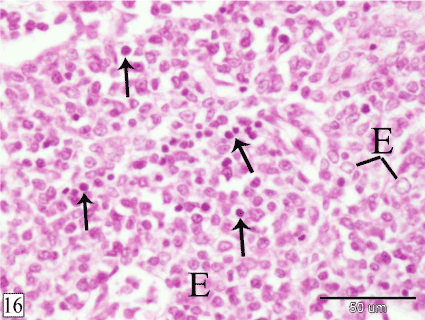
Figure 16. Sagittal section in a rabbit embryo at E 20 showing the lymphocytes (arrow) increase in number within the thymus. Epithelial cells (E). (H&E, X 400)
At E 24, the lobules of the thymus increase in size on the expense of the interlobular spaces. The interlobular blood vessels become larger and some blood vessels could be observed within the lobules (Figure 17).
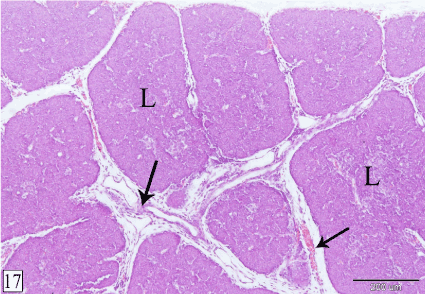
Figure 17. Cross section in a rabbit thymus at E 24 showing the thymus becomes dense as the lobules (L) become larger. Notice, the interlobular blood vessels (arrow) become large and some of them extend within the lobules. (H&E, X 100)
At E 26, the demarcation between the cortex and the medulla could be observed in some lobules. The cortex is darkly stained, contains high population of small lymphocytes and few epithelial cells. Macrophages could be rarely observed surrounded by lymphocytes. The medulla is paler staining consisting mainly of epithelial cells with small number of lymphocytes. The interlobular blood vessels enter the lobule from all its aspects to give off intralobular branches which gain the corticomedullary junction where they terminate as corticomedullary blood vessels that give off capillaries to the cortex and the medulla (Figures 18 and 19).
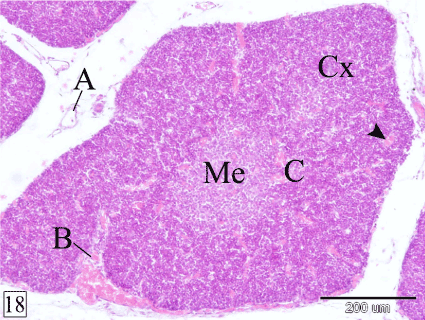
Figure 18. Cross section in a rabbit thymus at E 26 showing the demarcation between the cortex (Cx) and the medulla (Me). Interlobular (A), intralobular (B), corticomedullary (C) blood vessels and capillaries (arrow head). (H&E, X 100)
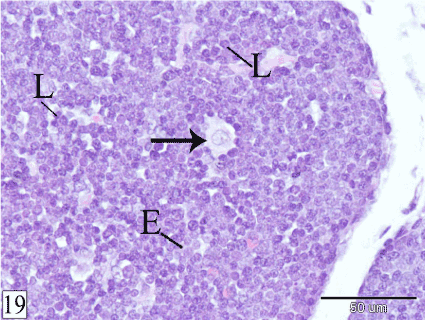
Figure 19. Cross section in a rabbit thymus at E 26 showing macrophage (arrow) within the cortex. Lymphocytes (L) and epithelial cells (E). (H&E, X 400)
At E 29, the demarcation between the cortex and the medulla becomes easily distinct in all lobules. At this age, Hassall's corpuscles could be observed within the medulla. They are few in number, small in size and show different stages of their formation. Some Hassall's corpuscles are represented by collection of swollen epithelial cells, other corpuscles consist of few layers of concentrically arranged epithelial cells with centrally located keratin substance (Figure 20).
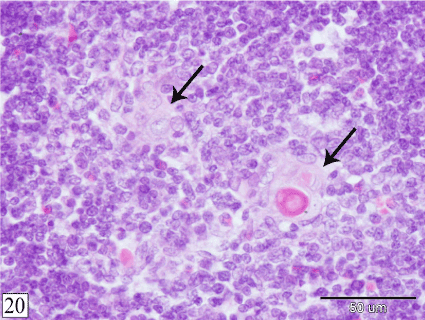
Figure 20. Cross section in a rabbit thymus at E 29 showing Hassal's corpuscles (arrow) within the medulla at different stages of their formation. (H&E, X 400)
At 1 and 2 weeks postnatally, the Hassall's corpuscles increase in size and number. Inwardly to the concentric layers, dead epithelial cells with pyknotic nuclei could be observed followed by centrally located keratin substance. Two Hassall's corpuscles are fused in a single and larger corpuscle could be seen (Figures 21 and 22).
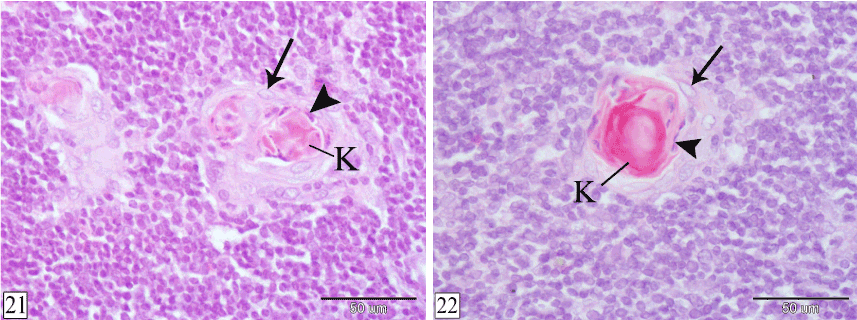
Figures 21&22. Cross sections in rabbit thymus at 1 week (Figure 21) and 2 weeks (Figure 22) postnatal showing the structure of the Hassal's corpuscles. Concentric layers of epithelial cells (arrow), dead epithelial cells with pyknotic nuclei (arrow head) and keratin substance (K). (H&E, X 400)
Grossly, the shape of the left lobe of the thymus is quadrilateral in outline with extended narrow craniomedial angle in the neck while the right lobe is triangular in outline with its base cranially directed and extended narrow craniomedial angle in the neck. The dorsal aspect of both lobes is concave showing the cardiac impression which is larger on the left lobe. In addition, the right lobe shows a pulmonary impression laterally. Medially, the left lobe slightly overlaps the right one and are connected only by small amount of interlobar connective tissue. The ventral aspect is convex and related to the sternum. The lobulation of the thymus could be observed grossly (Figures 23 and 24).
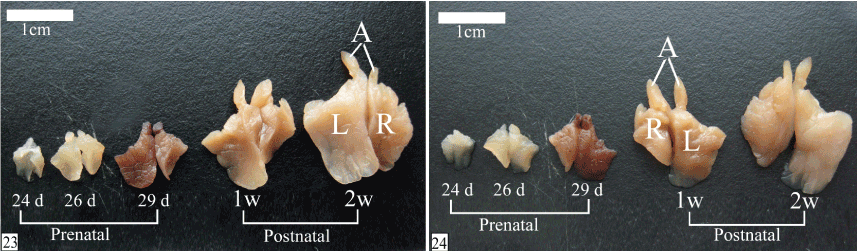
Figures 23&24. Photographs showing the shape of the thymus from E 24 to 2 weeks postnatally. Left lobe (L), right lobe (R) and craniomedial angle (A). Notice, the lobulation of the gland could be observed grossly. (23, dorsal view & 24, ventral view)
At E 24, the left lobe of the thymus extends from the level of the 5th cervical vertebra to the 2nd rib while the right lobe extends from the 5th cervical vertebra to the 1st intercostal space. The thymus gradually migrates caudally where at 2 weeks postnatally, the left lobe extends from the level of the 6th cervical vertebra to the 3rd intercostal space and the right lobe extends from the 7th cervical vertebra to the 2nd intercostal space (Figure 25).
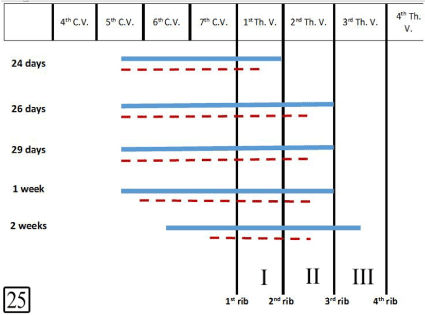
Figure 25. Diagram showing the position, extension and migration of the left and right lobes of the thymus at E 24, 26, 29 and 1 week, 2 weeks postnatal. Left lobe (continued line), right lobe (dotted line), cervical vertebra (C.V.), thoracic vertebra (Th.V.) and intercostal spaces (I-III)
Concerning the weight of the thymus, at E 24 it is about 25 mg. and increases gradually to reaches about 476 mg. at 2 weeks postnatally. The length of the left lobe increases from 6.83 mm. at E 24 to 22.73 mm. at 2 weeks postnatally while the right lobe increases in length from 6.07 mm. to 17.84 mm. respectively. The width of the thymus at E 24 is about 4.29 mm. and increases up to 14.5 mm. at 2 weeks postnatally (Table 2, Figures 26-28).
Table 2. Weight, Length and Width of the thymus and its lobes
|
Age |
Weight (mg) |
Length (mm) |
Width (mm) |
Thymus |
Left lobe |
Right lobe |
Thymus |
Left lobe |
Right lobe |
Thymus |
Left lobe |
Right lobe |
Prenatal |
24 day |
25 |
15 |
10 |
6.83 |
6.83 |
6.07 |
4.29 |
3.29 |
2.44 |
26 day |
61 |
32 |
29 |
9.62 |
9.62 |
8.95 |
4.89 |
4.29 |
3.83 |
29 day |
85 |
48 |
37 |
10.53 |
10.53 |
8.05 |
6.93 |
5.3 |
4.55 |
Postnatal |
1 week |
223 |
121 |
102 |
16.62 |
16.62 |
13.46 |
10.83 |
8.43 |
6.03 |
2 weeks |
476 |
260 |
216 |
22.73 |
22.73 |
17.84 |
14.5 |
12.11 |
8.22 |
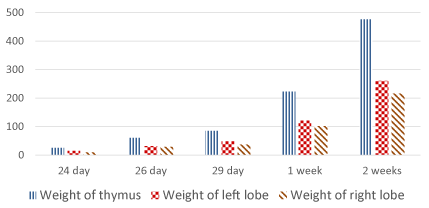
Figure 26. Weight of the thymus and its lobes (mg)
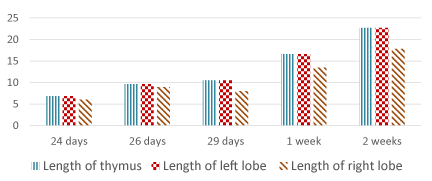
Figure 27. Length of the thymus and its lobes (mm)
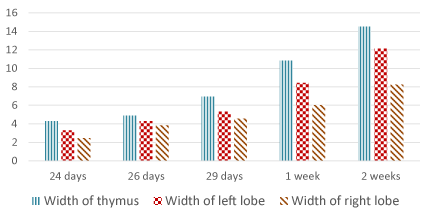
Figure 28. Width of the thymus and its lobes (mm)
The rabbit thymus develops bilaterally from the endoderm of the third pharyngeal pouch and the surrounding mesenchyme. These results are in accordance with Badertscher in pig, Ackerman and Knouff in hamster, Arey and Sadler in human and Schummer et al. in domestic mammals but other authors (Patten in pig; Venzke in domestic animals; Dijkstra and Sminia in rodents; Shimosato and Mukai; Lele et al. in human) stated that the thymus originates from the third and fourth pharyngeal pouches . The rabbit thymus develops very late, at E 10 (about the third of gestation period) as in dog where the epithelial anlage develops at day 23 of gestation . In hamster, the thymus primordium arises between the 9th and 10th day of embryonic development (about the half of gestation period) while, in human it develops so early in the 5th or 6th gestational week .
Concerning the migration of the thymus, at E 12, the thymus extends laterally and remains connected to the third pharyngeal pouch by a stalk. At E 14, it is detached from the pharynx and slightly converges with its fellow to be located ventral to the larynx. The thymus gains the level of the 6th cervical vertebra at E 15 and reaches the thoracic inlet at E 16. It reaches the cranial mediastinum and the two lobes become in contact with each other medially at E 18. At E 24, the thymus extends more caudally, under the cranial aspect of the heart where cardiac impression could be observed. The left lobe partially overlaps the right one and are connected only by small amount of interlobar connective tissue. In the same respect, in rodents the thymus migrates into the thorax around day 15 but in human, it occupies the cranial mediastinum at the 8th week [15,16,20]. Consequently, migration is an essential part of thymus embryonic development (at least in mammals), since the processes of pharyngeal detachment, organ separation and migration do not occur in birds and fish, as the thymus remains attached to the pharynx in these animals and does not migrate [21].
Although the cellular and molecular mechanisms that control thymus migration are not well understood, a study made by Gordon et al. indicated that an epithelial-mesenchymal transition does not play a role in this process [22]. However, several lines of evidence support a role for neural crest-derived mesenchyme during thymus migration. NCCs are themselves a migratory population, and it is therefore possible to imagine that they ‘pull’ the epithelial thymus lobes along. In a study by Foster et al., NCC-specific deletion in mice resulted in failed thymus migration and the formation of ectopic thymi [23].
In the present work, the thymus originates from two germinal layers; endoderm and mesoderm similar to that observed by Dijkstra and Sminia in rodents while, in guinea pig the thymus originates from the three germinal layers as, the extremity of the cephalic lobule is of ectodermal origin [12,24]. Similarly, the pig has an independent additional superficial thymus which is derived solely from the ectoderm [3]. Correspondingly, according to Shimosato and Mukai as well as Lele et al., the human thymus contains elements derived from all three germinal layers [13,16].
In mammals, the thymus consists of three parts; thoracic, cervical and cranial. In some mammals, the thymus has only one part either the cervical part as in guinea pig or the thoracic part as in dog, man, horse and cat [3,24]. In rabbit, the thymus has two parts; thoracic and cervical as observed in the current investigation. Three parts of the thymus (thoracic, cervical and cranial) could be observed in ruminants, pig and camel. The parts of the thymus are either continued with each other as in the present work or connected by parenchymatus isthmus; craniocervical and cervicothoracic as in ruminants, pig and camel [3,25,26]. From the above mentioned discussion we can suggest that in ruminants, pig and camel, the thymus has the highest degree of extension and the least degree of migration. On the contrary, in the present work (rabbit), horse, dog, cat and man, the thymus shows the highest degree of migration.
The thoracic part of the thymus consists of either one lobe as in ruminants and camel or two lobes as in the present work, pig, horse, dog, cat and man [3,25]. These two lobes are either partially overlapped as observed in the present work, partially fused in dog, pig, horse and man as mentioned by Schummer et al. or connected by a connective tissue isthmus as reported by Terszowski et al. in mice [3,27]. In rat, the two lobes are fused into a single thymus gland which is surrounded completely by a continuous connective tissue capsule [28]. The lobes of the thoracic part are asymmetrically in size where the left lobe is larger than the right one as in the present work and horse but in dog, the right lobe is the larger [3]. According to the present study, the left lobe of the thymus extends more caudally than the right one and they have cardiac and pulmonary impressions. Similar results were mentioned by Evans in dog [2].
Regarding the structure of the thymus, the results of the present investigation show that at E 10, the thymus primordium consists of endodermal thickening surrounded by mesenchyme. At E 12, it is composed entirely of epithelial cells surrounded by mesenchyme containing few lymphocytes. At E 14 & E 15, the bulk of the gland is formed of epithelial cells containing sporadic lymphocytes. At E 20, the lymphocytes within the thymus increase in number while at E 24, they form the bulk of the gland. From the previous results we can suppose that the first lymphocytes appear in the rabbit thymus are derived from invasion of lymphocytes from the surrounding mesenchyme. Our work supports that of Badertscher in pig, Smith in mouse as well as Ackerman and Hostetler in rabbit who concluded that mesenchymal lymphocytes infiltrate the epithelial anlage of the thymus [4,11,29]. In addition, experimental studies by Moore and Owen using tissue recombination and grafting in chick and mouse demonstrated that the lymphocytes in the thymus are hematopoietic in origin and derived extrinsically. They concluded that, the epithelial component of the thymic rudiment, rather than producing lymphoid cells itself, secretes factors that attract lymphocytes to the thymus [30]. On the contrary, Auerbach in mouse as well as Ackerman and Knouff in chick and hamster believed that, the lymphocytes originate in situ by the direct transformation of undifferentiated epithelial cells comprising the thymus parenchyma [5-8]. Schummer et al. added that, mesenchymal cells of mesodermal origin migrate into the thymus stimulating the formation of thymocytes (lymphocytes of epithelial genesis) [3].
The rabbit thymus acquires the lobulated appearance at E 14. The lobules become separated from each other by wide interlobular spaces at E 15 where the bulk of the lobule is composed of epithelial tissue. At E 26, some lobules begin to differentiate into peripheral cortex and central medulla. The bulk of the cortex consists of lymphocytes while that of the medulla is epithelial cells. At E 29, each lobule has its own cortex and medulla. On the other hand, in rat and man, the thymus is partially subdivided into lobules separated by connective tissue septa and having an interconnected medulla. However, the mouse thymus does not have sublobulation [1].
According to our observations, demarcation between the cortex and the medulla in rabbit thymus begins at E 26 in some lobules and becomes distinct in all lobules at E 29. The cortex is darkly stained consisting mainly of small lymphocytes overshadow epithelial cells and sparse macrophages. The medulla is pale with predominant epithelial cells and few lymphocytes. In human, various authors have reported different timing for differentiation of the cortex and medulla being at the 9th (Ajita et al.), 13th (Varga et al.), 14th (Prabavathy) or 16th (Lakshmi et al.) week of gestation while in the rat it occurs at day 15 of fetal life (Vicente et al.) [19,28, 31-33].
The rabbit thymus is surrounded by thin capsule at E 20 where the interlobular mesenchyme becomes highly vascular. At E 24, the interlobular blood vessels become larger and some blood vessels appear within the lobules. At E 26, The interlobular blood vessels enter the lobule from all its aspects to give off intralobular branches which gain the corticomedullary junction where they terminate as corticomedullary blood vessels which give off capillaries to the cortex and the medulla while, Banks mentioned that, the thymic arteries follow the course of the interlobular connective tissue septa and enter the organ substance at the corticomedullary junction [34].
The Hassall's corpuscles are large, acidophilic, rounded bodies consisting of central degenerated hyaline mass surrounded by concentrically arranged epithelial-reticular cells. They are unique to thymus [35]. The Hassall's bodies are structurally organized from medullary reticulo-epithelial cells, which usually undergo hypertrophy prior to their inclusion in the outer cell layer of the corpuscles [36]. In the present work, Hassall's corpuscles could be observed very late at the end of gestation period (E 29) while in dog, according to Bodey et al. and Bodey and Kaiser, it is found at day 38 of gestation however, in human they appear as early as the 8th (Fawcett), 13th (Varga et al.), 14th (Prabavathy), 15th (Ajita et al.) or 18th (Lakshmi et al.) gestational week [18,19,31-33,36,37]. Hassall's corpuscles are rare in rodent species when compared with humans and primates. In the mouse they are very small and not readily visualized without immunostaining. In rats they can form whorls of flattened cells surrounding cell debris or concentrically arranged keratin. In mice these structures are less well defined and do not form keratin in their centers [1].
- Pearse G (2006) Normal structure, function and histology of the thymus. Toxicol Pathol 34: 504-514. [Crossref]
- Evans HE (1993) The Thymus. In Miller’s Anatomy of the dog. 3rd (edn). WB Saunders Company, Philadelphia, USA.
- Schummer A, Wilkens H, Vollmerhaus B, Habermehl KH (1981) Thymus. In The Circulatory System, the Skin, and the Cutaneous Organs of the Domestic Mammals. Verlag Paul Parey, Berlin, Germany.
- Ackerman GA, Hostetler JR (1969) Morphological Studies of the Embryonic Rabbit Thymus: The in situ Epithelial Versus the Extrathymic Derivation of the Initial Population of Lymphocytes in the Embryonic Thymus. Anat Rec 166: 27-46.
- Ackerman Ga, Knouff Ra (1964) Lymphocyte Formation in The Thymus of The Embryonic Chick. Anat Rec 149: 191-215. [Crossref]
- Ackerman Ga, Knouff Ra (1965) The Epithelial Origin of The Lymphocytes in The Thymus of The Embryonic Hamster. Anat Rec 152: 35-53. [Crossref]
- Auerbach R (1960) Morphogenetic interactions in the development of the mouse thymus gland. Dev Biol 2: 271-284. [Crossref]
- Auerbach R (1961) Experimental analysis of the origin of cell types in the development of the mouse thymus. Dev Biol 3: 336-354. [Crossref]
- Harris HF (1900) On the rapid conversion of haematoxylin into haematein in staining reactions. J Appl Microsc Lab Methods 3: 777-780.
- Arey LB (1965) Developmental anatomy. A text book and laboratory manual of embryology. 7th (edn). WB Saunders Co. Philadelphia. USA.
2021 Copyright OAT. All rights reserv
- Badertscher JA (1915) The development of the thymus in the pig. II. Histogenesis. Am J Anat 17: 437-493.
- Dijkstra C, Sminia T (1990) Normal Anatomy, Histology, Immunohistology, Ultrastructure, Rat. In: Hematopoietic System. Monographs on Pathology of Laboratory Animals (T. Jones, J. Ward, U. Mohr, and R. Hunt, eds.), Springer-Verlag, Berlin, Germany.
- Lele SM, Lele MS, Anderson VM (2001) The thymus in infancy and childhood: embryologic, anatomic and pathologic considerations. Chest Surg Clin N Am 11: 233–253. [Crossref]
- Patten BM (1964) Foundations of Embryology. 2nd (edn). Mc Graw-Hill Book Company. New York, USA.
- Sadler TW (2000) Langman's Medical Embryology. 6th (edn). Williams and Wilkins. Philadelphia, USA.
- Shimosato Y, Mukai K (1997) Tumors of the thymus and related lesions. In: Shimosato Y, Mukai K, eds. Atlas of tumor pathology: tumors of the mediastinum, fasc 21, ser 3. Washington, Armed Forces Institute of Pathology.
- Venzke WG (1975) Thymus. In Sisson and Grossman’s, The Anatomy of the Domestic Animals. 5th (edn). WB Saunders Company, Philadelphia, USA.
- Bodey B, Calvo W, Prummer O, Fliedner TM, Borysenko M (1987) Development and histogenesis of the thymus in dog. A light and electron microscopical study. Dev Comp Immunol 11: 227-238. [Crossref]
- Varga I, Pospisilova V, Jablonska-Mestanova V, Galfiova P, Polak S (2011) The thymus: picture review of human thymus prenatal development. Bratisl Lek Listy 112: 368-376. [Crossref]
- Suster S, Rosai J (1992) Thymus. In Histology for Pathogists (S. Sternberg, ed.), Raven Press, New York, USA.
- Grevellec A, Tucker AS (2010) The pharyngeal pouches and clefts: Development, evolution, structure and derivatives. Semin Cell Dev Biol 21: 325-332. [Crossref]
- Gordon J, Patel SR, Mishina Y, Manley NR (2010) Evidence for an early role for BMP4 signaling in thymus and parathyroid morphogenesis. Dev Biol 339: 141-154. [Crossref]
- Foster KE, Gordon J, Cardenas K, Veiga-Fernandes H, Makinen T, et al. (2010) EphB-ephrin-B2 interactions are required for thymus migration during organogenesis. Proc Natl Acad Sci USA 107: 13414-13419. [Crossref]
- Klapper CE (1946) The development of the pharynx of the guinea pig with special emphasis on the morphogenesis of the thymus. Am J Anat 78: 139-179.
- Abdelaziz S (1992) Prenatal development of thymus in camel (Camelus dromedarius). Zag Vet J 20: 762-777.
- Aly AE (1986) Some anatomical studies on the camel's thymus (Camelus dromedarius). Zag Vet J 13: 34-46.
- Terszowski G, Müller SM, Bleul CC, Blum C, Schirmbeck R, et al. (2006) Evidence for a functional second thymus in mice. Science 312: 284-287. [Crossref]
- Vicente A, Varas A, Sacedon R, Zapata AG (1996) Histogenesis of the epithelial component of rat thymus: An ultrastructural and immunohistological analysis. Anat Rec 244: 506-519. [Crossref]
- Smith C (1965) Studies On The Thymus Of The Mammal. Xiv. Histology And Histochemistry Of Embryonic And Early Postnatal Thymuses Of C57bl-6 And Akr Strain Mice. Am J Anat 116: 611-629. [Crossref]
- Moore MA, Owen JJ (1967) Experimental studies on the development of the thymus. J Exp Med 126: 715-726. [Crossref]
- Ajita RK, Singh TN, Singh YI, Singh LC (2006) An insight into the structure of the thymus in human foetus - a histological approach. J Anat Soc India 55: 45-49.
- Lakshmi KV, Rao BN, Padmini MP (2012) Histo-morphogenesis of thymus in human fetuses. International Journal of Basic and Applied Medical Sciences 2: 78-82.
- Prabavathy G (2014) Histogenesis of human fetal thymus in different gestational age groups. National Journal of Clinical Anatomy 3: 117-121.
- Banks WJ (1993) Applied veterinary histology 3rd (edn). Mosby Year Book, St. Louis, USA.
- Hassan AU, Rasool Z (2014) The Hassal of Thymus: Hassals Corpuscle Histological and Histopathological Perspective. Sch J App Med Sci 2: 147-148.
- Bodey B, Kaiser HE (1997) Development of Hassall's bodies of the thymus in humans and other vertebrates (especially mammals) under physiological and pathological conditions: Immunocytochemical, electron microscopic and in vitro observations. In Vivo 11: 61-85. [Crossref]
- Fawcett DW (1994) A Text Book of Histology. 12th (edn). Chapman and Hall, New York, USA.






















