To describe the feasibility of preserving the lesser occipital nerve (LON) and great auricular nerve (GAN) in post-auricular incision in ear surgery. Methods. After the distribution of the LON and GAN was identified from cadavers, the clinical study was performed in 34 patients, who were divided into conventional group and modified group according to incision type. All patients underwent sensory testing and subjective evaluation of auricular numbness after surgery at different times. Results. The auricular branches of the LON went into the post-auricular groove at the same height of inferior crus of antihelix most frequently. The vertical dimension from the intersection of the highest auricular branch of the GAN and post-auricular groove to intertragic notch ranged from 5.7 mm to 4.2 mm. Preservation of the LON and GAN reduced sensory loss in modified group. Conclusions. Preservation of the LON and GAN in the modified post-auricular incision can reduce postoperative auricular numbness.
lesser occipital nerve, great auricular nerve, post-auricular incision, sensory loss, ear surgery
Post-auricular incisions are commonly performed for a range of ear surgery, including tympanoplasty and mastoid surgery. In most of the cases, surgery is performed via post-auricular incision, and sensory loss in auricular area is developed after surgery. Although the sensory loss could relieve over time slowly, it is reported that 69% of patients undergoing primary surgery experience numbness postoperatively, 26% have continued numbness after at least eight months, and 3% are constantly aware of the sensory deficit and feel distressed [1].
Under such circumstances, reducing postoperative sensory loss in postauricular incision is significantly important to improve quality of patients’ life. However, it may be because the complication of post-auricular incision is too common and can be relieved over time. There is a lack of solution, the problem does not cause enough attention, and there is no related report.
Sensory loss after post-auricular incision is associated with auricular sensory nerves transection. The auricular sensory nerves include LON, GAN, auriculotemporal nerve, auricular branch of vagus nerve and post-auricular branch of facial nerve [2]. Among them, the last two nerves just have little impact on the sensation of auricle [3]. And the auriculotemporal nerve is in front of the auricle [4], so it would not get hurt in a post-auricular incision. Therefore, it is the most important to preserve LON and GAN to reduce postoperative sensory loss. Peled et al. [5] had studied anatomic knowledge of LON, but they regarded the occipital protuberance as anatomic landmark. There is a lack of positional relationship between LON and auricle. Although the distribution of GAN has been reported [6], the position of the branches of GAN going into auricle is still unknown because of many variations.
The objective of this study was to propose a strategy of protecting LON and GAN in ear surgery through an anatomic study and to confirm the feasibility and effect of the strategy through a prospective interventional nonrandomized controlled clinical study.
Anatomic study
A total of 15 sides of formalin-fixed or fresh necks from Chinese cadavers (all are unilateral specimens) were used in this study. Five fresh frozen cadavers head dissections (3 males and 2 females) were performed specifically looking for branches of LON. Ten cadavers head dissections (8 males and 2 females) were performed specifically looking for branches of GAN. To measure the distribution of LON and GAN, 15 heads were dissected. To clarify the position of LON and GAN, the relationship of the nerves with constant anatomic landmarks and structures was examined: inferior crus of antihelix and intertragic notch. For LON, the positional relationship between auricular branch of LON and inferior crus of antihelix was observed. For GAN, the vertical dimension (VD) from the intersection of the highest auricular branch (Nh) of GAN and post-auricular groove to intertragic notch was measured (Figure 1), which is defined as “-” if the intersection is above Line 2, and as “+” if below.
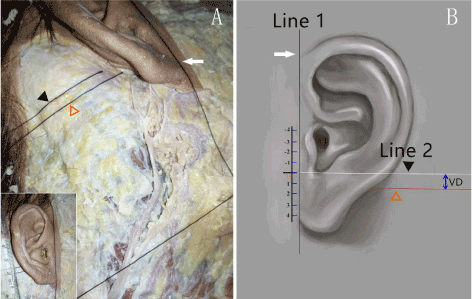
Figure 1. Connect the leading edge of helix and otobasion inferius to make a straight line, name it Line 1. Make a straight line through the intertragic notch which is perpendicular to Line 1, name it Line 2. Measure the VD from the intersection point of Nh and post-auricular groove to Line 2.
Clinical study
This study included 34 patients (14 males and 20 females) underwent tympanoplasty or mastoidectomy via post-auricular incision for chronic suppurative otitis media or for middle ear cholesteatoma between September 2016 and January 2017 at the Sun Yat-Sen memorial hospital, Sun Yat-Sen University. The mean age of the patients was 37.45 ± 11.45 years. Inclusion criteria: adults over 18 years of age, and with indications of middle ear surgery. Exclusion criteria included: history of head and neck surgery, history of trauma or psychosis, preoperative auricular abnormal sensation or congenital auricular abnormalities, infection after surgery, neurological diseases. These patients were divided into 2 groups: with a conventional post-auricular incision (conventional group) and with a modified post-auricular incision according to the preceding anatomic results (modified group).
The conventional post-auricular incision was made from the superior margin of auricular attachment to the mastoid tip. The distance between incision and post-auricular groove was about 2 mm. In modified group, the incision was made just like the conventional post-auricular incision, but the inferior margin did not extend beyond the intertragic notch. After removing the skin and superficial fascia, the auricular branch of LON was exposed clearly at the same height of inferior crus of antihelix. Then, a retractor was used to prevent the injury to the nerve during surgery (Figure 2).
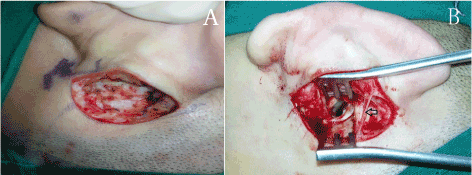
Figure 2. (A) In conventional group, the auricular branch of LON is cut off, (B) in modified group, the auricular branch of LON is exposed clearly at the same height of inferior crus of antihelix.
According to the distribution characteristics of LON and GAN, 9 areas were selected as the test areas (Figure 3). The sensation of each area was assessed before surgery and 1 day, 10 days, 1 month, 3 months after surgery, using the Semmes–Weinstein monofilaments (SWM) and the Pin Prick test (PP). The SWM used Baseline tactile monofilament evaluator. One set of the tool consists of 6 monofilaments. There were 6 levels: 0.07 g, 0.4 g, 2 g, 4 g, 10 g and 300 g, and were respectively recorded as: level 1, 2, 3, 4, 5, 6. Beginning from the lightest monofilament, the value of the monofilament that caused the patient to feel sensation for the first time was recorded as the minimal sensory threshold. The pain sensation was evaluated by PP test. With closing eyes, each area was stimulated in a random order with pointed instrument and blunt instrument, 4 times in each area. Patient was asked whether the feeling was sharp or blunt every time. It was regarded as normal pain when correct response times ≥ 3. In addition, the patient was asked to record the sensation that he felt on a scale of 0 point (no numbness) to 10 points (extreme numbness), using the visual analog scale (VAS).
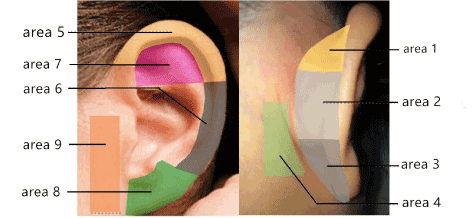
Figure 3. The auricle is divided into 9 zones. The superior medial surface of auricular area is designated as area 1, the middle medial surface of auricular area as area 2, the inferior medial surface of auricular area as area 3, the mastoid as area 4, the superior helix and scapha area as area 5, the inferior helix and scapha as area 6, the crus of antihelix as area 7, the anterior surface of earlobe as area 8, the preauricular area as area 9
Statistical analysis
All the data were collected prospectively. Statistical analyses were performed using SPSS software version 19.0. The VAS scores, results of SWM test were analyzed by Wilcoxon rank sum test, and results of PP test were analyzed by Fisher’s exact test. Statistical significance was accepted for the p < 0.05.
Anatomic study
Distribution of LON
In five specimens, LON emerged from the posterior border of sternocleidomastoid muscle (SCM), and was located behind external jugular vein and GAN in every case. After emergent, LON ascended beside the posterior border of SCM to mastoid and occipitalia (Figure 4). In four specimens, the auricular branches of LON were found, and went through the post-auricular area in superficial fascia to post-auricular groove at the same height of inferior crus of antihelix (Figure 5). The auricular branches of LON were not found in one specimen. The auricular branch had fibers from greater occipital nerve in one specimen.
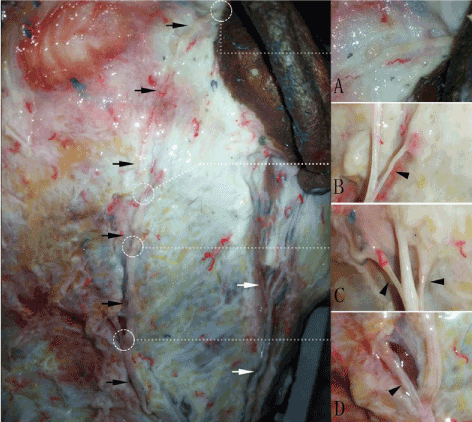
Figure 4. The auricular branches of LON. LON is shown with black arrow, GAN is shown with white arrow. (A) showed the position where the auricular branches of LON went int ear. (B-D) showed mastoid and occipital branches of LON
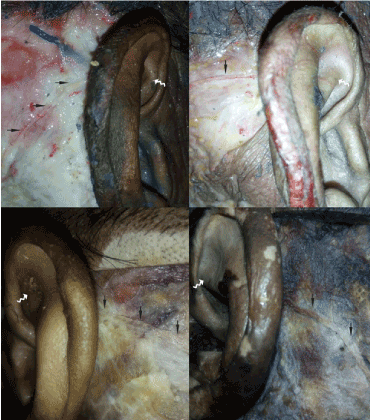
Figure 5. The auricular branches of LON go into auricle at the same height of inferior crus of antihelix. LON is shown with solid black arrow; the inferior crus of antihelix is shown with wavy white arrow
Distribution of GAN
In ten specimens, GAN ascended toward the parotid gland and mastoid from the posterior border of SCM. GAN was located behind external jugular vein in every case. In its course on SCM, GAN divided into 1-3 small branches to the area of parotid gland. The main trunk went on to the post-auricular area and divided into 2-6 branches, which distributed to the mastoid or auricular area (Figure 6). The distribution of GAN in the post-auricular area had a very great variation. Four specimens can find mastoid branches. When it came to the auricular branches, one specimen had two auricular branches, four specimens had three auricular branches, and five specimens had four auricular branches (Figure 7). The VD of the intersection of Nh and post-auricular groove ranged from 5.7–4.2 mm. Six specimens (60.0%) were below the Line 2, and four specimens (40.0%) were above.
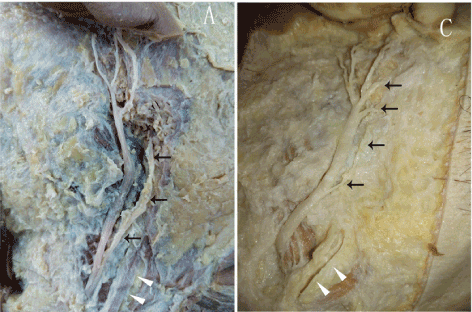
Figure 6. The distribution of post-auricular branches of GAN: parotid gland branches of GAN are shown with black arrow; external jugular vein is shown with white arrow
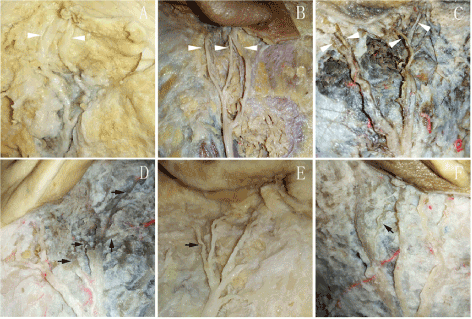
Figure 7. The distribution of post-auricular branches of GAN: (A) 2 auricular branches of GAN; (B) 3 auricular branches of GAN; (C) 4 auricular branches of GAN; (D-F) the mastoid branches of GAN are shown with solid black arrow
Clinical study
The mean subjective VAS scores of 34 patients was 0 before surgery. The results of VAS scores were divided into four degrees: no numbness (0), mild numbness (1-3), moderate numbness (4-6), severe numbness (7-10). The proportion of patients with no numbness and mild numbness in the modified group was higher than that in the conventional group (Table 1). There was a significant difference between VAS scores of modified group and conventional group (Wilcoxon rank sum test, all p < 0.05). That was to say, the subjective numbness of modified group was lighter than conventional group after surgery.
Table 1. The number of patients with four numbness degree in modified group and conventional group after surgery
VAS |
NO. of patients 1 day postoperatively |
NO. of patients 10 days postoperatively |
NO. of patients 1 month postoperatively |
NO. of patients 3 months postoperatively |
Convention group |
Modified group |
Conventional group |
Modified group |
Conventional group |
Modified group |
Conventional group |
Modified group |
|
0 |
0 |
3 |
0 |
4 |
0 |
7 |
1 |
11 |
|
3-Jan |
2 |
6 |
4 |
5 |
5 |
8 |
14 |
6 |
|
6-Apr |
13 |
8 |
11 |
8 |
11 |
2 |
2 |
0 |
|
10-Jul |
2 |
0 |
2 |
0 |
1 |
0 |
0 |
0 |
The SWM test results of 34 patients in the 9 areas before surgery were level 1. The loss of touch sensation occurred mainly in area 1 and 5 in modified group, and in area 1, 2 and 5 in conventional group after surgery. Wilcoxon rank sum test was used to compare the sensory threshold in area 1, 2 and 5 between conventional group and modified group (Table 2). The sensory loss degree of modified group was significantly lighter than conventional group in these three areas. The recovery in modified group was faster than conventional group.
Table 2. The results of SWM and Wilcoxon rank sum test
|
sensory threshold ≥ 2 |
p |
Area |
Period
after
surgery |
Modified group
(n = 17) |
Conventional group
(n = 17) |
Modified group VS
conventional group |
Modified group
Preoperatively VS postoperatively |
Conventional group
Preoperatively VS postoperatively |
Area 1 |
1 day |
7 (41.2%) |
17 (100%) |
0 |
0.041 |
0 |
10 days |
7(41.2%) |
17 (100%) |
0 |
0.041 |
0 |
1 month |
3 (17.6%) |
17 (100%) |
0 |
0.394 |
0 |
3 months |
0 (0.0%) |
9 (52.9%) |
0 |
* |
0.003 |
Area 2 |
1 day |
3 (17.6%) |
12 (70.6%) |
0.001 |
0.394 |
0 |
10 days |
2 (11.8%) |
13 (76.5%) |
0 |
0.056 |
0 |
1 month |
0 (0.0%) |
11 (64.7%) |
0 |
* |
0.001 |
3 months |
0(0.0%) |
4 (23.5%) |
0.094 |
* |
0.079 |
Area 5 |
1 day |
8 (47.1%) |
16 (94.1%) |
0.001 |
0.018 |
0 |
10 days |
8 (47.1%) |
16 (94.1%) |
0 |
0.008 |
0 |
1 month |
4 (23.5%) |
14 (82.4%) |
0 |
0.245 |
0.001 |
3 months |
0 (0.0%) |
7 (41.2%) |
0 |
* |
0.031 |
SWM: Semmes Weinstein monofilaments test; *: the postoperative data were the same as preoperative data.
The PP test results of 34 patients in the 9 areas before surgery were normal. After surgery, the loss of pain sensation occurred mainly in area 1 and 5 in modified group, and in area 1, 2 and 5 in conventional group. Fisher’s exact test was used to compare the pain sensation between conventional group and modified group (Table 3). The recovery of pain sensation in modified group was faster than conventional group, and the recovery of pain sensation is faster than touch sensation compared with SWM test results.
Table 3. Results of Pin Prick test and Fisher’s exact test.
Area |
Normal Pin Prick test |
p |
Period after surgery |
modified group
(n = 17) |
conventional
group
(n = 17) |
modified group VS
conventional group |
modified group
preoperatively VS postoperatively |
conventional group
preoperatively
VS
postoperatively |
Area 1 |
1 day |
11 (64.7%) |
1 (5.9%) |
0 |
0.009 |
0 |
10 days |
11 (64.7%) |
3 (17.6%) |
0.007 |
0.009 |
0 |
1 month |
13 (76.5%) |
4 (23.5%) |
0.003 |
0.051 |
0 |
3 months |
17 (100%) |
15 (88.2%) |
0.242 |
0.242 |
0.242 |
Area 2 |
1 day |
14 (82.4%) |
6 (35.3%) |
0.007 |
0.114 |
0 |
10 days |
15 (88.2%) |
9 (52.9%) |
0.029 |
0.242 |
0.001 |
1 month |
17 (100%) |
14 (82.4%) |
0.114 |
* |
0.114 |
3 months |
17 (100%) |
17 (100%) |
* |
* |
* |
Area 5 |
1 day |
13 (76.5%) |
2 (11.8%) |
0 |
0.051 |
0 |
10 days |
12 (70.6%) |
2 (11.8%) |
0.001 |
0.022 |
0 |
1 month |
15 (88.2%) |
6 (35.3%) |
0.002 |
0.242 |
0 |
3 months |
17 (100%) |
15 (88.2%) |
0.242 |
0.242 |
0.242 |
*: two sets of data were the same
Sensory loss in the auricular area after surgery performed via post-auricular incisions is very common, which is mainly because of transection of auricular branches of the LON and GAN. So, if we hope to reduce postoperative auricular numbness, it is most important to protect the LON and GAN in surgery. LON innervates occipitalia, mastoid and auricle. In present study, the topographic of the LON is the same as the previous studies [5]. We pay attention to the auricular branch of LON, especially its position relationship with inferior crus of antihelix in the postauricular groove. Because a postauricular incision is made 4 mm posterior to the post-auricular groove in a curvilinear line, the auricular branch of LON is very likely to be injured. Previous studies have shown that, the auricular branch of LON occurred frequently [6-8]. The auricular branch may be having the fibers from the greater occipital nerve [7]. In our present study, the dissection of specimens had verified it. Fujimaki et al. [9] had developed a surgical procedure for preserving the LON during suboccipital craniotomy with microvascular decompression for hemifacial spasm, and occipital and post-auricular numbness was alleviated significantly. Therefore, if we want to protect the LON during the post-auricular incision, we can try to identify and preserve the LON at the same height of inferior crus of antihelix.
The GAN originated from the second and third cervical nerves. There is lots of reports about the innervation of the GAN. It ascends from the posterior border of SCM muscle midpoint, the position can be slightly higher or lower, winds around the SCM muscle surface, and extends along the SCM muscle surface to the auricular lobule, divides into several branches to parotid gland, inferior part of masticatory muscle, the auricular and mastoid skin [7,10]. Murphy et al. [11] observed that GAN was behind the external jugular vein on the surface of the SCM muscle and accompanied with the external jugular vein. The dissection of 10 specimens from the present study was consistent with the results of previous studies. In order to avoid auricular numbness after surgery, lots of related studies have been done [12-14]. However, these studies focus mainly on plastic surgery and related operations of parotid gland, and there is a lack of attention to post-auricular incision. Previous study has shown that GAN divided into several branches, to control corresponding parts of mastoid and auricle [6]. But the position of GAN going into the auricle in post-auricular groove, there is no reported. The conventional post-auricular incision usually goes down to the mastoid tip, with a great variation of GAN in post-auricular area [6], it is difficult to preserve the branches of GAN. We attempted a unique way in our study, through analyzing the relationship between the position of the highest auricular branch of GAN going into auricle in post-auricular groove and intertragic notch, reform the inferior margin of post-auricular incision to preserve GAN. According to the results of dissection, the modified post-auricular incision was made by preserving LON at the height of inferior crus of antihelix and being higher than intertragic notch. In addition, because the range of VD from 5.7 mm to -4.2 mm, if the incision can be above intertragic notch more than 4.2 mm, the effect may be better.
The clinical results showed that the strategy can reduce sensory loss after post-auricular incision. In sensory test, two superficial sensations were adopted: touch and pain. Touch represents for the A-β fibers, and pain represents for A- δ and C fibers [15]. The SWM test was used to evaluate touch. It’s a reliable, simple and repeatable test method which is widely used in plastic, trauma surgery and rehabilitation [16,17]. Kang et al. [18] also used it to assess the auricular sensation and suggested that it can provide relatively objective and accurate information. The Pin-Prick test was used to evaluate pain, and it is used in studies of auricular numbness about parotid gland surgery [15,19,20]. Other sensations were not used, such as two-point discrimination or temperature sensation. Because two-point discrimination depends on the quantity and the density of the receptors, and it can’t reflect the recovery of nerves. The temperature test reflects A-δ and C sensory fibers just like pain sensation, but the actual measurement results are not accurate [21].
The SWM and PP test results showed that sensory loss occurred mainly in area 1, 2 and 5, it may be related to the injury to auricular branch of LON and GAN. LON divides branches into superior medial surface of auricle, superior helix and scapha. In present study, the sensory loss was not obvious in crus of antihelix area. It may because GAN and auriculotemporal nerve also innervate this area [22,23], and LON has a weak effect on the area. In addition, previous studies have shown that the branch of GAN which innervated medial surface of auricle was higher than another GAN branches [6]. Therefore, there is a higher possibility of this branch being injured in post-auricular incision than other branches. The GAN mainly innervates area 2 and 3, so it is considered that sensory loss in area 2 is related to the injury to the branch of GAN. It is worth mentioning that the innervation of GAN and LON is not invariable in medial surface of auricle. The LON can innervate area 2, and even the whole medial surface of auricle. The GAN also can innervate area 1 sometimes. Therefore, the sensory loss in medial surface of auricle may be related to these 2 nerves [22,24]. In addition, although GAN innervates area 2 and 3, there was no statistical difference in area 3 in both groups preoperatively and postoperatively. The reason may be related to the position of the branch of GAN is so low that it is difficult to be injured. It was mentioned in the study of Yang et al. [6], the branch of GAN which innervated medial surface of auricle is different from the branches innervating area 6, 8 and 9, and the latter divided into branches at the position near otobasion inferius. Although the conventional post-auricular incision is close to the mastoid tip, it is not close to otobasion inferius so that the injury to the branch is relatively rare. In conventional group, it need 1 month to recover to the preoperative level in area 2, while there was no statistical difference in modified group postoperatively and preoperatively. It was probably because the inferior margin of incision didn’t exceed the intertragic notch so that the auricular branch of GAN is preserved. The sensory loss occurred mainly in area 1, 2 and 5, and modified group was significantly better than conventional group. The results showed that it can effectively reduce auricular sensory loss by protecting LON and GAN according to the strategy in this study.
Sensory loss still occurred in area 1 and 5 in modified group postoperatively. It may be related to the stretch injury to nerves, the injury to nutrient vessels and tissues. The mechanism of auricular sensory recovery may be related to the regeneration of nerve fibers, the collateral innervation of other auricular nerves13 and the psychological adjustment of patients [18]. Previous studies have shown that there are abundant collateral innervations, and auricular nerves overlap usually [22,25]. The recovery speed in area 2 was faster than area 1 and 5 may be related to this situation. Pain recovery was faster than touch in present study, there is also a similar research from Hu et al. [15]. In previous studies, the auricular sensation needed 0.5~1 year to recover to preoperative levels after the injury to the posterior branch of the GAN [20,26]. Touch can recover to preoperative level after 3 months in the study of Kang et al. [18], they selected area 2 as measuring area. In present study, the touch did not recover to preoperative level in area 1 and 5 in conventional group after 3 months while area 2 had recovered to preoperative level. The differences between these results may be related to different measuring areas.
In clinical trial part of this study, the value of VAS is subjective, Semmes–Weinstein monofilaments and Pin Prick test also are not completely objective. Because it is difficult to acquire the informed consent from patients to attempt a new kind of incision, this trial is not blinded or randomized. Due to both these aspects, the test results may be not objective enough.
It is possible to preserve LON and GAN in post-auricular incision, which can reduce postoperative auricular numbness. In post-auricular incision, it can preserve LON during surgery, with special attention being paid to the course of LON at the height of inferior crus of antihelix. GAN can be preserved effectively, with the inferior margin of post-auricular incision being higher than the intertragic notch. If the incision can be higher than the intertragic notch more than 4.2 mm, the effect may be better.
- Frampton SJ, Pringle M (2011) Cutaneous sensory deficit following post-auricular incision. J Laryngol Otol 125: 1014-1019. [Crossref]
- Min H, Lee H, Lee Y, Jeong J, Cho S, et al. (2007) Is it necessary to preserve the posterior branch of the great auricular nerve in parotidectomy? Otolaryngol Head Neck Surg 137: 636-641. [Crossref]
- Lekakis G (2003) Philipp Friedrich Arnold, Ludvig Levin Jacobson and their contribution to head and neck anatomy. J Laryngol Otol 117: 28-31. [Crossref]
- Loughner BA, Larkin LH, Mahan PE (1990) Nerve entrapment in the lateral pterygoid muscle. Oral Surg Oral Med Oral Pathol 69: 299-306. [Crossref]
- Peled Z, Pietramaggiori G, Scherer S (2016) Anatomic and compression topography of the lesser occipital nerve. Plast Reconstr Surg Glob Open 4: e639. [Crossref]
- Yang H, Kim H, Hu K (2015) Anatomic and histological study of great auricular nerve and its clinical implication. J Plast Reconstr Aesthet Surg 68: 230-236. [Crossref]
- Ella B, Langbour N, Caix P, Midy D, Deliac P, et al. (2015) Transverse cervical and great auricular nerve distribution in the mandibular area: a study in human cadavers. Clin Anat 28: 109-117. [Crossref]
- Tubbs R, Fries F, Kulwin C, Mortazavi M, Loukas M, et al. (2016) Modified skin incision for avoiding the lesser occipital nerve and occipital artery during retrosigmoid craniotomy: potential applications for enhancing operative working distance and angles while minimizing the risk of postoperative neuralgias and intraoperative hemorrhage. J Clin Neurosci 32: 83-87. [Crossref]
- Fujimaki T, Son J, Takanashi S, Ishii T, Furuya K, et al. (2007) Preservation of the lesser occipital nerve during microvascular decompression for hemifacial spasm. Technical note. J Neurosurg 107: 1235-1237. [Crossref]
- Biglioli F, D'Orto O, Bozzetti A, Brusati R (2002) Function of the great auricular nerve following surgery for benign parotid disorders. J Craniomaxillofac Surg 30: 308-317. [Crossref]
- Murphy R, Dziegielewski P, O'Connell D, Seikaly H, Ansari K (2012) The great auricular nerve: an anatomic and surgical study. J Otolaryngol Head Neck Surg 41: S75-S77. [Crossref]
- McKinney P, Katrana D (1980) Prevention of injury to the great auricular nerve during rhytidectomy. Plast Reconstr Surg 66: 675-679. [Crossref]
- Brown JS, Ord RA (1989) Preserving the great auricular nerve in parotid surgery. Br J Oral Maxillofac Surg 27: 459-466. [Crossref]
- Grosheva M, Shabli S, Volk G, Sommer B, Ludwig L, et al. (2017) Sensation loss after superficial parotidectomy: A prospective controlled multicenter trial. Head Neck 39: 520-526. [Crossref]
- Hu J, Ye W, Zheng J, Zhu H, Zhang Z (2010) The feasibility and significance of preservation of the lobular branch of the great auricular nerve in parotidectomy. Int J Oral Maxillofac Surg 39: 684-689. [Crossref]
- Bell-Krotoski JA, Fess EE, Figarola JH, Hiltz D (1995) Threshold detection and Semmes-Weinstein monofilaments. J Hand Ther 8: 155-162. [Crossref]
- Levin S, Pearsall G, Ruderman R (1978) Von Frey's method of measuring pressure sensibility in the hand: an engineering analysis of the Weinstein-Semmes pressure aesthesiometer. J Hand Surg Am 3: 211-216. [Crossref]
- Kang H, Ahn S, Jeon S, Hur D, Kim J, et al. (2012) Sensation recovery of auricle following chronic ear surgery by retroauricular incision. Eur Arch Otorhinolaryngol 269: 101-106. [Crossref]
- Vasquez N, Gall A, Ellaway P, Craggs M (2013) Light touch and pin prick disparity in the International Standard for Neurological Classification of Spinal Cord Injury (ISNCSCI). Spinal Cord 51: 375-378. [Crossref]
- Vieira M, Maia A, Ribeiro J (2002) Randomized prospective study of the validity of the great auricular nerve preservation in parotidectomy. Arch Otolaryngol Head Neck Surg 128: 1191-1195. [Crossref]
- Suen DT, Chow TL, Lam CY, Wong ES, Lam SH (2007) Sensation recovery improved by great auricular nerve preservation in parotidectomy: a prospective double-blind study. ANZ J Surg 77: 374-376. [Crossref]
- Peuker ET, Filler TJ (2002) The nerve supply of the human auricle. Clin Anat 15: 35-37. [Crossref]
- Schmidt BL, Pogrel MA, Necoechea M, Kearns G (1998) The distribution of the auriculotemporal nerve around the temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 86: 165-168. [Crossref]
- Pantaloni M, Sullivan P (2000) Relevance of the lesser occipital nerve in facial rejuvenation surgery. Plast Reconstr Surg 105: 2594-2599; discussion 600-603. [Crossref]
- Kiyokawa J, Yamaguchi K, Okada R, Maehara T, Akita K (2014) Origin, course and distribution of the nerves to the posterosuperior wall of the external acoustic meatus. Anat Sci Int 89: 238-245. [Crossref]
- Ryan WR, Fee WE (2009) Long-term great auricular nerve morbidity after sacrifice during parotidectomy. Laryngoscope 119: 1140-1146. [Crossref]







