Objective: Barlow disease is a still challenging pathology for heart surgeons. Aim of the present study is to report 5-year follow-up results of mitral valve repair in Barlow disease from a single large volume center.
Methods: Between January 1st, 2008 and December 31st, 2011, 85 consecutive patients (54 men and 31 women) underwent mitral repair of Barlow mitral valve disease Mean age was 59 ±14 years (range: 28-85 years). Before surgery 47% of patients were in NYHA functional class III or IV. Reconstructive techniques were posterior leaflet quadrangular resection with (50.6%) or without (37.8%) leaflet sliding plasty, implantation of Gore-Tex artificial chordae (53.0%), and mitral annuloplasty, that was performed in all patients. Concomitant procedures included tricuspid valve repair, aortic valve repair or replacement, coronary artery bypass graft surgery, and modified maize procedure.
Results: There were no perioperative in-hospital deaths. Intraoperative TEE did not disclose any early failure of valve repair. Postoperative minor complications were detected in 19 patients. Two patients died during follow-up. At five-year follow-up in the 83 surviving patients NYHA functional class improved significantly (p<0,0001). Significant postoperative atrial and ventricular remodeling occurred after surgery. 48 (57.8%) patients had no residual regurgitation and 24 (28.9%) showed trivial or mild mitral regurgitation. Mild to moderate regurgitation was found in 10 (12%), only one developed severe mitral regurgitation. Chordal elongation and anterior leaflet flail were related to recurrence of mitral regurgitation.
Conclusion: Repair of the mitral valve is currently the surgical treatment of choice for complex degenerative mitral valve disease and should be pursued even in the most advanced forms of Barlow disease. Careful selection of patients, early surgical intervention, optimization of surgical techniques including use of large size annuloplasty rings and artificial chordae, along with surgical expertise in mitral valve repair are the main determinants to decrease the risk of recurrent mitral regurgitation although progression of disease may be responsible for most of reinterventions.
Barlow disease is related to myxoid degeneration of all components of mitral valve apparatus [1]. The disease is defined according to the criteria described by Carpentier as: bileaflet prolapse > 2 mm; billowing valve with excess tissue and thickened leaflets ≥ 3 mm; severe anular dilatation [2]. Chordal elongation or rupture, anular and papillary muscle calcification may be present [2,3]. At present mitral valve repair is considered the surgical gold standard: repair is more frequently associated with reverse left ventricular remodeling and restoration of left ventricular function in comparison to valve replacement. Moreover, valve repair prevents prosthetic valve related complications. Previous investigations showed a decrease of peri operative mortality, increased long-term survival and freedom from re-intervention [4,5]. Due to anatomical complexity of the disease however surgical valve repair is usually performed only in near half of patients [6,7]. Guidelines encourage referral of patients with Barlow disease to centers with surgeons experienced in mitral valve repair in order to achieve to decrease the number of replaced valves [8-10]. The goals of reconstructive surgery are preservation or restoration of normal leaflet motion, creation of a large surface of coaptation, and stabilization of the entire annulus with a remodeling annuloplasty. Reconstructive surgery should be performed early in patients with severe mitral regurgitation (EROA ≥ 0.40 cm2) with reparable valves, before the occurrence of clinical symptoms, atrial fibrillation, pulmonary hypertension, and left ventricular dysfunction [11,12]. Present investigation reports 5-year follow-up results of mitral valve repair in 85 consecutive patients with Barlow disease underwent mitral valve repair at Heart surgery department of AOU Careggi. Factors related to late recurrence of mitral valve regurgitation were evaluated.
Between January 1st, 2008 and December 31st, 2011, 85 consecutive patients (54 men and 31 women; mean age 59 ±14 years) with the features of Barlow disease underwent mitral valve repair at Heart Surgery Department of Azienda Ospedaliera Universitaria Careggi, Firenze. Barlow disease was diagnosed by preoperative echocardiogram and confirmed by surgical inspection. Patients presenting features of fibroelastic deficiency, Marfan disease and mild degenerative mitral valve prolapse were excluded from the study.
Transthoracic (TTE) and transesophageal echocardiography (TEE) were performed in all patients before surgery and the echocardiograms were reviewed by an expert cardiologist. Intraoperative TEE monitoring was performed during intervention in all cases. In table 1 are reported anatomic features of mitral valves.
Table 1. Anatomic features of mitral valves (n=85)
Excess Tissue* |
85 (100) |
Bileaflet Prolapse* |
85 (100) |
Leaflet Thickening* |
85 (100) |
Anular Dilatation* |
85 (100) |
Anular Calcification |
28 (33.0) |
Chordae Elongation |
31 (36.5) |
Chordae Rupture |
31 (36.5) |
PM Elongation |
1 (1.2) |
PM Calcification |
3 (3.5) |
Multiple Clefts |
7 (8.2) |
Flail AML |
8 (9.4) |
Flail PML |
32 (37.6) |
Abbreviations: EROA: Effective Regurgitant Orifice Area; AP: Anterior-Posterior; PM: Papillary Muscle; AML: Anterior Mitral Leaflet; PML: Posterior Mitral Leaflet.
* Fundamental criteria to define Barlow’s Disease on surgical inspection.
Surgical technique
The operation was performed through full sternotomy in 94.1%, whereas in 5 patients access was obtained with a minimally invasive approach. Mitral valve was accessed through a left atriotomy in the interatrial groove and the repair was performed according to the principles described by Carpentier and coll. [2,13,14]. Prolapse of the posterior leaflet was corrected in most cases by quadrangular resection, combined in 43 (50.6%) patients with a leaflet sliding plasty to reduce the risk of postoperative Systolic Anterior Motion (SAM) [14,15,16]. Mitral ring annuloplasty was performed in all patients. Mitral valve reconstruction was completed by cleft correction, when necessary. Associated procedures performed included tricuspid valve repair, aortic valve replacement, aortic valve repair, coronary artery bypass graft surgery, and a modified maze procedure. Operative details are reported in Table 2. Mean cross clamp (CCL) and Cardiopulmonary Bypass (CPB) times were 92.2 ± 32.7 minutes and 114.6 ± 36.5 minutes, respectively.
Table 2. Operative data.
Mitral Valve Repair |
n (%) |
None |
9 (10.6) |
PMLQR |
76 (89.4) |
PMLTR |
0 (0.0) |
AMLTR |
0 (0.0) |
Sliding Plasty |
43 (50.6) |
Cleft Repair |
7 (8.2) |
Paracommissural E2E |
2 (2.3) |
Prostethic Ring |
85 (100) |
Ring type |
|
CE |
18 (21.2) |
CEP |
24 (28.2) |
CEPII |
31 (36.5) |
Medtronic |
11 (12.9) |
Cosgrove |
1 (1.2) |
Ring size (mm) [range] |
36.5±2.1 [32-40] |
Artificial Chordae |
45 (53.0) |
AML |
23 (27.1) |
PML |
5 (5.9) |
AML + PML |
17 (19.9) |
Abbreviations: PMLQR: Posterior Mitral Leaflet Quadrangular Resection; PMLTR: Posterior Mitral Leaflet Triangular Resection; AMLTR: Anterior Mitral Leaflet Triangular Resection; E2E: Edge To Edge; CE: Carpentier-Edwards Ring (Edwards Lifesciences, Irvine, CA); CEP: Carpentier Physio I Ring (Edwards Lifesciences, Irvine, CA); CEPII: Carpentier Physio II Ring (Edwards Lifesciences, Irvine, CA); AML: Anterior Mitral Leaflet; PML: Posterior Mitral Leaflet; CPB: Cardiopulmonary Bypass; CCL: (Aortic) Cross Clamp.
Intraoperative transesophageal echocardiography was performed in all patient after weaning from cardiopulmonary bypass to detect any residual mitral regurgitation or the presence of left ventricular outflow tract obstruction.
Follow-up
Patients were followed up periodically after surgery in an outpatient clinic with clinical assessment, Electrocardiogram and a trans-thoracic echo (TTE). TTE examinations were performed by a single operator with a Sonos 5500 (Philips Medical System, Rotterdam, The Netherlands) ultrasound system. Mitral valve regurgitation was graded as none, mild, mild to moderate, moderate, moderate to severe and severe. Mean follow-up was 876 days (interquartile range: 192-1633 days) and was closed on July 1st, 2011.
Statistical analysis
Statistical analysis was performed using and SPSS 22.0 software (Chicago, IL, USA).
Data are presented as mean ± standard deviation for continuous variable, as number and percentage for categorical variables, and as median [interquartile range] for non-parametric variables. Unpaired Student’s t-test was used to determine differences between mean values for continuous variables. Pearson χ2 test or Fisher’s exact tests were used to analyze baseline differences between categorical variables. Survival rate was estimated using Kaplan-Meier curve. A p value < 0.05 was considered to indicate a significant difference. The role of each variable in predicting the outcome of surgical repair was estimated using logistic regression analysis.
Clinical and echocardiographic characteristics of patients include in the study are reported in Table 3. Forty- seven percent were in NYHA functional class III or IV before surgery. Seventy-seven (90.6%) patients had severe mitral valve regurgitation, the others (9.4%) moderate to severe.
Table 3. Clinical and echocardiographic characteristics
Number of Patient |
85 |
Age (years) [range] |
59 ±14 [28-85] |
Gender M/F |
54/31 (63.5/36.5) |
BSA (m2) [range] |
1.84±0.20 [1.35-2.38] |
Clinical Parametres |
n (%) |
NYHA Class |
|
I |
27 (31.8) |
II |
18 (21.2) |
III |
30 (35.3) |
IV |
10 (11.7) |
Atrial Fibrillation |
27 (31.8) |
Hypertension |
27 (31.8) |
Diabetes |
1 (1.2) |
COPD |
2 (2.3) |
Chronic Renal Disease |
2 (2.3) |
Redo |
1 (1.2) |
Echocardiographic Parametres |
|
EDD (mm) |
58.02±6.14 [40-73] |
ESD (mm) |
34.81±6.88 [21-60] |
ST (mm) |
10.32±1.42 [7-14] |
WT (mm) |
10.04±1.27 [7-14] |
EDV (ml) |
152.40±43.91 [52-287] |
ESV (ml) |
55.84±19.54 [15-132] |
EDVI (ml/m2) |
82.30±19.36 [31.5-138] |
ESVI (ml/m2) |
30.26±9.36 [9-71] |
LVEF (%) |
62.32±7.11 [45-76] |
LAD M-mode (mm) |
47.95±6.47 [36-67] |
LA Area (cm2) |
28.50±7.46 [17-50] |
PAP mean (mmHg) |
37.08±15.88 [20-120] |
Vena Contracta (cm) |
0.74±0.16 [0.3-1.1] |
EROA (cm2) |
0.58±0.26 [0.25-1.6] |
Anulus AP (mm) |
43.05±3.92 [33-55] |
Abbreviations: M/F: Male/Female; BSA: Body Surface Area; NYHA: New York Heart Association; COPD: Chronic Obstructive Pulmonary Disease; EDD: End-Diastolic Diameter; ESD: End-Systolic Diameter; ST: Septum Thickness; WT: Wall Thickness; EDV: End-Diastolic Volume; ESV: End-Systolic Volume; EDVI: End-Diastolic Volume Index; ESVI: End-Systolic Volume Index; LVEF: Left Ventricular Ejection Fraction; LAD: Left Atrium Diameter; LA: Left Atrium; PAP: Pulmonary Artery Pressure.
There were neither intraoperative nor postoperative in-hospital deaths. Intraoperative TEE did not disclose any early failure of valve repair. Postoperative minor complications were detected in 19 (22.4%) patients (Table 4): 2 patients (2.3%) underwent surgical revision for bleeding and 4 (4.7%) had pericardial effusion. In addition, 13 patients developed postoperative atrial fibrillation.
Table 4. Early Complications.
None |
66 (77.7) |
Revision for Bleeding |
2 (2.3) |
Post-operative SAM |
0 (0.0) |
Drainage for Pericardial Effusion |
4 (4.7) |
Post-operative AF Onset |
13 (15.3) |
Abbreviations: SAM: Systolic Anterior Motion, AF: Atrial Fibrillation.
Transthoracic echocardiogram performed before discharge did not disclose more than trivial mitral regurgitation in 79 patients (92.9%) and none showed more than mild residual regurgitation. There was no evidence of SAM in any patient. At discharge 5 (5.9%) patients were in atrial fibrillation.
Two patients died during follow-up; the first had a stroke 790 day after surgery and the second died from a Parkinson’s disease complication 15 months after surgery. Six patients experienced new hospitalization for heart disease (2 patients for heart failure and 4 for AF). Freedom from endocarditis was 100% during follow-up. In Figure 1 is shown event free survival during follow-up.
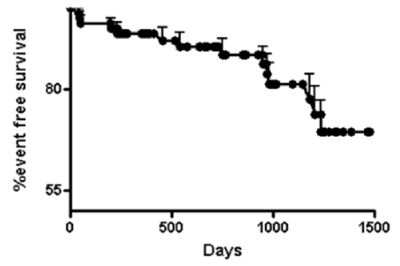
Figure 1. Event free survival after mitral valve repair
At the latest clinical examination, NYHA functional class improved significantly (χ2=23,32; p<0,0001): 73 (88.0%) patients were in class I and 10 (12.0%) patients in class II. Seventy-five (87.9%) patients were in sinus rhythm whereas 10 (12.1%) were treated with oral anticoagulation therapy for atrial fibrillation.
Transthoracic echocardiography (Table 5) showed a significant decrease of left ventricular diameters and volumes in comparison to preoperative data, suggesting a significant remodeling after surgery. A substantial decrease in left atrial size was also found (Table 5).
Table 5. Comparison between preoperative and follow-up echocardiographic data.
|
Preoperative Data |
Follow-up Data |
t |
P |
LVEF
(%) |
62.32±7.11 |
58.50±5.87 |
3.80 |
0.0002 |
EDD
(mm) |
58.02±6.14 |
51.52±5.18 |
7.35 |
<0.0001 |
ESD
(mm) |
34.81±6.88 |
31.25±4.77 |
3.87 |
0.002 |
EDV
(ml) |
152.40±43.91 |
131.10±38.77 |
3.31 |
0.0011 |
ESV
(ml) |
55.84±19.54 |
55.64±21.30 |
0.04 |
n.s. |
EDVI (ml/m2) |
82.30±19.36 |
70.88±18.32 |
3.89 |
0.0001 |
ESVI (ml/m2) |
30.26±9.36 |
30.05±10.27 |
0.90 |
n.s. |
LAD M-mode (mm) |
47.95±6.47 |
40.95±5.87 |
7.26 |
<0.0001 |
LA Area (cm2) |
28.50±7.46 |
19.28±4.04 |
9.81 |
<0.0001 |
Abbreviations: LVEF: Left Ventricular Ejection Fraction; EDD: End-Diastolic Diameter; ESD: End-Systolic Diameter; EDV: End-Diastolic Volume; ESV: End-Systolic Volume; EDVI: End-Diastolic Volume Index; ESVI: End-Systolic Volume Index; LAD: Left Atrium Diameter; LA: Left Atrium.
At the end of follow-up 48 (57.8%) patients had no residual regurgitation and 24 (28.9%) showed trivial or mild mitral regurgitation. Moderate regurgitation was found in 10 (12%), only one developed severe mitral regurgitation (Figure 2). Mean transvalvular gradient was 2.67 ± 0.88 mmHg (range:1.2-5 mmHg).
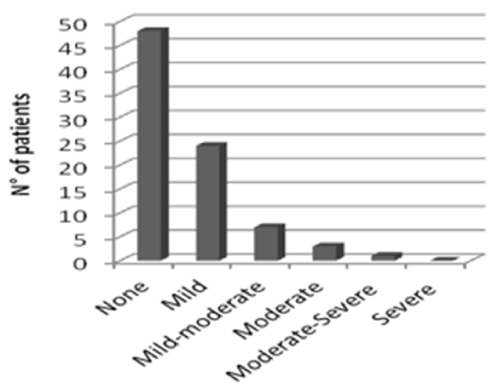
Figure 2. Degree of mitral regugitation at follow-up
Recurrent mitral regurgitation was more frequent in patients with chordal elongation or rupture and with anterior leaflet flail (Table 6). Patients with recurrent mitral regurgitation were older (mean age 66.8 ± 13.0 years vs. to 58.1 ± 12.6) and had a larger preoperative left ventricular end-diastolic diameter (62.4 ± 4.9 mm vs. 57.2 ± 5.1 mm p=0.009) and end-diastolic volume index (93.9 ± 18.8 ml/m2 vs. 80.1 ± 18.9 ml/m2, p=0.03). The effect of association of specific surgical repair techniques and of other associated surgical procedures performed on mid-term and long-term results did not show any relation with clinical outcome.
Table 6. Univariate analysis, Fisher Exact Test.
Mitral valve lesion |
Group 1 (n=11) |
Group 2 ( n=72) |
p |
Anular Calcification |
4 (36.4%) |
20 (27%) |
0.74 |
Chordae Elongation |
7 (63%) |
24 (33%) |
0.0166 |
Chordae Rupture |
8 (72.7%) |
24(33%) |
0.0163 |
Flail AML |
4 (36.4%) |
4 (6%) |
0.0096 |
Flail PML |
6 (54.5%) |
26 (36%) |
0.188 |
Abbreviations: AML: Anterior Mitral Leaflet; PML: Posterior Mitral Leaflet.
Mitral valve repair is the technique of choice to correct MV regurgitation due to Barlow disease with excellent results in reference centers with large activity volumes [18]. A systematic, standardized approach including intraoperative TEE is fundamental to achieve a qualitative evaluation of anatomic changes of mitral valve in order to plan surgical strategy. Results from present investigation suggest that mitral valve repair in Barlow disease is associated with low operatory risk and low incidence of perioperative complications. Long term related mortality is negligible while a significant left atrial and ventricular remodeling and functional improvement has been found at 5-year follow-up. About 13% of patients showed moderate to severe recurrent mitral regurgitation at last echocardiographic examination, none required re-intervention. Chordal elongation or rupture and anterior leaflet flail were associated with recurrent mitral regurgitation.
A similar incidence of recurrent mitral valve regurgitation was reported by Flameng at al [19]. In 348 patients that underwent mitral valve repair for degenerative valve incompetence, they found at 5 years a 17.8% recurrence rate of mitral regurgitation (>2/4). Recurrence rate was higher in patients with Barlow disease than in patients with fibroelastic deficiency. Factors related to recurrence of MV regurgitation were performing chordal shortening, the nonuse of sliding plasty and the nonuse of an annuloplasty ring.
Gillinov et al. [20] demonstrated that late failure presents two hazard phases: an early peaking phase in the first year followed by a slow-rising late risk phase. Surgical techniques may be more frequently responsible for early recurrence while valve-related factors leading to recurrent regurgitation from chordal rupture or elongation may be related to late recurrence. It is well recognized that the process of tissue degeneration, involving mainly the chordae, goes on after surgical correction.
A new pathology was found in 80 (55%) patients who needed surgical re-intervention for recurrent mitral regurgitation [21] . Failure of the initial repair was found in 61 (42%) patients. The mitral valve was re-repaired in 64 (44%) patients and replaced in 81 (56%) patients. Mitral re-repair was performed in almost half of patients and was associated with better functional results and longer survival. Other authors reported that mitral valve disease progression was the main cause of repair failure, in particular in presence of AML flail valve [22]. The implantation of artificial chordae probably can prevent, at some extent, the progress of the disease; we used artificial chordae in more than a half of cases, and this proportion is increasing in more recent experience.
Repair of the mitral valve is currently the best surgical option for complex degenerative mitral valve disease and should be pursued even in the most advanced forms of Barlow disease. Careful selection of patients, early surgical intervention, optimization of surgical techniques including use of large size annuloplasty rings and artificial chordae, along with surgical expertise in mitral valve repair are the main determinants to reduce the risk of recurrent mitral regurgitation and to ensure a better outcome.
- Anyanwu AC, Adams DH (2007) Etiologic classification of degenerative mitral valve disease: Barlow’s disease and Fibroelastic deficiency. Semin Thorac Cardiovasc Surg 19: 90-96. [Crossref]
- Carpentier A (1983) Cardiac valve surgery--the "French correction". J Thorac Cardiovasc Surg 86: 323-337. [Crossref]
- Carpentier A, Chauvaud S, Fabiani JN, Deloche A, Relland J, et al. (1980) Reconstructive surgery of mitral valve incompetence: ten-year appraisal. J Thorac Cardiovasc Surg 79: 338–348. [Crossref]
- Suri RM1, Schaff HV, Dearani JA, Sundt TM 3rd, Daly RC, et al. (2006) Survival advantage and improved durability of mitral repair for leaflet prolapse subsets in the current era. Ann Thorac Surg 82: 819 –827. [Crossref]
2021 Copyright OAT. All rights reserv
- David TE1, Ivanov J, Armstrong S, Christie D, Rakowski H. (2005) A comparison of outcomes of mitral valve repair for degenerative disease with posterior, anterior and bileaflet prolapsed. J Thorac Cardiovasc Surg; 130: 1242-1249. [Crossref]
- Savage EB, Ferguson TB Jr, Di Sesa VJ (2003) Use of mitral valve repair: analysis of contemporary United States experience reported to the Society of Thoracic Surgeons National Cardiac Database. Ann Thorac Surg 75: 820–825. [Crossref]
- Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C et al. (2003) A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 24: 1231–1243. [Crossref]
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, et al. (2014) 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 148: e1–e132. [Crossref]
- Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). (2007) Eur J Cardiothorac Surg 42: S1–44. [Crossref]
- Hartzell V. Schaff, Rakesh M. Suri, DPhil, and Maurice Enriquez-Sarano, “Indications for surgery in degenerative mitral valve disesase”, Seminars of Thoracic and Cardiovascular Surgery 19: 97-102.
- David TE, Ivanov J, Armstrong S, Rakowski H (2003) Late outcomes of mitral valve repair for floppy valves: implications for asymptomatic patients. J Thorac Cardiovasc Surg; 125: 1143–1152. [Crossref]
- Carpentier AF, Lessana A, Relland JY, Belli E, Mihaileanu S, et al. (1995) The "physio-ring": an advanced concept in mitral valve annuloplasty. Ann Thorac Surg 60: 1177-1185. [Crossref]
- David H. Adams, Anelechi C. Anyanwu (2006) Current concepts in mitral valve repair for degenerative disease. Heart Failure Review 11: 241–257.
- Jebara VA, Mihaileanu S, Acar C, Brizard C, Grare P, et al. (1993) Left ventricular outflow tract obstruction after mitral valve repair. Results of the sliding leaflet technique. Circulation 88: II-30–34 [Crossref]
- Ani C. Anyanwu, and David H. Adams, Bileaflet repair for Barlow syndrome, Seminars in Thoracic and Cardiovascular Surgery, Volume 22, Number 2, 2010.
- Adams DH, Anyanwu AC, Rahmanian PB, Abascal V, Salzberg SP, et al. (2006) Large annuloplasty rings facilitate mitral valve repair in Barlow's disease. Ann Thorac Surg 82: 2096-2100. [Crossref]
- Khan RA , Mittnacht AJC, Anyanwu, AC Systolic Anterior Motion as a result of relative “undersizing” of a mitral valve annulus in a patient with Barlow’s disease , Anesthesia and Analgesia, International Anesthesia Research Society Vol. 108, No. 4, April 2009.
- Castillo JG, Anyanwu AC, Fuster V, Adams DH (2012) A near 100% repair rate for mitral valve prolapse is achievable in a reference center: implications for future guidelines. J Thorac Cardiovasc Surg 144: 308–312. [Crossref]
- Flameng W, Meuris B, Herijgers P, Herregods MC (2008) Durability of mitral valve repair in Barlow disease versus fibroelastic deficiency. J Thorac Cardiovasc Surg 135: 274-282. [Crossref]
- Gillinov AM, Cosgrove DM, Blackstone EH, Diaz R, Arnold JH, et al. (1998) Durability of mitral valve repair for degenerative disease. J Thorac Cardiovasc Surg 116: 734-743. [Crossref]
- Suri RM, Schaff HV, Dearani JA, Sundt TM 3rd, Daly RC, et al. (2006) Recurrent mitral regurgitation after repair: should the mitral valve be re-repaired? J Thorac Cardiovasc Surg 132: 1390-1397. [Crossref]
- Coutinho GF, Correia PM, Branco C, Antunes MJ2. (2016) Long-term results of mitral valve surgery for degenerative anterior leaflet or bileaflet prolapse: analysis of negative factors for repair, early and late failures, and survival. Eur J Cardiothorac Surg 50: 66-74. [Crossref]


