Following electrical cardioversion, some adult patients with pre-existing atrial arrhythmias (usually atrial fibrillation) can present mitral insufficiency. However, to our knowledge, this effect has not been previously described in pediatric patients. In the present work, we report three cases of pediatric patients followed at our Hospital (Hospital de Niños Pedro de Elizalde ex “Casa Cuna”, Buenos Aires, Argentina), who presented transient mitral insufficiency after electrical cardioversion.
Electrical cardioversion, pediatric patients, arrhythmias
ECV: Electrical Cardioversion; ECG: Electrocardiogram; IV: Intravenous
Electrical cardioversion (ECV), i.e. electric shocks applied by means of a cardiac defibrillator, is a procedure commonly used to treat arrhythmias at risk of death, as well as arrhythmias that do not respond to drug treatment. After this procedure, approximately 50% of adult patients present cardiac stunning, evidenced in the first 3 hours and up to 96 hours after the procedure [1], and may even evolve into cardiogenic shock [2]. This myocardial stunning usually requires support with inotropes.
In pediatric patients, ECV is one of the methods used to restore the sinus rhythm after intravenous medication has failed to control supraventricular tachyarrhythmias. Although the adverse effects of this procedure are well known in adult patients, here we report transient mitral insufficiency following ECV, an effect which, to our knowledge, had not yet been described in pediatric-aged patients. One of the hypotheses is that this mitral insufficiency could be due to left ventricular myocardial stunning, which in 50% of cases occurs during the 4-96 hours following ECV. Another hypothesis is that it may be due to transient ischemia of the papillary muscles, which leads to transient deterioration of the mitral valve function.
Eight-year-old patient diagnosed with supraventricular tachycardia (SVT) at 6 months of life and treated with atenolol until 3 years of life. Due to persistence of paroxysmal episodes, he required medical intervention with ECV on multiple occasions, and is changed to flecainide, with favorable response to it.
Previous evaluation with transthoracic echocardiography had shown no structural heart disease, and surface ECG had shown no pre-excitation or other pathological findings.
The child is hospitalized again due to an episode of SVT, which fails to be controlled with the passage of two intravenous (IV) doses of adenosine and an IV loading dose of amiodarone. Thus, ECV is performed at 1 Joule/kg, achieving control.
In the child’s subsequent cardiac control, the transthoracic Doppler echocardiogram showed evidence of mild to moderate mitral insufficiency. Subsequent echocardiographic controls showed retrograde flow (with a total duration of 96 hours).
Sixteen-day-old patient, weighing 3 kg, without perinatological history of relevance, transferred to the Neonatology Department due to tachycardia. In the ECG, the patient is diagnosed with atrial flutter, which required ECV at 2 Joule/Kg, which allowed restoring the sinus rhythm and hemodynamic control of the patient.
In the child’s subsequent cardiac control, the color Doppler echocardiogram showed multi-fenestrated atrial septal defects, with no hemodynamic impact, and mild mitral insufficiency with slight biventricular dilation.
During the echocardiographic control at one month of age, the patient showed no valve insufficiency.
Two-month-old patient hospitalized in the pediatric intensive care unit due to severe low acute respiratory infection, which required mechanical ventilatory assistance.
The cardiology control performed at admission showed ostium secundum atrial septal defects, without evidence of mitral insufficiency (Figure 1).
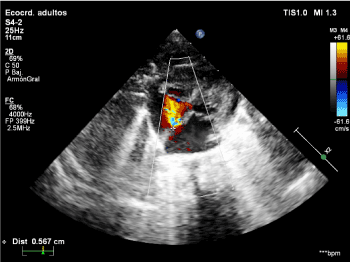
Figure 1. Color Doppler echocardiogram at the patient's admission, showing ostium secundum atrial septal defects and no mitral insufficiency.
During hospitalization, the patient presented an episode of SVT, interpreted as atrial flutter, and thus ECV was performed at 1 Joule/Kg and 1.5 Joule/Kg, without favorable response. Subsequently, loading doses of amiodarone are given, which allowed restoring the sinus rhythm.
Subsequent control with color Doppler echocardiogram showed moderate mitral insufficiency (Figure 2), which reverted 72 h later.
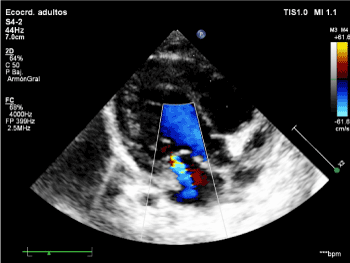
Figure 2. Color Doppler echocardiogram following ECV showing moderate mitral insufficiency.
One of the several hypotheses that have attempted to explain the left ventricle stunning phenomenon observed in 50% of adult patients after ECV is that it could be due to a change in the functionality of the left ventricle, which could also lead to severe mitral insufficiency. This transient severe mitral insufficiency can also be explained by local microvascular dysfunction caused by diffuse spasms or vasoconstriction, as well as by direct action of the electric shocks. Electric shocks are intended to depolarize the membranes of most heart cells, so as to resynchronize the electrical activity of the heart. However, these can cause reversible or irreversible tissue damage, due to electroporation, a phenomenon known since the 1940s, when Goldman D.E [3], described a sharp increase in membrane conductance, which generates hyperpolarization of the membrane, demonstrating the formation of volcano-like pores in cell membranes [4]. During the first 20 ms of electroporation, the pore quickly expands from 20 to 120 nm in diameter, and, after several seconds, begins to shrink and reseal. The electrophysiological changes that occur during this process include excitability depression, which in turn results in depolarization of the cell membrane during the diastolic interval [5], a reduction in the range of action potentials, and an increase in the intracellular calcium concentration [6].
Basic data from electrophysiology research indicate that defibrillation shocks are accompanied by adverse effects likely associated with electroporation. These include: transient ventricular ectopia, tachycardia or ventricular fibrillation induction [7], a decrease in the heart’s excitability and mechanical function [8], bradycardia, complete heart block and increased stimulation threshold [9], mechanical atrial and ventricular dysfunction (stunning), in direct relation to the intensity of shocks [10], significant increase in serum troponin levels in patients after discharges of ECV [11] and decreased rate of myocardial lactate extraction by mitochondria [12].
Although electroporation has the potential to affect all heart structures, certain heart regions have been shown to be more sensitive to the effects of ECV than others. For example, when exposed to the same electrical stimulus intensity, the degree of the condition is lower in the epicardium than in the endocardium, and, within the latter, trabecular structures and papillary muscles have shown greater susceptibility, which may cause a transient suppression of excitability and conduction [13,14], which lasts from seconds to hours, with the consequent mechanical repercussions (e.g. transient mitral insufficiency).
In the pediatric cases here reported, who received electrical shocks calculated at a rate of 1 to 2 Joule/Kg and presented no pathology of the mitral valve in previous echocardiograms, the finding of mitral insufficiency following ECV (with an average duration of 96 h, showing ad integrum restitution in subsequent controls) allows suggesting that this transient alteration is due to the direct action of the passage of the electric current through the heart muscle, with special impact on the papillary muscles, as described in previous publications. In addition to coinciding with the duration, this suggests that the formerly described phenomena, such as reversible electroporation, should be taken into account to try to explain recent findings in the pediatric population.
The authors declare that they have no conflicts of interest.
- Gowda RM, Misra D, Khan IA, Schweitzer P (2003) Acute pulmonary edema after cardioversion of cardiac arrhythmias. Int J Cardiol 92: 271-274. [Crossref]
- Kontoyannis DA, Nanas JN, Toumanidis ST, Stamatelopoulos SF (2000) Severe cardiogenic shock, after cardioversion, reversed by the intraaortic balloon pump. Intensive Care Med 26: 649. [Crossref]
- Goldman DE (1943) Potential, impedance, and rectification in membranes. J Gen Physiol 27: 37-50. [Crossref]
- Chang DC, Reese TS (1990) Changes in membrane structure induced by electroporation as revealed by rapid-freezing electron microscopy. Biophys J 58: 1-12. [Crossref]
- Al-Khadra A, Nikolski V, Efimov IR (2000) The role of electroporation in defibrillation. Circ Res 87: 797-804. [Crossref]
- Krauthamer V, Jones JL (1997) Calcium dynamics in cultured heart cells exposed to defibrillator-type electric shocks. Life Sci 60: 1977-1985. [Crossref]
- Waldecker B, Brugada P, Zehender M, Stevenson W, Welens HJ (1986) Ventricular arrhythmias after precordial electric shock. Am J Cardiol 57: 120-123.
- Kodama I, Shibata N, Sakuma I, Mitsui K, Iida M, et al. (1994) Aftereffects of high-intensity DC stimulation on the electromechanical performance of ventricular muscle. Am J Physiol 267: H248-H258 [Crossref]
- Eysmann SB, Marchlinski FE, Buxton AE, Josephson ME (1986) Electrocardiographic changes after cardioversion of ventricular arrhythmias. Circulation 73: 73-81. [Crossref]
- Babbs CF, Tacker WA, VanVleet JF, Bourland JD, Geddes LA (1980) Therapeutic indices for transchest defibrillator shocks: effective, damaging, and lethal electrical doses. Am Heart J 99: 734-738. [Crossref]
- Hasdemir C, Shah N, Rao AP, Acosta H, Matsudaira K, et al. (2002) Analysis of troponin I levels after spontaneous implantable cardioverter defibrillator shocks. J Cardiovasc Electrophysiol 13: 144-150. [Crossref]
- Osswald S, Trouton TG, O’Nunain SS, Holden HB, Ruskin JN, et al. (1994) Relation between shock-related myocardial injury and defibrillation efficacy of monophasic and biphasic shocks in a canine model. Circ Res 90: 2501-2509.
- Yabe S, Smith WM, Daubert JP, Wolf PD, Rollins DL, et al. (1990) Conduction disturbances caused by high current density electric fields. Circ Res 66: 1190-1203.
- Al-Khadra A, Nikolski V, Efimov IR (2000) The role of electroporation in defibrillation. Circ Res 87: 797-804.


