The aim of the study was to assess the lower limits of midluteal plasma progesterone (P4) and estradiol (E2) concentrations in patients who achieved pregnancy with timed intercourse (TI) or intrauterine insemination (IUI) without a human menopausal gonadotropin (hMG) stimulation. We included 297 women who became pregnant with TI or IUI without hMG stimulation, and assessed midluteal plasma P4 and E2 concentrations and pregnancy outcomes, retrospectively. In this cohort, letrozole was used in 26 cycles, and was not used in 271 cycles (twin pregnancies and ectopic pregnancies were excluded). Mean midluteal plasma P4 and E2 concentrations were 14.6 ng/mL and 194.3 pg/mL, respectively, without a letrozole cycle, and 12.8 ng/mL and 127.7 pg/mL, respectively, with letrozole. E2 with a letrozole cycle was significantly lower than that without letrozole (P<0.01). The 5 percentiles of P4 and E2 were 5.6 ng/mL and 74.7 pg/mL, respectively, without a letrozole cycle, and 5.9 ng/mL and 28.2 pg/mL, respectively, with letrozole. A correlation between E2 and P4 was observed in cycles without, but not with letrozole. Miscarriage was not related to E2 or P4 concentrations. These values may be useful for the evaluation of necessary values for pregnancy with not only TI or IUI, but also natural cycle-frozen embryo transfer with the same protocol.
estradiol, implantation, natural cycle, pregnancy, progesterone
The midluteal plasma progesterone (P4) concentration is considered to be one of the best markers for ovulation and luteinization because P4 may reflect the functions of the corpus luteum [1-3]. However, minimum values for P4 to achieve a natural cycle pregnancy have not yet been established. With our best knowledge, only one previous study reported reference values for midluteal P4 in 192 patients who achieved pregnancy with human menopausal gonadotropin (hMG)-stimulated cycles [4]. Since that study included 48 multiple pregnancies and hMG (150 IU) was administered daily, the mean value for P4 was relatively high at 29.07 ng/mL. The minimum value for full-term singleton pregnancy (N=72) was concluded to be 10.83 ng/mL [4]. Besides P4, estradiol (E2) is regarded as a marker for ovulation and luteinization [5]. We conducted this study in order to identify minimum values for P4 and E2 in patients who became pregnant with timed intercourse (TI) or intrauterine insemination (IUI) without hMG stimulation. If it is possible to establish these values, they might be useful for evaluations of the necessary values for pregnancy with not only TI or IUI, but also natural cycle-frozen embryo transfer (FET) with the same protocol.
A single-center retrospective study was performed. All patients gave written informed consent for this study. Institutional Review Board (IRB) approval was obtained from Jinjukai Ishikawa Hospital (the mother organization of Reproduction Clinic Osaka).
After opening of our institute on October 2013, we have been analyzing midluteal plasma P4 and E2 for all women who hoped for children in all cycles of TI or IUI. Since the purpose of this study is to determine the lower limit of P4 and E2 in order to achieve pregnancy, we excluded the cycles without pregnant. Between October 2013 and December 2016, 307 women who became pregnant with TI or IUI were included in the present study. We included a natural cycle or minimum ovarian stimulation for follicle growth. Ovulation induction cycles with hMG were excluded. The minimum ovarian stimulation included cyclofenil (300-600 mg x 5 days, Aska Pharmaceutical Co., Ltd., Tokyo, Japan), clomifene (50 mg x 3-5 days, Fuji Pharma Co., Ltd., Tokyo, Japan), and letrozole (2.5 mg x 2-4 days, Novartis Pharma K.K., Tokyo, Japan). Follicular development was monitored by vaginal ultrasonography. Cycles with 3 or more developing follicles were canceled. In all cycles, gonadotropin releasing hormone agonist (GnRHa, buserelin acetate 600 μg, Fuji Pharma Co., Ltd) was administered as a trigger. Ovulation was confirmed by ultrasound and serum samples were collected on the 7th day after ovulation. Serum P4 and E2 concentrations were measured by a clinical laboratory company (Medic Co., Shiga, Japan) using a chemiluminescent enzyme immunoassay (progesterone and estradiol II, Architect®, Abott Japan Co., Ltd., Tokyo, Japan). Cycles with luteal support were excluded. We retrospectively evaluated the minimum levels of E2 and P4 in the cycle achieving pregnancy. Miscarriage was defined as a missing or undetectable heart beat at 12 weeks of gestation, while ongoing pregnacy was defined as a confirmed heart beat beyond 12 weeks of gestation.
The Student’s t-test (non-homogeneity, two-sided) was used for comparisons between two groups. Correlations were detected by Pearson’s test. Significance was defined as P <0.05.
In this cohort, 307 women (all Japanese) resulted in pregnancy. After exclusion of 4 twin pregnancies and 6 ectopic pregnancies, 297 cycles were evaluated for analyses (cyclofenil 75 cycles, clomifene 132 cycles, letrozole 26 cycles, no medication 64 cycles). Since a previous study reported that letrozole decreases E2 levels [6], we divided all the pregnant cycles into two groups with or without letrozole (Table 1). No significant difference was observed in BMI between the two groups; however, the age of the letrozole group (31.2 years) was significantly younger than that without letrozole (33.6 years, P<0.01). Mean midluteal plasma P4 and E2 concentrations were 14.6 ng/mL and 194.3 pg/mL, respectively, without a letrozole cycle, and 12.8 ng/mL and 127.7 pg/mL, respectively, with letrozole. E2 with a letrozole cycle was significantly lower than that without letrozole (P<0.01). The 5 percentiles of P4 and E2 were 5.6 ng/mL and 74.7 pg/mL, respectively, without a letrozole cycle, and 5.9 ng/mL and 28.2 pg/mL, respectively, with letrozole. The lowest P4 and E2 levels were 2.3 ng/mL and 44.2 pg/mL, respectively, without a letrozole cycle, and 4.6 ng/mL and 23.4 pg/mL, respectively, with letrozole. When we divided non-letrozole cycles into cyclofenil, clomifene and no medication, or when we divided all cycles into TI (130 cycles) or IUI (167 cycles), similar results were obtained. Therefore, we did not divide those groups.
Table 1 Patients' profile and endocrine parameters
|
letrozole (-)(n=271) |
letrozole(+) (n=26) |
P value |
Age (years) |
33.6 ± 4.0 |
31.2 ± 3.0 |
<0.01 |
BMI (kg/m2) |
19.6 ± 5.4 |
20.5 ± 3.6 |
NS |
P4 (ng/mL) |
14.6 ± 7.8 |
12.8 ± 4.1 |
NS |
5 percentile P4 |
5.6 |
5.9 |
NA |
lowest P4 |
2.3 |
4.6 |
NA |
E2 (pg/mL) |
194.3 ± 120.0 |
127.7 ± 84.6 |
<0.01 |
5 percentile E2 |
74.7 |
28.2 |
NA |
lowest E2 |
44.2 |
23.4 |
NA |
Values are indicated by means ± standard deviations.
The Student’s t-test (non-homogeneity, two-sided) was used for comparisons between the two groups.
A correlation between E2 and P4 was observed in cycles without letrozole (R=0.60, P<0.01), but not in cycles with letrozole (R=0.13). According to the scatter plot for E2 and P4 in letrozole-free cycles (Figure 1), miscarriage (red squares) was not associated with either E2 or P4 concentrations. However, P4 in miscarriage group was slightly reduced compared with ongoing pregnancy group (blue circles) without statistical significance.
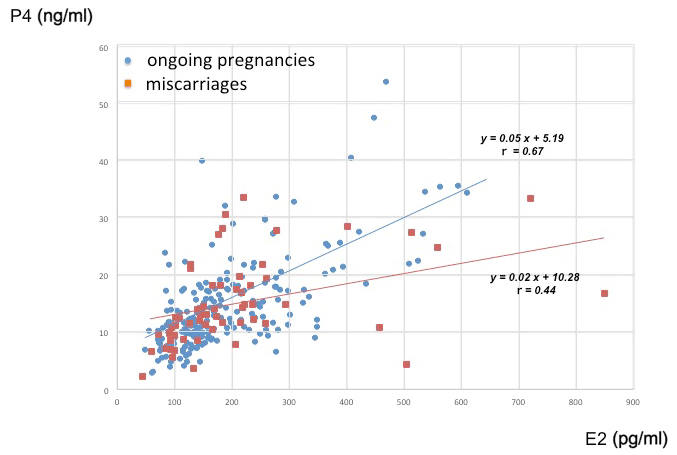
Figure 1. Scatter plot for E2 and P4 concentrations in letrozole-free cycles. Red squares indicate miscarriages (missing or undetectable heart beat at 12 weeks of gestation), and blue circles indicate ongoing pregnancies as a confirmed heart beat beyond 12 weeks of gestation.
Our results suggest that the lower limits (5 percentiles) of midluteal plasma P4 and E2 in patients who became pregnant with TI or IUI without hMG stimulation were 5.6 ng/mL and 74.7 pg/mL, respectively. This is the first study to report reference values for P4 and E2 in patients with pregnancy without an hMG stimulation. Only one previous study showed the minimum value of midluteal P4 in 72 patients who achieved a singleton pregnancy in stimulated cycles (hMG 150 IU daily) with timed intercourse as 10.8 ng/mL [4]. We set the lower limit of P4 and E2 as 5 percentile, because the World Health Organization (WHO) determined semen quality parameters as 5 percentile for the lower reference limit [7].
As minimum values, ovulation and luteinization are confirmed when midluteal P4 was more than 1.8~5.0 ng/mL [8-12]; however, it has yet to be established whether these values are adequate for pregnancy. Since pregnancy is the best evidence for adequate ovulation and luteinization, reference values for P4 and E2 need to be evaluated in pregnant cycles only. Therefore, we did not evaluate or compare the values for P4 and E2 in non-pregnant women, who may had other factors not to achieve pregnancy.
Recent evidence was obtained to show reference values for P4. The nucleolar channel system (NCS) in the endometrium is a marker for endometrial receptivity [13]. An NCS study in the endometrium revealed that the minimum P4 level was 4.0 ng/mL [14]. This value indicated ovulation only; it did not indicate the necessary value for pregnancy. Since there is no linear correlation between the prevalence of NCS and P4 levels, this level simply indicated the P4 threshold level. Thus, endometrial receptivity may require 4.0 ng/mL or more of P4.
In terms of a luteal phase deficiency, there is currently no standard P4 value during the luteal phase in normal fertile women [15-17]. Therefore, whether the minimum P4 concentration defines fertile luteal function remains unknown. Moreover, corpus luteum functions vary from cycle to cycle because the corpus luteum changes from cycle to cycle [1,2]. Since it currently remains unclear whether women have a luteal phase deficiency, a minimum value may be useful as a treatment tool.
Limitations that may have contributed to the present results include the following. This was a retrospective observational study. A prospective study may be difficult to perform to assess minimum values for pregnancy because data from pregnant patients only are needed for analyses. Furthermore, since P4 levels may fluctuate in the 90-minute period during the midluteal phase (2.3-40.1 ng/mL) [18], a single value may not be sufficient. However, since the aim of the present study was to assess the minimum values of P4 and E2, fluctuations may not have influenced the data obtained.
The lower limits of midluteal plasma P4 and E2 concentrations in patients who achieved pregnancy with TI or IUI without an hMG stimulation were 5.6 ng/mL and 74.7 pg/mL, respectively.
This research was supported by Jinjukai Ishikawa Hospital (the mother organization of Reproduction Clinic Osaka). We acknowledge Medical English Services in Kyoto (reference number: 184813) for their professional English editing services.
- Ecochard R, Bouchard T, Leiva R, Abdulla S, Dupuis O (2017) Characterization of hormonal profiles during the luteal phase in regularly menstruating women. Fertil Steril 108: 175-182. [Crossref]
- Direito A, Bailly S, Mariani A, Ecochard R (2013) Relationships between the luteinizing hormone surge and other characteristics of the menstrual cycle in normally ovulating women. Fertil Steril 99: 279-285. [Crossref]
- Babalioğlu R, Varol FG, Ilhan R, Yalçin O, Cizmecioğlu F (1996) Progesterone profiles in luteal-phase defects associated with recurrent spontaneous abortions. J Assist Reprod Genet 13: 306-309. [Crossref]
- Sallam HN, Sallam A, Ezzeldin F, Agamia AF, Abou-Ali A (1999) Reference values for the midluteal plasma progesterone concentration: evidence from human menopausal gonadotropin-stimulated pregnancy cycles. Fertil Steril 71: 711-714. [Crossref]
- Schliep KC, Mumford SL, Hammoud AO, Stanford JB, Kissell KA, et al. (2014) Luteal phase deficiency in regularly menstruating women: prevalence and overlap in identification based on clinical and biochemical diagnostic criteria. J Clin Endocrinol Metab 99: 1007-1014. [Crossref]
- Bedaiwy MA, Mousa NA, Casper RF (2009) Aromatase inhibitors prevent the estrogen rise associated with the flare effect of gonadotropins in patients treated with GnRH agonists. Fertil Steril 91: 1574-1577. [Crossref]
- World Health Organization (2010) Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. World Health Organization, Geneva.
- Askalani H, Smuk M, Sugar J, Delvoye P, Robyn C, et al. (1974) Serum progesterone in nonpregnant women. I. Comparative study of serum progesterone concentration and urinary pregnanediol excretion. Am J Obstet Gynecol 118: 1054-1063. [Crossref]
- Nadji P, Reyniak JV, Sedlis A, Szarowski DH, Bartosik D (1975) Endometrial dating correlated with progesterone levels. Obstet Gynecol 45: 193-194. [Crossref]
- Israel R, Mishell DR Jr, Stone SC, Thorneycroft IH, Moyer DL (1972) Single luteal phase serum progesterone assay as an indicator of ovulation. Am J Obstet Gynecol 112: 1043-1046. [Crossref]
- Leiva R, Bouchard T, Boehringer H, Abulla S, Ecochard R (2015) Random serum progesterone threshold to confirm ovulation. Steroids 101: 125-129. [Crossref]
- Saxena BN, Poshyachinda V, Dusitsin N (1976) A study of the use of intermittent serum luteinizing hormone, progesterone and oestradiol measurements for the detection of ovulation. Br J Obstet Gynaecol 83: 660-664. [Crossref]
- Guffanti E, Kittur N, Brodt ZN, Polotsky AJ, Kuokkanen SM, et al.(2008) Nuclear pore complex proteins mark the implantation window in human endometrium. J Cell Sci 121: 2037-2045. [Crossref]
- Nejat EJ, Szmyga MJ, Zapantis G, Meier UT (2014) Progesterone threshold determines nucleolar channel system formation in human endometrium. Reprod Sci 21: 915-920. [Crossref]
- Practice Committee of the American Society for Reproductive Medicine (2015) Current clinical irrelevance of luteal phase deficiency: a committee opinion. Fertil Steril 103: 27-32.[Crossref]
- Mesen TB, Young SL (2015) Progesterone and the luteal phase: a requisite to reproduction. Obstet. Gynecol. Clin North Am 42: 135-151. [Crossref]
- Daya S, Ward S (1988) Diagnostic test properties of serum progesterone in the evaluation of luteal phase defects. Fertil Steril 49: 168-170. [Crossref]
- Filicori M, Butler JP, Crowley WF Jr (1984) Neuroendocrine regulation of the corpus luteum in the human. Evidence for pulsatile progesterone secretion. J Clin Invest 73: 1638-1647. [Crossref]

