Purpose: Complications after surgery are still matter of concern. In this study we assessed mid-regional proadrenomedullin (MR-proADM) in comparison to white blood cell count (WBC), C-reactive protein (CRP) and procalcitonin (PCT) for prediction or detection of postoperative infectious complications.
Methods: This prospective study investigated in 47 adult patients undergoing elective orthopaedic, abdominal, vascular surgery or angioplasty the MR-proADM, WBC, CRP and PCT plasma concentrations prior to anaesthesia (at baseline), and on the 1st, 3rd, 5th and 7th postoperative day. Pearson correlation coefficient calculation, normality test by Kolmogorov-Smirnov and non-parametric tests (Kruskal-Wallis test, Wilcoxon test), were used for the statistical analysis. Data are mean ± standard deviation or median (range) where appropriate. The accuracy of biomarkers in the diagnosis of postoperative complications was assessed by comparing the area under the receiver operating characteristics curve (AUC-ROC).
Results: All baseline biomarker concentrations were comparable between the four groups: MR-proADM 0.77 ± 0.37 nmol*l−1, WBC 7.0±0.60 nl−1, CRP 5 (1-50) mg*l−1 and PCT 0.10, (0.05-1.53) ng*ml−1. MR-proADM was higher with increasing American Society of Anaesthesiology (ASA) classification (0.55 (0.37-0.91) nmol*l−1 in patients with ASA classification <3, and 0.77 (0.45-2.50) nmol*l−1 in patients with ASA classification ≥3, p<0.01) and correlated with the baseline plasma creatinine (R2=0.71). Angioplasty had no significant influence on MR-proADM.
In patients with postoperative infectious complications, MR-proADM was significantly higher (1.71 (0.67-4.02) vs. 0.87 (0.43-2.98) nmol*l−1 on the 1st day, 1.81 (0.67-19.9) vs. 0.83 (0.43-1.57) nmol*l−1 on the 3rd day) and rose earlier compared to other biomarkers.
Conclusions: MR-proADM might have a promising role in the early detection of postoperative complications and could be useful in identifying patients with need for higher level of care and treatment.
biomarker, MR-proADM, procalcitonin, perioperative risk, postoperative complications
Abbreviations
ASA, American Society of Anaesthesiologists’; BMI, body mass index; CRP, C-reactive protein; EDTA, ethylenediaminetetraacetic acid; GOLD, Global Initiative on Obstructive Lung Disease; MR-proADM, mid regional proadrenomedullin; NYHA, New York Heart Association; PCT, procalcitonin; SD, standard deviation; UTI, urinary tract infection; WBC, white blood cell count
Adrenomedullin, a 52 amino acid peptide, is a member of the calcitonin peptide family and is present in numerous tissues and organs. It has been shown to have a variety of physiological functions, including immune-modulating, direct bactericidal, diuretic and potent vasodilatory activity. Its potent vasodilatory and hypotensive response is elicited through an initial increase in cyclic adenosine monophosphate levels, and a subsequent production of nitric oxide, its widespread production helping maintain the blood supply to individual organs [1-6].
The importance of adrenomedullin in homeostasis is illustrated by its central role in the up- and down-regulation of cytokines and other mediators, as well as its own stimulatory and inhibitory effect on cytokine production. Interleukin (IL)-1β and tumour necrosis factor (TNF) are two of the most potent stimulators for adrenomedullin production and adrenomedullin itself is up-regulated by hypoxia, bacterial products and shear stress [6-8].
The ubiquitous and important functional role of adrenomedullin results in its clinical use in many diverse indications as marker for the early prediction of organ dysfunction and outcome [9-15].
The reliable measurement of adrenomedullin is challenging due to its short half life of 22 minutes thanks to its rapid degradation by proteases and its binding to the factor H [5]. The increased stability of its precursor molecule, MR-proADM, allows it to be reliably measured as a surrogate biomarker for the unstable ADM in 1:1 ratio, thus allowing changes in biomarker concentrations to be determined [16]. In healthy conditions, MR-proADM circulates at low concentrations; however, plasma levels are significantly up-regulated in many diseased states, such as pulmonary hypertension, heart failure, renal failure, ischaemic injury, lower respiratory diseases and bacterial infection, in proportion to disease severity. Healthy individuals have detectable levels of MR-proADM of approximately 0.4 nmol*l−1, but this can increase significantly, depending on individual disease conditions [2-16].
Since so far little data are available in the operative medicine, we focused on the evaluation of MR-proADM in the operative field. Complications after surgery, for example bacterial infections, are still an important cause of death [17]. They continue to impose diagnostic and therapeutic challenges, due to the difficulty to determine, if the occurring event is related to a physiological postoperative inflammatory response or is caused by another pathological process. It can often be difficult to determine which patients will develop short-term postoperative infectious complications, based on diagnostic biomarker levels and clinical signs alone. A biological marker that can predict infectious complications before clinical signs and symptoms develop could be of great clinical value. MR-proADM might help to differentiate the infectious origin in patients with systemic inflammatory response syndrome and organ dysfunction. In a previous study Valenzuela-Sánchez et al. demonstrated that MR-proADM is a biomarker of organ failure, stratifying the mortality risk in patients with sepsis in emergency department (ED) and ICU. The MR-proADM levels were more effective than procalcitonin (PCT) and C-reactive protein (CRP) levels to determine an unfavorable outcome and the risk of mortality in patients with sepsis admitted to the ICU. MR-proADM proved useful in patients diagnosed with organ dysfunction of infectious etiology, its level in septic patients was several times higher than in patients without sepsis [18]. Angeletti et al. [19] compared MR-proADM levels in septic patients and non-septic patients with SIRS with similar results [19].
The major aim of our pilot project was to assess whether MR-proADM is able to identify patients with postoperative organ dysfunction and infectious complication with the need of higher level of care and treatment and to compare its performance with white blood cell count (WBC), C-reactive protein (CRP) and procalcitonin (PCT). We also aimed to investigate whether mechanical manipulation of the endothelial surface in patients with peripheral arterial angioplasty without major surgery is associated with an induction of MR-proADM increase.
With approval of the ethics committee of the University of Witten/Herdecke (No.17/2013) we conducted a small scale preliminary study in order to evaluate the feasibility of measuring MR-proADM in the perioperative setting, to assess the postoperative risk for complications with the help of MR-proADM, to guide the patient management and to determine an appropriate sample size prior to performance of a full-scale research project.
The study took place in one university hospital and 2 teaching hospitals in Germany. Sixty-eight adult patients undergoing non-cardiac surgery were screened for the study. Inclusion criteria were: written informed consent by the patient and a planned surgery. The exclusion criteria were: age <18 yrs, emergency surgery, pregnancy, preoperative infections or organ dysfunctions. Of the 68 screened patients 21 were excluded due to known or suspected infections, surgical emergency, incomplete data or pre-existing altered organ function. Preoperative altered organ function was defined in order to exclude patients as follows: heart failure using NYHA (New York Heart Association) criteria, renal disease using the RIFLE criteria, chronic pulmonary disease using the GOLD (Global Initiative on Obstructive Lung Disease) criteria.
All patients were anaesthetized and treated postoperatively according to each hospital’s standard operating procedure. All patients were evaluated by the assigned study physician for potential clinical signs of postoperative infections, sepsis or organ dysfunctions. A postoperative infection or organ dysfunction was determined based on medical records, microbiology and clinical examination. The definition of sepsis was based on the criteria established by the American College of Chest Physicians and the Society of Critical Care Medicine Consensus Conference Committee (known also as “Bone’s criteria”) [20]. Cardiovascular dysfunction was defined as hypotension with vasopressor support postoperatively and a heart rate higher than 90 beats/min. Respiratory dysfunction was defined as respiratory rate higher than 20 breaths/min or arterial carbon dioxide tension below 32 mmHg. Postoperative kidney dysfunction was defined as serum creatinine of more than 2.0 mg*dl−1 or diuresis of less than 0.5 ml*kg−1*h−1. Pneumonia was diagnosed if infiltrates were present on the chest radiograph combined with positive culture from sputum or bronchial fluid. Urinary tract infections were diagnosed by the evidence of leukocyturia and growth of pathogens in the urine culture. Postoperative kidney dysfunction was defined as serum creatinine of more than 2.0 mg*dl−1 or diuresis of less than 0.5 ml*kg−1*h−1. Standard supportive care, surgical procedures (drainage of abscesses, etc.) and broad-spectrum antibiotics were provided to septic patients.
Blood samples (each time two samples) were drawn immediately prior to induction of anaesthesia (baseline) and on 1st, 3rd, 5th and 7th day thereafter. In the angioplasty group an additional blood sample was obtained through the arterial introducer immediately after the procedure (day 0.1). For blood collecting we used the S-Monovette® 2.6 ml EDTA tubes (Sarstedt AG&Co., Nuembrecht, Germany). The blood samples were processed as follows: one sample was immediately centrifuged at 4000 g force for 15 min, was stored at -20°C, collected and sent pseudonymized to BRAHMS AG Hennigsdorf Germany, for the measurement of MR-proADM level using the high sensitive BRAHMS Kryptor system. The second sample was used to determine PCT, CRP and WBC on site on the day of sampling.
The reference values for the determined biomarkers were: for WBC 3.5-9.8 nl−1, for CRP<5 mg*l−1, for PCT< 0.2 ng*ml−1 and for MR-proADM 0.4 nmol*l−1. There is little data available about cut-off values for MR-proADM, WBC, CRP and PCT in the postoperative setting. In previous studies [11,12] the optimal cut-off point for MR-proADM was 1 nmol*l−1 for patients with organ dysfunction of infectious etiology. The AUC to determine the presence of sepsis in the studied group was 0.977. In a small prospective study Mokart et al. [21] found that after major surgery a PCT cut-off point set at 1.1 ng*ml−1 yielded a sensitivity of 81% and a specificity of 72% in diagnosing septic complications, in association with the occurrence of SIRS on day 1 these values reached 100% and 86%, respectively. A CRP cut-off value of >93 mg*l−1 yielded a sensitivity of 63%, specificity of 72% for septic complications, when associated with SIRS reached 100% and 80% respectively. Elevated or too low (WBC count <4 nl−1 or >12 nl−1) is a nonspecific inflammatory marker and one of the SIRS criteria. Therefore, it is not surprising that WBC count has a poor diagnostic performance for postoperative infectious and organ complications.
In this study complications were recorded for 7 days postoperatively. For statistical evaluation, patients were allocated to two groups on the basis of postoperative complications:
I. Patients without complications;
II. Patients with postoperative complications. By the severity of complications, this group was subdivided into 3 subgroups:
- Patients with a localized infection;
- Patients with sepsis;
- Patients with organ dysfunction(s).
Statistical analysis
Data were tested for normality by Kolmogorov-Smirnov test with Lilliefors correction. All data normally distributed are given as mean ± standard deviation (SD) (p>0.05 for normal distribution). Non-normally distributed data are given as median and quartiles. Pearson correlation coefficient calculation and non-parametric tests were used for the statistical analysis. We analyzed the correlation between the different biomarkers. Kruskal-Wallis test was used to compare values of the biomarkers among the different patient-groups. If a significant result (p<0.05) was found, Wilcoxon rank sum test was used for the pair-wise comparison. Statistical analyses were performed using IBM® SPSS® Statistics 22 (IBM Corporation 2013) for Windows. The accuracy of biomarkers in the diagnosis of postoperative complications was assessed by comparing the area under the receiver operating characteristics curve (AUC-ROC).
Forty-seven (15 male, 32 female) patients were included into the final statistical analysis. The groups were similar in age and body mass index, differed in coexisting diseases and gender. All patients of the abdominal surgical group had a diagnosed malignancy; all patients of the vascular group had an underlying cardiovascular disease. Patients’ characteristics are presented in Table 1.
Table 1. Patients’ baseline characteristics. Normally distributed data are given as mean ± SD (p > 0.05 for normal distribution), non-normally distributed data are given as median and quartiles. n= number; BMI = body mass index; ASA = American Society of Anaesthesiologists’ Classification; MR-proADM= mid-regional proadrenomedullin; PCT= procalcitonin; CRP= C-reactive protein; WBC= white blood cell count.
|
Orthopaedic |
Abdominal |
Vascular |
Angioplasty |
p value |
(n=13) |
(n=9) |
(n= 13) |
(n=12) |
|
Age - years |
65 ± 12 |
66 ± 12 |
68 ± 9 |
65 ± 10 |
0.94 |
Sex – n (%) |
|
|
|
|
|
Male – n (%) |
4 (30.8) |
8 (88.9) |
12 (92.3) |
8 (66.7) |
|
Female – n (%) |
9 (69.2) |
1 (11.1) |
1 (7.7) |
4 (33.3) |
|
Coexisting conditions – n (%) |
|
|
|
|
|
Cardiovascular disease |
9 (69.2) |
3 (33.3) |
10 (76.9) |
6 (50) |
|
Malignancy |
1 (7.7) |
9 (100) |
1 (7.7) |
2 (16,7) |
|
Diabetes mellitus |
1 (7.7) |
2 (22.2) |
3 (23.1) |
3 (25) |
|
Neurologic disease |
1 (7.7) |
0 (0) |
6 (46.2) |
2 (16.7) |
|
Screening lab |
|
|
|
|
|
Haemoglobin (g/dl) |
13.6 ± 1.5 |
10.6 ± 2.0 |
13.5 ± 1.9 |
13.6 ± 1.7 |
0.01 |
Serum creatinine (mg/dl) |
1.16 ± 1.03 |
1.01 ± 0.36 |
0.86 ± 0.25 |
0.99 ± 0.33 |
0.37 |
Quick (%) |
93 ± 10 |
87 ± 11 |
88 ± 13 |
102 ± 24 |
|
Baseline measurements |
|
|
|
|
|
MR-proADM (nmol/l) |
0.85 ± 0.55 |
0.86 ± 0.43 |
0.67 ± 0.20 |
0.72 ± 0.22 |
0.76 |
PCT (ng/ml) |
0.20 (0.2-1.53) |
0.10 (0.10-0.20) |
0.05 (0.05-0.10) |
0.10 (0.10-0.20) |
|
CRP (mg/l) |
5 (3-31) |
9 (1-50) |
5 (5-18) |
5 ( 5-26) |
0.68 |
WBC (/nl) |
5.6 ± 2.1 |
8.0 ± 2.9 |
7.2 ± 2.2 |
7.7 ± 1.8 |
|
ASA score – n (%) |
|
|
|
|
|
1 |
3 (23.1) |
0 (0) |
0 (0) |
0 (0) |
|
2 |
5 (38.5) |
5 (55.6) |
3 (23.1) |
4 (33.3) |
|
3 |
5 (38.5) |
3 (33.3) |
10 (76.9) |
8 (66.7) |
|
4 |
0 (0) |
1 (11.1) |
0 (0) |
0 (0) |
|
BMI (kg/m2) |
29 ± 5 |
25 ± 4 |
24 ± 4 |
26 ± 3 |
0.07 |
<18.5 |
0 (0) |
0 (0) |
1 (7.7) |
0 (0) |
|
19.0-24.9 |
3 (23.1) |
6 (66.7) |
7 (53.8) |
3 (25) |
|
25.0-29.9 |
5 (38.5) |
2 (22.2) |
3 (23.1) |
8 (66.7) |
|
30.0-34.9 |
3 (23.1) |
1 (11.1) |
2 (15.4) |
1 (8.3) |
|
35.0-39.9 |
1 (7.7) |
0 (0) |
0 (0) |
0 (0) |
|
>40.0 |
1 (7.7) |
0 (0) |
0 (0) |
0 (0) |
|
Duration of surgery (min) |
80 (58-124) |
225 (146-304) |
160 (75-280) |
52 (20-90) |
<0.01 |
Type of anaesthesia – no. (%) |
|
|
|
|
|
General |
4 (31) |
0 (0) |
10 (77) |
0 (0) |
|
Regional |
3 (23) |
0 (0) |
1 (8) |
0 (0) |
|
Local |
0 (0) |
0 (0) |
0 (0) |
100 (100) |
|
Combined |
6 (46) |
9 (100) |
2 (15) |
0 (0) |
|
MR-proADM was significantly higher with increasing American Society of Anaesthesiology (ASA) classification. In detail, MR-proADM concentration was 0.55 (0.37-0.91) nmol*l−1 in patients with ASA classification <3, and 0.77 (0.45-2.50) nmol*l−1 in patients with ASA classification ≥3 (Figure 1). In comparison the distribution of PCT, CRP and WBC was the same across ASA categories.
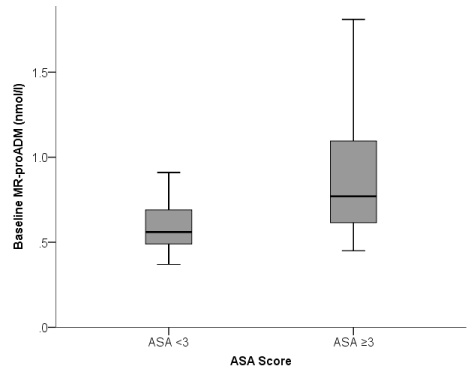
Figure 1. Box plot representing the baseline MR-proADM comparing the groups ASA <3 (n=20) and ASA ≥3 (n=27). The Wilcoxon test revealed statistical significance (p<0.01). ASA, American Society of Anaesthesiologists’ Classification, MR-proADM= mid-regional proadrenomedullin, “o”= outlier.
MR-proADM correlated with the baseline plasma creatinine (p<0.001), age (p=0.014), body mass index (p=0.011) (Table 2).
Table 2.Correlations between patients’ age, BMI, baseline serum creatinine, haemoglobin and biomarkers of inflammation.BMI = body mass index; MR-proADM= mid-regional proadrenomedullin; PCT= procalcitonin; CRP= C-reactive protein; WBC= white blood cell count.
|
|
MR-proADM |
PCT |
CRP |
WBC |
Age (years) |
Pearson correlation |
0.356 |
0.125 |
0.056 |
-0.137 |
p value |
0.014 |
0.404 |
0.711 |
0.358 |
BMI (kg/m2) |
Pearson correlation |
0.369 |
0.128 |
-0.145 |
-0.386 |
p value |
0.011 |
0.391 |
0.331 |
0.007 |
Haemoglobin (g/dl) |
Pearson correlation |
-0.250 |
0.112 |
-0.288 |
-0.292 |
p value |
0.091 |
0.452 |
0.050 |
0.047 |
Serum creatinine (mg/dl) |
Pearson correlation |
0.842 |
0.035 |
-0.116 |
-0.039 |
p value |
<0.001 |
0.814 |
0.437 |
0.796 |
Baseline MR-proADM (Figure 2), CRP, PCT and WBC were not different between the orthopaedic, abdominal and vascular surgical group (Table 1). All measured biomarker increased postoperatively compared to baseline. Among these patient-groups the highest postoperative MR-proADM levels were measured in the abdominal surgical group (Figure 2). Patients who underwent abdominal surgery had significantly higher MR-proADM levels compared to the other groups (p <0.05, Wilcoxon test). There were no significant differences between the orthopaedic and vascular surgery groups.
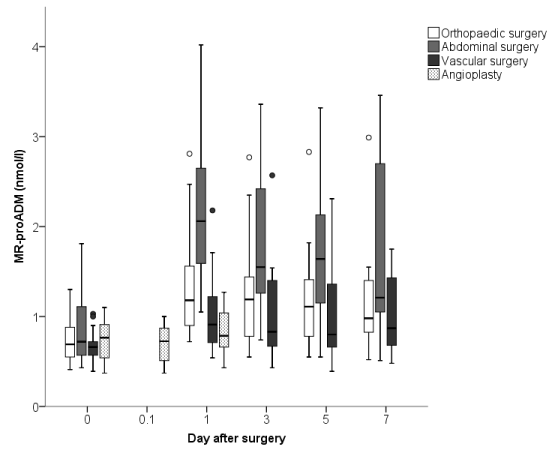
Figure 2. Box plot representing the course of MR-proADM in the first postoperative week in different surgery-groups. MR-proADM= mid-regional proadrenomedullin; day 0.1= blood sample obtained through the arterial introducer immediately after angioplasty; “o”= outlier.
Patients undergoing angioplasty (n=12) developed no increase in MR-proADM at 1 hour after the procedure compared to baseline (median 0.68 (range 0.37-1.0) nmol*l−1 vs. 0.75 (range 0.37-1.1) nmol*l−1). The day after the procedure they continued to have unchanged MR-proADM concentrations (median 0.78 (0.43-1.22) nmol*l−1), none of these patients developed complications and most of them were discharged on the first day after procedure.
In our study, 4 out of 47 patients developed localized bacterial infections: one patient developed urinary tract infection (UTI) after hip replacement surgery, 2 patients had UTI after vascular surgery and one patient of the vascular group had a central venous catheter associated infection. Two patients had bloodstream infections with sepsis. The cause of sepsis was in one case an anastomotic leakage after bowel resection, in the other case pneumonia after vascular surgery. Eleven patients presented on the first postoperative day signs of organ dysfunction (heart rate >90*min−1, respiratory rate>20*min−1, serum creatinine of more than 2.0 mg*dl−1 or diuresis of less than 0.5 ml*kg−1*h−1) without any proof of infection. The remaining patients had no complications.
WBC and CRP did not differ among the studied patient-groups (Kruskal-Wallis test, Table 3). However, patients with postoperative complications (infectious or non-infectious) developed significantly higher MR-proADM and PCT levels in comparison to patients without complications (p<0.01 on the 1st, 3rd and 5th day and p=0.04 on the 7th day, Figure 3). In patients with postoperative organ dysfunction both MR-proADM and PCT reached peak concentrations on the first postoperative day. In patients with postoperative sepsis MR-proADM rose to its maximum on day 3 (median 10.8 (range 2.57-19.1) nmol*l−1). PCT reached its highest levels in sepsis later, on the 5th postoperative day (Table 3). Patients with a localized infection did not have significantly higher MR-proADM or PCT levels in comparison to patients without complications (p=0.47 on day 1, p=0.72 on day 3, Wilcoxon test).
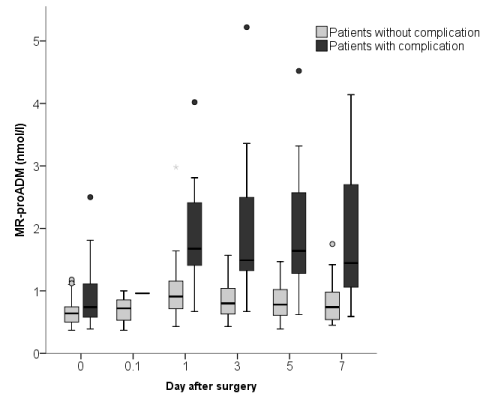
Figure 3. MR-proADM in the different complication-groups. MR-proADM= mid-regional proadrenomedullin; day 0.1= blood sample obtained through the arterial introducer immediately after angioplasty; “o”= outlier.
Table 3. Course of biomarkers in the different event-groups from baseline to postoperative day 7; MR-proADM= mid-regional proadrenomedullin; PCT= procalcitonin; CRP= C-reactive protein; WBC= white blood cell count. Data are presented as median (minimum, maximum).
Study day |
Patients without complications
(n=30) |
Localized infection
(n=4) |
Patients with
sepsis
(n=2) |
Organ dysfunction
(n=11) |
p value |
Day 0 (= Baseline) |
|
|
|
|
|
MR-proADM (nmol/l) |
0.61 (0.37-1.12) |
0.63 (0.39-1.0) |
0.88 (0.74-1.03) |
1.01 (0.57-2.50) |
0.01 |
PCT (ng/ml) |
0.1 (0.05-1.53) |
0.08 (0.05-0.2) |
0.1 (0.1-0.1) |
0.1 (0.1-0,2) |
0.58 |
CRP (mg/l) |
5 (1-31) |
5 (3-15) |
7 (5-9) |
9 (3-50) |
0.40 |
WBC (/nl) |
6.2 (3.5-10.4) |
5.3 (4.7-10.8) |
8.7 (7.5-9.9) |
7.5 (4.0-13.1) |
0.57 |
Day 0.1* |
|
|
|
|
|
MR-proADM (nmol/l) |
0.61 (0.37-0.89) |
- |
- |
0.87 (0.72-1.0) |
- |
PCT (ng/ml) |
0.1 (0.05-0.2) |
- |
- |
0.1 (0.05-0.1) |
- |
CRP (mg/l) |
5 (5-31) |
- |
- |
7 (5-12) |
- |
WBC (/nl) |
6.1 (5.2-7.8) |
- |
- |
8.2 (8.2-9.0) |
- |
Day 1 |
|
|
|
|
|
MR-proADM (nmol/l) |
0.87 (0.37-2.98) |
1.1 (0.67-2.18) |
1.67 (1.64-1.71) |
2.13 (0.77-4.02) |
<0.01 |
PCT (ng/ml) |
0.2 (0.05-12.8) |
0.2 (0.1-0.43) |
0.7 (0.1-1.3) |
0.7 (0.1-15.26) |
0.04 |
CRP (mg/l) |
29 (2-114) |
59 (24-89) |
47 (45-50) |
70 (6-162) |
0.01 |
WBC (/nl) |
9.,4 (6.2-18.2) |
8.7 (7.2-13.9) |
15.2 (11.3-19.3) |
10 (7.3-12.2) |
0.47 |
Day 3 |
|
|
|
|
|
MR-proADM (nmol/l) |
0.83 (0.43-1.57) |
1.05 (0.67-1.43) |
10.8 (2.57-19.1) |
2.24 (1.26-3.36) |
<0.01 |
PCT (ng/ml) |
0.2 (0.1-4.3) |
0.2 (0.1-0.21) |
1.15 (0.2-2.1) |
1.07 (0.5-5.31) |
0.03 |
CRP (mg/l) |
114 (10-256) |
157 (100-174) |
142 (117-167) |
142 (93-189) |
0.21 |
WBC (/nl) |
8.8 (4.1-15.1) |
8.0 (5.1-10.1) |
11.7 (9.6-13.8) |
9 (4.6-10.7) |
0.24 |
Day 5 |
|
|
|
|
|
MR-proADM (nmol/l) |
0.8 (0.39-1.47) |
1.01 (0.62-1.39) |
8.4 (2.31-14.6) |
1.79 (1.15-3.32) |
<0.01 |
PCT (ng/ml) |
0.2 (0.05-2) |
0.15 (0.1-2.4) |
45.95 (2.7-89.2) |
0.6 (0.2-1.72) |
0.02 |
CRP (mg/l) |
56 (22.3-139) |
96 (38-230) |
285 (234-337) |
71 (37-380) |
0.14 |
WBC (/nl) |
8.3 (4.9-13.8) |
6.4 (6.1-9.0) |
13.2 (10.0-16.4) |
7.3 (5.3-17.8) |
0.43 |
Day 7 |
|
|
|
|
|
MR-proADM (nmol/l) |
0.92 (0.48-1.75) |
0.95 (0.59-1.43) |
5.99 (1.46-10.5) |
1.58 (1.1-3.46) |
0.01 |
PCT (ng/ml) |
0.2 (0.05-1.75) |
0.15 (0.1-0.6) |
23.35 (0.7-46.0) |
0.4 (0.1-0.79) |
0.09 |
CRP (mg/l) |
40 (14-217) |
55 (19-92) |
137 (117-158) |
44 (28-276) |
0.10 |
WBC (/nl) |
7.8 (4.9-12.2) |
6.6 (6.1-9.1) |
12.2 (11.8-12.6) |
7.3 (5.2-17) |
0.32 |
In patients with mild or moderate organ dysfunction (n=11) MR-proADM was significantly higher than in patients without complications (p=0.01). These elevated baseline MR-proADM increased slightly on the first day after surgery (p=0.1, Wilcoxon test) and then remained unchanged throughout the study.
The accuracy of biomarkers in distinguishing patients with postoperative infectious complications or organ dysfunction (with higher need of monitoring and treatment) from patients without any postoperative complication was assessed by comparing ROC curves (Figure 4-8). On day 1, at a cut-off point set at 1.2 nmol*l−1 MR-proADM yielded a sensitivity of 81.3% and a specificity of 83.9%. On day 3 these values reached 92.3%% and 81.8%, respectively (Table 4 and 5). At a cut-off point set at 1.0 ng*ml−1, PCT yielded at day 1 a sensitivity of 37.5 % and a specificity of 87.1%. On day 3 these values reached 38.5%% and 81.8%, respectively (Table 4 and 5). At a cut-off point set at 71 mg*l−1 on day1, CRP proved to be similar valuable in the prognosis of postoperative organ failure or infectious complication as PCT. On day 3 a CRP value >71 mg*l−1 showed a very good sensitivity (100%) and a specificity of 72.7% for postoperative organ dysfunction or infectious complications (Table 4 and 5). WBC count had a poor diagnostic performance.
We investigated the utility of MR-proADM in comparison to PCT, CRP and WBC in the diagnosis of postoperative infectious complications and identification of patients at risk to develop organ dysfunctions. The topic of this study is of interest because it is desirable to identify patients in risk of postoperative complications in order to optimize the treatment and reduce the length of stay at hospital. Based on this data, MR-proADM was found to have a good performance in identifying or even predicting patient’s infectious and non-infectious complications regardless of the underlying disease. In predicting postoperative sepsis MR-proADM was earlier elevated compared to PCT and CRP.
In a large number of healthy individuals [16,22] MR-proADM was found to follow a Gaussian distribution with a mean (SD) of 0.33 (±0.07) nmol*l−1, respectively a median level of 0.41 (range 0.23-0.64) nmol*l−1.
Several previous studies investigated MR-proADM in non-operative patients. In a large cohort study of 501 patients with chronic heart failure [13] patients’ baseline MR-proADM value was in median 0.64 (0.11-3.30) nmol*l−1. In 146 patients with lower respiratory tract infection [23], baseline MR-proADM concentration was 1.12 (0.81-2.11) nmol*l−1. In 128 febrile critically ill patients in the Emergency Department [24] MR-proADM has a median value of 0.85 (0.50-1.68) nmol*l−1. A previous study in 101 critically ill patients [25] showed in patients with SIRS a median MR-proADM level of 1.1 (0.3-3.7) nmol*l−1, in patients with sepsis 1.8 (0.4-5.8) nmol*l−1, in patients with severe sepsis 2.3 (1.0-17.6) nmol*l−1 and in patients with septic shock 4.5 (0.9-21) nmol*l−1.
A recent cohort study of 388 septic patients [26] demonstrated that PCT and MR-proADM are complementary markers in establishing the correct diagnosis and prognosis of severe sepsis and septic shock. Determined in the first 24 h of ICU admission PCT showed higher diagnostic value, MR-proADM exhibited a relevant prognostic benefit, especially used together with the APACHE-II score.
Another recent study in septic patients [27] MR-proADM was not superior to PCT or CRP regarding the prediction of disease severity or mortality, however the combination of SOFA, APACHE II scores and MR-proADM was efficient to predict prognosis and mortality rate in severe sepsis or septic shock patients. Other authors [28] concluded that the combination of PCT with other markers should expedite the diagnosis and treatment of sepsis optimizing clinical management.
Only a few previous studies investigated MR-proADM in the operative field, focusing on cardiac surgery. A prospective observational cohort study [29] in a single-center academic medical hospital analyzed in 746 consecutive patients undergoing elective cardiac surgery the value of preoperative levels of inflammatory biomarkers. The conclusion of the study was that in elective cardiac surgery, preoperative levels of MR-proADM and CT-proET-1 are predictors of 30-day mortality and could improve the predictive accuracy of the currently used EuroSCORE. Another study [30] included 153 patients suffering from severe aortic stenosis and identified MR-proADM as a novel predictor of mortality in patients undergoing transcatheter aortic valve replacement.
Compared to the given reference range the preoperative MR-proADM levels in our patients were higher (0.77±0.37 nmol*l−1). Thus, our data suggest that preoperative MR-proADM levels in elective surgical patients are slightly higher than in healthy adults. This may be explained by the fact that our patients suffered from various co-morbidities (Table 2). In detail, orthopaedic and vascular patients had a high rate of cardiovascular disease and 100% of the abdominal surgical patients had an underlying cancer diagnosis. There is evidence [15] that MR-proADM is a plausible prognostic biomarker in critically ill patients with malignancy.
According to our results, MR-proADM is higher with increasing ASA classification. The ASA score is used to assess the patient's pre-operative physical status, and to quantify the amount of physiological reserve that a patient possesses at the time at which they are assessed for a surgical procedure [31]. One major limitation of the ASA score is the subjective measure conferred by the practitioner rather than an objective measure determined by the presence of specific disease states. The correlation between ASA classification and postoperative mortality has been shown in previous studies. Wolters et al. [32] examined the strength of association between ASA score and postoperative complications in 6301 surgical patients and found that the risk of complication was influenced mainly by ASA score 4 (risk odds ratio=4.2) and ASA score 3 (risk odds ratio=2.2). In the EuSOS study with 46539 patients, the ASA score was again identified as an independent factor associated with mortality: the ASA score 3 had an odds ratio of 1.51, the ASA score 4 of 6.75 [17]. So far, the correlation between ASA score and MR-proADM has never been studied before. Our finding (Figure 1) might support the role of MR-proADM in the operative medicine. It also demonstrates, that this new biomarker reflects the general health status. The advantages of MR-proADM are its objective measurement and reliability. It might be an additional tool for more accurate risk stratification. Comparing the ROC curves of the preoperative inflammatory markers in identifying patients at risk to develop organ dysfunctions with higher need of monitoring in the first 7 postoperative days (Figure 4) MR-proADM had the highest prognostic value with an AUC of 87% (95% confidence interval 75.7-97.6). However, adequate future clinical studies are required to validate MR-proADM for risk stratification.
We found a linear correlation between the baseline MR-proADM levels and plasma creatinine (R2=0.71). Previous publications [4, 14] already described increased values of MR-proADM in patients with renal dysfunction. For instance, Suberviola et al. [11] described only slightly higher levels of MR-proADM in patients with plasma creatinine of > 2 mg*dl−1. In comparison to our results, Christ-Crain et al. [25] found a similar but somewhat weaker correlation between plasma creatinine and MR-proADM in 101 critically ill medical patients (R²=0.58; p<0.001). It remains unclear, whether increased MR-proADM in renal dysfunction is related to an overproduction or a reduced renal elimination.
With respect to abdominal surgical patients, the only data are available on adrenomedullin (ADM; MR-proADM is a more stable precursor of ADM). Fujioka [33] reported in 18 patients undergoing major abdominal and thoraco-abdominal surgery increased postoperative plasma adrenomedullin levels. However, this author did not find significant differences between different types of surgical or anaesthetic procedures. Notably, plasma concentrations of adrenomedullin significantly correlated with the duration of surgery which might have been related to the larger cellular damage due to prolonged surgery time. For comparison, our data revealed a poor correlation (R2=0.22) between MR-proADM on the postoperative day 1 and duration of surgery and non-significant correlations on the postoperative day 3 (R2=0.04) and later on.
In general, major surgery and trauma do significantly activate the immune system [34, 35]. Due to the activation of the immune system and elevation of biomarkers for sepsis and cytokines, post-traumatic complications such as new-onset postoperative infections are difficult to diagnose [35]. Clinically, biomarkers like WBC, CRP and PCT are commonly used to diagnose postoperative complications, e.g. infections. However, all of them have their limitations in sensitivity and specificity.
So far, MR-proADM was found to be well correlated with severity of sepsis and mortality [11, 37] and has been adopted as a parameter to predict the severity of heart failure [10], lower respiratory tract infections [23, 38-41] or sepsis [25]. Its use might additionally be of advantage in planning and guiding treatment but only in combination with other biomarkers, e.g., PCT. MR-proADM might be a very useful tool in the perioperative medicine in detecting postoperative complications. In our study, patients with postoperative complications (infectious or non-infectious) developed significantly higher MR-proADM and PCT levels in comparison to patients without complications (Figure 3). MR-proADM in plasma correlated with the severity of the postoperative complication. During the early postoperative period, on day 1 and day 3, MR-proADM had the highest discriminative value with AUC of 87.4 and 88.8% respectively in diagnosing patients with organ dysfunction and septic complications during the first 7 postoperative days.
These findings may aid early therapeutic intervention in high-risk surgical patients. In patients without postoperative events or with a localized postoperative infection the MR-proADM did not change significantly (Table 3). In patients with a postoperative organ dysfunction, MR-proADM nearly doubled in the course of the first to third postoperative day. However, MR-proADM levels significantly increased, up to tenfold above baseline levels, in patients who developed sepsis. Compared to other measured biomarker, plasma MR-proADM raised in sepsis earlier, thus giving potential to initiate diagnostic and therapeutic measures at an earlier stage.
Severity in septic patients is considered when perfusion disorders or organ dysfunction are present . Early identification of patients with developing organ dysfunction could help initiate early treatment and improve prognosis. The widespread production of MR-proADM helping maintain the blood supply to individual organs Compared to other measured biomarker, plasma MR-proADM raised in sepsis earlier, thus giving potential to initiate diagnostic and therapeutic measures at an earlier stage.
Our study has several limitations. First, the small number of enrolled subjects may limit the generalizability of the results. Out of 47 patients only 6 developed postoperative complications, 2 patients (4.3%) developed bloodstream infections with sepsis, 4 patients (8.5%) had localized infections. The therapeutic decisions were made by the assigned study physician according to each hospital’s standard operating procedure based upon clinical signs and measured PCT, CRP and WBC on site, without considering the MR-proADM results which were obtained from the external lab several months later. Other limitations might be the differences in interventions and care applied to the study groups in the different settings.
In the perioperative setting, MR-proADM has a promising role in the early detection or identification of infectious complications and organ dysfunction and might be a useful tool in identifying patients with the need of higher level of care and treatment. MR-proADM might be an additional very useful tool for accurate risk stratification. Our data are of importance because they demonstrate that using MR-proADM in the early postoperative period it is possible to detect organ dysfunctions, which could indicate a disturbed perfusion due to postoperative infectious complication. Compared to other measured biomarker, plasma MR-proADM raised in septic complications earlier, thus giving potential to initiate diagnostic and therapeutic measures at an earlier stage. Identification of patients with the need of early therapeutic intervention may favourably influence outcome.
In order to elaborate a perioperative risk-stratification using the MR-proADM a larger prospective clinical study is planned.
- Preoperatively MR-proADM increased with higher ASA classification.
- Postoperative MR-proADM concentration correlated with the occurence of postoperative organ dysfunctions.
- MR-proADM raised in septic complications earlier.
- MR-proADM correlated with plasma creatinine.
- MR-proADM had the highest discriminative value with AUC of 87.4 and 88.8% respectively in diagnosing patients with organ dysfunction and septic complications during the first 7 postoperative days.
- Peripheral arterial angioplasty without major surgery is not associated with an induction of MR-proADM release.
- For adequate perioperative risk stratification with the help of MR-proADM further larger studies are needed.
Preliminary results were presented as poster at the Hauptstadtkongress (HAI-Congress) in Berlin, Germany on 18th September 2014, entitled “Eignung des Laborparameters Proadrenomedullin (proADM) für die perioperative Risikostratifizierung- eine Pilotstudie” (PO1;1.4). The abstract was published as: Egyed E, Defosse J, Schröder S, Hering R, Lefering R, Wappler F, Sakka S: Eignung des Laborparameters Proadrenomedullin (proADM) für die perioperative Risikostratifizierung- eine Pilotstudie. Anästhesiologie & Intensivmedizin 2014; 55: 476.
EE helped to design the study, collected and analysed the data, performed the statistical analysis and drafted the manuscript. JMD helped to design the study, to interpret the data and to draft the manuscript. SS, SR, RH and SB helped to design the study and collecting data. FW participated in the design of the study and the statistical analysis, did the critical revision of this manuscript. SGS conceived the study, participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
The authors declare that they have no competing financial interests. The study was realized without any financial support.
The authors declare that they have no non-financial competing interests.
Thermo Fisher Scientific/B.R.A.H.M.S. GmbH analyzed the biomarker samples.
- Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, et al. (1993) Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun 192: 553-560. [Crossref]
- Hinson JP, Kapas S, Smith DM (2000) Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev 21: 138-167. [Crossref]
- Linscheid P, Seboek D, Zulewski H, Keller U, Müller B (2005) Autocrine/paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology 146: 2699-2708. [Crossref]
- Nishikimi T (2007) Adrenomedullin in the kidney-renal physiological and pathophysiological roles. Curr Med Chem 14: 1689-1699. [Crossref]
- Pio R, Martinez A, Unsworth EJ (2001) Complement factor H is a serum-binding protein for adrenomedullin, and the resulting complex modulates the bioactivities of both partners. J Biol Chem 276: 12292-12300.
- http://www.MR-proADM.com
- Eto T (2001) A review of the biological properties and clinical implications of adrenomedullin and proadrenomedullin N-terminal 20 peptide (PAMP), hypotensive and vasodilating peptides. Peptides 22: 1693-1711. [Crossref]
- Kubo A, Minamino N, Isumi Y, Katafuchi T, Kangawa K, et al. (1998) Production of adrenomedullin in macrophage cell line and peritoneal macrophage. J Biol Chem 273: 16730-16738. [Crossref]
- Wong HK, Cheung TT, Cheung BM (2012) Adrenomedullin and cardiovascular diseases. JRSM Cardiovasc Dis 1. [Crossref]
- Krüger S, Ewig S, Giersdorf S, Hartmann O, Suttorp N, et al. (2010) Cardiovascular and inflammatory biomarkers to predict short- and long-term survival in community-acquired pneumonia: Results from the German Competence Network. Am J Respir Crit Care Med 182: 1426-1434. [Crossref]
- Suberviola B, Castellanos-Ortega A, Llorca J, Ortiz F, et al. (2012) Prognostic value of proadrenomedullin in severe sepsis and septic shock patients with community-acquired pneumonia. Swiss Med Wkly 19: 142. [Crossref]
- Guignant C, Voirin N, Venet F, Poitevin F, Malcus C, et al. (2009) Assessment of pro-vasopressin and pro-adrenomedullin as predictors of 28-day mortality in septic shock patients. Intensive Care Med 35: 1859-1867. [Crossref]
- von Haehling S, Filippatos GS, Papassotiriou J, Cicoira M, Jankowska EA, et al. (2010) Mid-regional pro-adrenomedullin as a novel predictor of mortality in patients with chronic heart failure. Eur J Heart Fail 12: 484-491. [Crossref]
- Al Shuaibi M, Bahu RR, Chaftari AM, Al Wohoush I, Shomali W, et al. (2013) Pro-adrenomedullin as a novel biomarker for predicting infections and response to antimicrobials in febrile patients with hematologic malignancies. Clin Infect Dis 56: 943-950. [Crossref]
- Debiane L, Hachem RY, Wohoush IA, Shomali W, Bahu RR, et al. (2014) The Utility of Proadrenomedullin and Procalcitonin in Comparison to C - reactive protein as Predictors of Sepsis and Bloodstream Infections in Critically Ill Patients With Cancer. Crit Care Med 42: 2500-2507. [Crossref]
- Morgenthaler NG, Struck J, Alonso C, Bergmann A (2005) Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem 51: 1823-1829. [Crossref]
- Pearse RM, Moreno RP, Bauer P, Pelosi P, Metnitz P, et al. (2012) Mortality after surgery in Europe: a 7 day cohort study. Lancet 380: 1059-1065. [Crossref]
- Valenzuela-Sánchez F, Valenzuela-Méndez B, Rodríguez-Gutiérrez JF, Estella-García Á, González-García MÁ (2016) New role of biomarkers: mid-regional pro-adrenomedullin, the biomarker of organ failure. Ann Transl Med 4: 329. [Crossref]
- Angeletti S, Battistoni F, Fioravanti M, Bernardini S, Dicuonzo G (2013) Procalcitonin and mid-regional pro-adrenomedullin test combination in sepsis diagnosis. Clin Chem Lab Med 51: 1059-1067. [Crossref]
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, et al. (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101: 1644-1655. [Crossref]
- Mokart D, Merlin M, Sannini A, Brun JP, Delpero JR, et al. (2005) Procalcitonin, interleukin 6 and systemic inflammatory response syndrome (SIRS): early markers of postoperative sepsis after major surgery. Br J Anaesth 94: 767-773. [Crossref]
- Smith JG, Newton-Cheh C, Hedblad B, Struck J, Morgenthaler NG, et al. (2009) Distribution and correlates of midregional proadrenomedullin in the general population. Clin Chem 55: 1593-1595. [Crossref]
- Albrich WC, Rüegger K, Dusemund F, Bossart R, Regez K, et al. (2011) Optimised patient transfer using an innovative multidisciplinary assessment in Kanton Aargau (OPTIMA I): an observational survey in lower respiratory tract infections. Swiss Med Wkly 141: 132-137. [Crossref]
- Travaglino F, De Berardinis B, Magrini L, Bongiovanni C, Candelli M, et al. (2012) Utility of Procalcitonin (PCT) and Mid regional pro-Adrenomedullin (MR-proADM) in risk stratification of critically ill febrile patients in Emergency Department (ED). A comparison with APACHE II score. BMC Infect Dis 12: 184. [Crossref]
- Christ-Crain M, Morgenthaler NG, Struck J, Harbarth S, Bergmann A, et al. (2005) Mid-regional pro-adrenomedullin as a prognostic marker in sepsis: an observational study. Crit Care 9: R816-824. [Crossref]
- Enguix-Armada A, Escobar-Conesa R, La Torre AG, De La Torre-Prados MV (2015) Usefulness of several biomarkers in the management of septic patients: C-reactive protein, procalcitonin, presepsin and mid-regional pro-adrenomedullin. Clin Chem Lab Med 54: 163-168. [Crossref]
- Akpinar S, Rollas K, Alagöz A, Seğmen F, Sipit T (2014) Performance evaluation of MR-proadrenomedullin and other scoring systems in severe sepsis with pneumonia. J Thorac Dis 6: 921-929. [Crossref]
- Angeletti S, Dicuonzo G, Fioravanti M, De Cesaris M, Fogolari M, et al. (2015) Procalcitonin, MR-Proadrenomedullin, and Cytokines Measurement in Sepsis Diagnosis: Advantages from Test Combination. Dis Markers 2015: 951532. [Crossref]
- Schoe A, Schippers EF, Ebmeyer S, Struck J, Klautz RJ, et al. (2014) Predicting mortality and morbidity after elective cardiac surgery using vasoactive and inflammatory biomarkers with and without the EuroSCORE model. Chest 146: 1310-1318. [Crossref]
- Csordas A, Nietlispach F, Schuetz P, Huber A, Müller B, et al. (2015) Midregional Proadrenomedullin Improves Risk Stratification beyond Surgical Risk Scores in Patients Undergoing Transcatheter Aortic Valve Replacement. PLoS One 10: e0143761. [Crossref]
- Fitz-Henry J (2011) The ASA classification and peri-operative risk. Ann R Coll Surg Engl 93: 185-187. [Crossref]
- Wolters U, Wolf T, Stützer H, Schröder T (1996) ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth 77: 217-222. [Crossref]
- Fujioka S (2001) Increased plasma concentration of adrenomedullin during and after major surgery. Surg Today 31: 575-579. [Crossref]
- Kashiwabara M, Miyashita M, Nomura T, Makino H, Matsutani T, et al. (2007) Surgical trauma-induced adrenal insufficiency is associated with postoperative inflammatory response. J Nippon Med Sch 74: 274-283. [Crossref]
- Desborough JP (2000) The stress response to trauma and surgery. Br J Anaesth 85: 109-117. [Crossref]
- Stubljar D, Skvarc M (2015) Effective Strategies for Diagnosis of Systemic Inflammatory Response Syndrome (SIRS) due to Bacterial Infection in Surgical Patients. Infect Disord Drug Targets 15: 53-56. [Crossref]
- Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, et al. (1998) Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Crit Care Med 26: 1793-1800. [Crossref]
- Schuetz P, Wolbers M, Christ-Crain M, Thomann R, Falconnier C, et al. (2010) Prohormones for prediction of adverse medical outcome in community-acquired pneumonia and lower respiratory tract infections. Crit Care 14: 106. [Crossref]
- Bello S, Lasierra AB, Mincholé E, Fandos S, Ruiz MA, et al. (2012) Prognostic power of proadrenomedullin in community-acquired pneumonia is independent of aetiology. Eur Respir J 39: 1144-1155. [Crossref]
- Albrich WC, Dusemund F, Rüegger K, Christ-Crain M, Zimmerli W, et al. (2011) Enhancement of CURB65 score with proadrenomedullin (CURB65-A) for outcome prediction in lower respiratory tract infections: derivation of a clinical algorithm. BMC Infect Dis 11: 112. [Crossref]
- Guertler C, Wirz B, Christ-Crain M, Zimmerli W, Mueller B, et al. (2011) Inflammatory responses predict long-term mortality risk in community-acquired pneumonia. Eur Respir J 37: 1439-1446. [Crossref]



