The unpredictable chronic mild stress (UCMS) mouse model of depression causes a variety of neuronal structural alterations ranging from dendritic spines loss and dendrite arbor retraction to spine proliferation in brain regions involved in the control of mood. Based on data showing that the plant stress hormone methyl jasmonate (MJ: methyl acetate-2,2-d2) mimics the effect of imipramine in rescuing the UCMS-induced depressive behavioral phenotype, here we examine whether the UCMS protocol used to demonstrate the antidepressant action of the compound triggers structural alterations in basolateral amygdala, hippocampus and prefrontal cortex which can be rescued by MJ treatment. Male C57BL/6 mice were injected with MJ (50 mg/kg) or saline (SAL) before each of the two daily exposures to UCMS administered over a 10-day period. Home cage, daily manipulated, SAL-injected mice served as controls (CTRL). On day 11, mice were sacrificed for Golgi-staining. Results show that in comparison with CTRL mice, SAL+UCMS mice exhibit a massive reduction in spine density and dendritic arbor extension/complexity in the three regions examined. Remarkably, MJ+UCMS mice show an alleviation of neuronal structural alterations in the three regions, with a more complete recovery observed in the prefrontal cortex. Our data provide further validation of the antidepressant action of the compound by revealing its efficacy in globally preventing the collapse in synaptic density and neuronal connectivity identified as key nodes of the mood circuitry.
dendritic arbor, dendritic spines, depression, Methyl Jasmonate, Unpredictable Chronic Mild Stress (UCMS)
Unpredictable chronic mild stress (UCMS) is a well-validated rodent model of depression [1-3] based on the presentation of multiple minor stressors which gradually induce a learned helplessness state and produce anhedonia-like symptoms [4]. Extensive evidence indicates that the UCMS-induced depressive phenotype associates with dendrite atrophy and spine loss in the hippocampus [5-7] and the prefrontal orbital cortex [8], and with a reduction of synaptic density [9] or an increase in spines [10] in the amygdala. Molecular dysregulations involved in these structural alterations include downregulation of brain-derived neurotrophic factor (BDNF), cAMP-response element binding protein (CREB), and calcium/calmodulin-dependent protein kinase II (CaMKII) pathways [11] with an established role in synaptic plasticity, and reduced expression of proteins implicated in cytoskeletal reorganization [12-14] and synaptic activity [15,16].
Methyl jasmonate-(MJ: methyl acetate-2,2-d2) is a phytohormone endowed with antidepressant properties. Specifically, MJ potentiates the toxic effect of yohimbine in the yohimbine lethality test, and reduces the immobility time in the forced swim (FS) and tail suspension tests [17-19]. Of note, MJ and imipramine similarly alleviate anhedonia in the sucrose preference test, prevent the increase in serum corticosterone levels, attenuate neuro-inflammation, and decrease oxidative stress markers in brain extracts [20].
Although the current challenge is to verify that drugs which reduce depressive symptoms also rescue the structural abnormalities of neurons, only few studies support this possibility [21-23]. Accordingly, here we examine if the UCMS protocol used to demonstrate the antidepressant action of the compound triggers a specific pattern of structural alterations of neurons in key brain regions of the mood circuitry - basolateral amygdala, hippocampus and prefrontal cortex - which can be rescued by MJ treatment.
Animals: We used male C57BL/6J@Ico (C57) mice which were 9-week-old at the beginning of the experiments. They were housed 5 per cage and maintained in a temperature-controlled facility (22 ± 1°C) on a 12:12 h light-dark cycle with free access to food and water. All experimental procedures were conducted in accordance with the official European Guidelines for the care and use of laboratory animals (86/609/EEC).
Methyl jasmonate treatment: 1,2 µl of Methyl jasmonate (MJ: methyl acetate-2,2-d2, Sigma-Aldrich) was dissolved in 1.2 µl of ethanol (95%). This solution was further diluted in 197.6 µl of distilled water to get a 200 µl of 50 mg/kg dose for each mouse of an average weight of 25 g. This dose was chosen on the basis of data showing that it produced maximal rescue of UCMS-induced behavioural indexes of depression [17]. Saline (SAL) was used as vehicle.
Experimental design and UCMS protocol: Mice (N=10 per group) were randomly assigned to the following three experimental conditions: SAL+UCMS (Stress), MJ+UCMS (MJ+Stress), and home cage+SAL (CTRL). The UCMS protocol was a revised version of the one described by Yalcin et al., 2008 [24] and Wilner et al., 2017 [3]. It consisted of exposing mice twice per day to one of the 10 stressors listed in table 1 over 10 consecutive days. The UCMS schedule was delivered on random time intervals to avoid habituation or foresight. Control mice did not experience the stressors but were handled daily in the experimental room for 5 minutes.
Golgi staining: On day 11, mice were deeply anaesthetized with a cocktail of Zoletil (800 mg/kg) and Rompum (200 mg/kg) and perfused transcardially with 0.9% saline solution (N = 7 mice per group). Brains were dissected and immediately immersed in a Golgi-Cox solution (1% potassium dichromate, 1% mercuric chloride, and 0.8% potassium chromate) at room temperature for 6 days. On the seventh day, brains were transferred in a 30% sucrose solution for cryoprotection and then sectioned with a vibratome. Coronal sections (100 μm) were collected and stained according to the method described by Gibb and Kolb (1998) [25]. Sections were stained through consecutive steps in water (1 minute), ammonium hydroxide (30 minutes), water (1 minute), developer solution (Kodak fix 100%, 30 minutes), and water (1 minute). Sections were then dehydrated through successive steps in alcohol at rising concentrations (50%, 75%, 95%, and
100%) before being closed with slide cover slips. Neurons were identified with a light microscope (Leica DMLB) under low magnification (20×/NA 0.5). In each region, six fully impregnated neurons (three per hemisphere) displaying dendritic trees without obvious truncations and isolated from neighboring impregnated neurons were retained for the analysis [26]. Because no interhemispheric difference was detected, the data were pooled so that 6 neurons per region and per animal were considered in each analysis. Measurements were carried out using a microscope (DMLB, Leica) equipped with a motorized stage and a camera connected to a software for morphological analyses allowing quantitative 3D analysis of complete dendritic arbor (Neurolucida 7.5; MicroBrightField, Inc.).
Morphological analysis
Spine density: Five 30-100 μm dendritic segments of secondary and tertiary branch order of CA1 pyramidal neuron basal and apical dendrites, BLA spiny neurons, and layer V prelimbic cortex pyramidal neurons were randomly selected and counted using Neurolucida software. Only protrusions with a clear connection of the head of the spine to the shaft of the dendrite were counted as spines. Statistical comparisons were made on single neuron values obtained by averaging the number of spines counted on segments of the same neuron. Inter-regional comparisons were made after spine density scores variations were normalized to the CTRL group in each region.
Dendritic Arbor: The dendritic arbor of BLA spiny neurons, and of CA1 and PFC pyramidal neurons (apical and basal) selected for spine density counts were traced by means of Neurolucida software. Three parameters were examined: total dendritic length, number of nodes, and number of endings. Dendritic arbor complexity was then evaluated by Sholl analysis. Briefly, in each region, the number of neuron dendrite intersections with concentric circles traced at increasing radial distances (segment radius: 25 μm) from the center of the soma was counted by adding up all values in each successive radius.
Statistical analysis: Two-way ANOVAs with group and brain region as main factors were used for statistical comparison of dendritic spines, dendrite length, number of nodes, number of endings and PV+ immuno- reactive puncta. Post hoc pair comparisons were carried out where necessary using the LSD test. In each region, the number of dendrite intersections with concentric circles was calculated and compared among groups by means of a two-way ANOVA with group and radius distance from the soma as main factors.
Methyl jasmonate rescues UCMS-induced dendritic spine loss
Data are shown in figure 1. Histograms (Figure 1D) report spine density data in each group x region condition. There was a significant effect of group [F2,129 = 330,4, p < 0.001], of region [F2,129 = 3,40, p < 0.05], and a significant group x region interaction [F4,129 = 3,08, p < 0.05]. Pair comparisons showed that SAL-injected mice exposed to UCMS exhibited significantly less spines than their counterparts non-exposed to UCMS (Stress vs CTRL, p< 0.001 for all regions). Remarkably, MJ treatment significantly increased dendritic spines in the stressed mice (MJ+Stress vs Stress, p < 0.001 for all regions). The treatment, however, produced only a partial rescue of spines, since they were still more numerous in the Control group (CTRL vs MJ+ Stress, p < 0.01 for all regions). Of note, the partial rescue was stronger in PFC where more spines were counted than in BLA and CA1 (PFC vs BLA, p < 0.001; PFC vs CA1, p < 0.05).
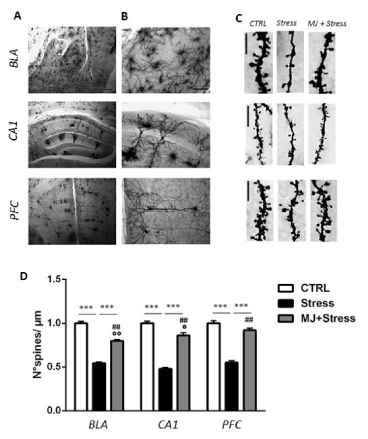
Figure 1. (A-B) Photomicrographs of a Golgi-stained sections of basolateral amygdala (BLA), dorsal hippocampus (CA1) and prefrontal cortex (PFC) at 5x (A) and 20x (B) magnification (scale bar: 250 μm (A); 50 μm (B). (C) Representative dendritic segments from BLA, CA1 and PFC neurons in control (CTRL), stressed (Stress), and treated (MJ+Stress) mice (scale bar: 5 μm). (D) Histograms showing spine density values (number of spines/μm) in BLA, CA1 and PFC neurons from CTRL, Stress, and MJ+Stress mice groups. Data are plotted as mean +/- s.e.m. *** p<0.001: Stress vs CTRL or MJ+Stress; ## p<0.01: CTRL vs MJ+Stress; °°p<0.01: BLA vs PFC, °p<0.05: CA1 vs PFC
Methyl jasmonate rescues UCMS-induced dendrite retraction
Dendrite length: (Figures 2D-2F) There was a significant effect of group [F2,130 = 87,56, p < 0.001], of region [F2,130 = 16,92, p < 0.01], but no effect of the group x region interaction [F4,130 = 0,92, p = 0.454]. Dendrites were significantly shorter in SAL-injected mice exposed to UCMS than in their counterpart non-exposed to UCMS (Stress vs CTRL, p < 0.001 for all regions). Remarkably, MJ significantly increased dendritic length in the stressed mice (MJ+Stress vs Stress, p < 0.001 for all regions). This increase partially rescued dendrite retraction in BLA and CA1 neurons since dendritic length values were still higher in the CTRL group than in the MJ+Stress group (BLA: p < 0.05; CA1: p < 0.001). Differently, a full rescue was found in PFC neurons (CTRL vs MJ+Stress, p = 0.40).
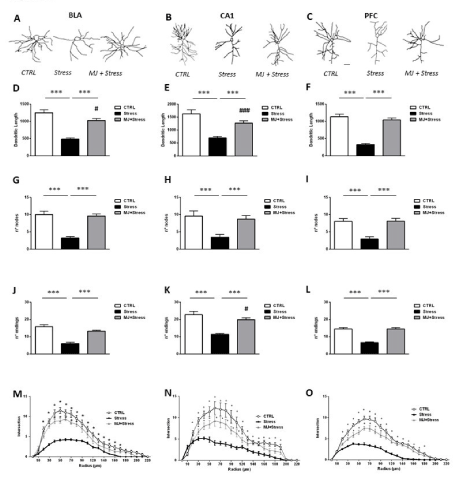
Figure 2. Representative traces of the dendritic arbor extension from basolateral amygdala (BLA) (A), dorsal hippocampus (CA1) (B) and prefrontal cortex (PFC) (C) in control (CTRL), stressed (Stress), and treated (MJ+Stress) mice (scale bar: 50 μm). Total dendritic length (D-F), number of nodes (G-I) and number of endings (J-L) counted in BLA, CA1 and PFC from control (CTRL), stressed (Stress), and treated (MJ+Stress) mice. (M-O) curves depict Sholl analysis data (number of dendrite intersections with concentric circles traced at increasing radial distances from the soma; segment radius: 25 μm) in BLA (M) CA1 (N) and PFC (O). Data are plotted as mean +/- s.e.m. *** p<0.001: Stress vs CTRL or MJ+Stress. ### p<0.001, #p<0.05: CTRL vs MJ+Stress
Number of nodes: (Figures 2G-2I) There was a significant effect of group [F2,130 = 41,72, p < 0.001] but no effect of region [F2,130 = 1,46, p = 0.238] or of the group x region interaction [F4,130 = 0,290, p = 0.887]. Less nodes were counted in SAL-injected mice exposed to UCMS than in their non-exposed counterparts (Stress vs Control, p < 0.001 for all regions). MJ fully rescued this morphological defect as more nodes were counted in the MJ+Stress group compared to the Stress group (p < 0.001 for all regions), and no difference in the number of nodes was found between CTRL and MJ+Stress groups (p > 0.1 for all regions).
Number of endings: (Figures 2J-2L) There was a significant effect of group [F2,130 = 81,37, p < 0.001] of region
[F2,130= 39,84, p > 0.001] but no effect of the group x region interaction [F4,130 = 1,03, p = 0.397]. Less endingswere counted in saline-injected mice exposed to UCMS than in their counterpart non –exposed to UCMS (Stress vs CTRL, p < 0.001 for all regions). MJ significantly increased the number of endings in neurons from all regions (MJ+Stress vs Stress p < 0.001). The number of endings was fully rescued in BLA and PFC neurons (CTRL vs MJ+Stress p > 0.05 for each region), but partially rescued in CA1 neurons as more endings were still counted in the CTRL group than in the MJ+Stress group (p < 0.05).
Dendritic arbor complexity: (Figures 2M-2O) The data are shown in figure 2. In each region, statistical analyses revealed a significant effect of group [BLA: F2,546 = 43,32; CA1: F2,860 = 56,60; PFC: F2,817 = 59,10, p < 0.001 for all comparisons], of radius [BLA: F13,546 = 91,69; CA1: F20,860= 175,5; PFC: F19,817 = 143,00, p < 0.001] and of the group x radius interaction [F26,546 = 6,27; CA1: F40,860 = 12.03; PFC: F38,817= 10,47, p < 0.001]. For BLA and CA1, post hoc comparisons showed that dendrites lying 30/40–110 μm from the soma intersected concentric circles significantly less in the Stress group than in the CTRL and MJ+Stress groups where the number of intersections was in the same range. For PFC, the same decreased number of intersections was found in the Stress group compared to the CTRL group but for dendrites lying farther from the soma (100-200 μm). Notably, the MJ + Stress group started to show more intersections than the Stress group already at the 60 μm radius. Altogether, these findings reveal that the treatment restored dendritic arbor complexity in all regions although a more complete recovery was observed in the BLA.
The main finding of this study is that pre-treatment with MJ globally rescues UCMS-induced structural neuronal abnormalities in cortico-limbic regions involved in the control of mood. Specifically, dendritic spine elimination and dendrite arbor retraction that developed concurrently in the BLA, CA1 and PFC of UCMS- exposed mice were either alleviated or entirely reverted by MJ treatment. For example, spine density and dendritic length were fully rescued in PFC but partially rescued in BLA and CA1. Differently, dendrite arbor complexity, estimated by the number of endings and the sholl analysis, was entirely reinstated in BLA but partially reinstated in CA1 and PFC. Together, these data show that MJ preserves cortico-limbic circuits from structural collapse upon UCMS exposure.
Dendritic spines are the subcellular elements which host the majority of glutamatergic excitatory synapses [27]. At a higher scale, single synaptic inputs are integrated at the level of dendrites to finely regulate neuronal connectivity and function [28]. Dysregulation of this integrative process due to aberrant dendritic morphology is a key feature of a variety of neurological and psychiatric diseases including anxiety and depression [29,30]. Consistently, rodent models of depression trigger structural alterations of neurons [16] and decrease synaptic proteins level [31] in brain regions involved in mood regulation although variations in the severity and localization of these dysfunctions have been reported. For example, coherently with the amygdaloid centered model of stress [32] UCMS administered for periods ranging between 1 to 3 months elicits spine loss and dendritic arbor retraction in hippocampus and medial prefrontal cortex [12,33] but increases spines in amygdala and nucleus accumbens [6,34]. On the functional level, it has been suggested that reduction of mPFC synaptic density decreases cortical activity, prevents fear extinction to develop but, at the same time, is permissive for amygdala hyperactivation due to the diminished control of PFC projections on BLA excitatory neurons. Differently, UCMS administered for longer duration (9 months) triggers PFC hyperexcitability [35,36] and decrease synaptic density in the amygdala [9,11], i.e., a pattern of finding reminiscent of the dissociative anhedonic subtype where the hyper-activation of PFC excessively dampens the activation of the amygdala [37,38].
Thus, by showing that a shorter (10 days) UCMS protocol produces spine loss and dendrite retraction in both cortical and subcortical regions, our data do not align with the above models, but with the Dorze et al. observation [39] that rats exposed to the single prolonged stress (SPS) model of PTSD exhibit a helplessness- like depressive phenotype associated with a concurrent retraction of BLA and PFC dendrites. Data showing that both UCMS and SPS decrease microtubule dynamics in the rat hippocampus [40] indicate that depression and PTSD act on the same cytoskeleton target.
Although the current challenge is to demonstrate that anti-depressants can stably modify brain structure, i.e., revert depression-associated structural alterations of neurons, only few in vivo studies support this possibility. Among those, a 4-week treatment with the natural polyphenols paeonol or resveratrol endowed with antidepressant properties were found to antagonize the UCMS-induced loss of spines in hippocampus and medial prefrontal cortex via activation of the scaffolding protein cofilin 1 [21,22]. More recently, imipramine administered before each session of a 21-day restraint stress protocol paradoxically reverted the opposed structural and molecular alterations detected in BLA and mPFC [23]. Specifically, the compound rectified spine density, pCAMKII/CREB levels and PSD-95 expression that were increased in BLA and decreased in PFC respectively, in strong discrepancy with the expected unidirectional effect of imipramine on transcription factors or scaffolding proteins. Instead, a possibility exists that the primary effect of the compound might be to normalize glutamatergic excitatory synaptic activity whose control on spine morphology and function has been extensively demonstrated [41]. Supporting this view, compelling evidence indicates that (i) chronic stress-induced enhancement of glutamate transmission remodels amygdala synapses in a circuit-specific manner [42] whereas (ii) monoaminergic-based antidepressants reduce glutamate release [43] and reverse neuronal structural abnormalities [44].
Together, our findings first show that a 10-day UCMS protocol disrupts spine density and dendrite extension at several nodes of the mood circuitry coherently with the structural alterations observed in a PTSD rodent model [39]. Then, they demonstrate that MJ, which mimics the effect of imipramine in reducing depressive symptoms [17,20] and enhancing monoamine activity [45] prevents spines and dendrites from collapsing upon UCMS exposure. In view of its neuroprotective action against a major neurological symptom of depression and its absence of side effects, MJ might be taken into consideration as a potential therapeutic agent, especially for treating PTSD-like depressive subtypes characterized by multiregional and unidirectional maladaptive remodeling of synapses and circuits.
OA and AP designed and performed the experiments, collected and analyzed the morphological data with the support of MD-I, EB and OMJ, carried out the statistical analyses and prepared the figures. MAT and SU were involved in funding acquisition and project administration. MAT wrote the manuscript.
OA was a visiting scientist at Laboratory of Psychobiology, FSL-CERC, Rome, Italy supported by an ISN (International Society for Neurochemistry)-CAEN (Committee for Aid and Education in Neurochemistry) 1A award. This work was funded by the Italian Ministry of Health (5XMille-2018).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
- Willner P, Muscat R, Papp M (1992) Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev 16: 525-534. [Crossref]
- Willner P (1997) Validity, reliability and utility of the chronic mild stress (CMS) model of depression: a ten- year review and evaluation. Psychopharmacology 134: 319-329. [Crossref]
- Willner P (2017) Reliability of the chronic mild stress model of depression: a user survey. Neurobiol Stress 6: 68-77.
- Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J, et al. (2012) Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev 36: 2085-2011.
- Gilabert-Juan J, Bueno-Fernandez C, Castillo-Gomez E, Nacher J, et al. (2016) Reduced interneuronal dendritic arborization in CA1 but not in CA3 region of mice subjected to chronic mild stress. Brain Behav 7: e00534.
- Qiao H, Li MX, Xu C, Chen HB, An SC, et al. (2016) Dendritic Spines in Depression: What We Learned from Animal Models. Neural Plast 2016: 8056370. [Crossref]
- Magalhães R, Novais A, Barrière DA, Marques P, Marques F, et al. (2019) A Resting-State Functional MR Imaging and Spectroscopy Study of the Dorsal Hippocampus in the Chronic Unpredictable Stress Rat Model. J Neurosci 39: 3640-3650.
- Xu C, Ma XM, Chen HB, Zhou MH, Qiao H, et al. (2016) Orbitofrontal cortex 5-HT2A receptor mediates chronic stress-induced depressive-like behaviors and alterations of spine density and Kalirin7. Neuropharmacology 109: 7-17.
- Zhang L, Luo J, Zhang M, Yao W, Ma X, et al. (2014) Effects of curcumin on chronic, unpredictable, mild, stress-induced depressive-like behaviour and structural plasticity in the lateral amygdala of rats. Int J Neuropsychopharmacol 17: 793-806.
- Padival M, Quinette D, Rosenkranz JA (2013) Effects of repeated stress on excitatory drive of basal amygdala neurons in vivo. Neuropsychopharmacology 38: 1748-1762. [Crossref]
- Zhou M, Liu Z, Yu J, Li S, Tang M, et al. (2018) Quantitative proteomic analysis reveals synaptic dysfunction in the amygdala of rats susceptible to chronic mild stress. Neuroscience 376: 24-39. [Crossref]
- Qiao H, An SC, Xu C, Ma XM (2017) Role of proBDNF and BDNF in dendritic spine plasticity and depressive-like behaviors induced by an animal model of depression. Brain Res 1663: 29-37.
- Marshall J, Zhou XZ, Chen G, Yang SQ, Li Y, et al. (2018) Antidepression action of BDNF requires and is mimicked by Gαi1/3 expression in the hippocampus. Proc Natl Acad Sci U S A 115: E3549-E3558.
- Fan C, Song Q, Wang P, Li Y, Yang M, et al. (2018) Neuroprotective Effects of Ginsenoside-Rg1 Against Depression-Like Behaviors via Suppressing Glial Activation, Synaptic Deficits, and Neuronal Apoptosis in Rats. Front Immunol 9: 2889.
- Yan W, Liu JF, Han Y, Zhang W, Luo YX, et al. (2018) Protein kinase Mζ in medial prefrontal cortex mediates depressive-like behavior and antidepressant response. Mol Psychiatry 23: 1878-1891.
- Luo J, Zhang L, Ning N, Jiang H, Yu SY (2013) Neotrofin reverses the effects of chronic unpredictable mild stress on behavior via regulating BDNF, PSD-95 and synaptophysin expression in rat. Behav Brain Res 253: 48-53. [Crossref]
- Umukoro S, Akinyinka AO, Aladeokin AC (2011) Antidepressant activity of methyl jasmonate, a plant stress hormone in mice. Pharmacol Biochem Behav 98 :8-11.
- Umukoro S, Aluko OM, Eduviere AT, Owoeye O (2016) Evaluation of adaptogenic-like property of methyl jasmonate in mice exposed to unpredictable chronic mild stress. Brain Research Bulletin 121: 105-114.
- Aluko OM, Umukoro S, Annafi O, Adewole FA, Omorogbe O, et al. (2015) Effects of methyl jasmonate on acute stress responses in mice subjected to forced swim and anoxic tests. Scientia Pharmaceutica 83: 635-644. [Crossref]
- Adebesin A, Ajayi AM, Olonode EO, Omorogbe O, Umukoro S, et al. (2017) Methyl jasmonate ameliorated unpredictable chronic mild stress-induced behavioral and biochemical alterations in mouse brain. Drug Res Dev 78: 3381-389.
- Zhu XL, Chen JJ, Han F, Pan C, Zhuang TT, et al. (2018) Novel antidepressant effects of Paeonol alleviate neuronal injury with concomitant alterations in BDNF, Rac1 and RhoA levels in chronic unpredictable mild stress rats. Psychopharmacology (Berl) 235: 2177-2191.
- Leem YH, Yoon SS, Jo SA (2020) Imipramine ameliorates depressive symptoms by blocking differential alteration of dendritic spine structure in amygdala and prefrontal cortex of chronic stress-induced
- Chen JJ, Shen JX, Yu ZH, Pan C, Han F, et al. (2021) Antidepressant Effects of Resveratrol are Accompanied by the Attenuation of Dendrite/Dendritic Spine Loss and the Upregulation of BDNF/p-cofilin1 Levels in Chronic Restraint Mice. Neurochem Res 46: 660-674.
- Yalcin I, Belzung C, Surget A (2008) Mouse strain differences in the unpredictable chronic mild stress: a four-antidepressant survey. Behav Brain Res 193: 140-143.
- Gibb R, Kolb B (1998) A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods 79: 1–4.
- Pignataro A, Middei S, Borreca A, Ammassari-Teule M (2013) Indistinguishable pattern of amygdala and hippocampus rewiring following tone or contextual fear conditioning in C57BL/6 mice. Front Behav Neurosci 7: 156.
- Gipson CD and Olive MF (2017) Structural and functional plasticity of dendritic spines - root or result of behavior? Genes Brain Behav 16: 101-117.
- Ledda F, Paratcha G (2017) Mechanisms regulating dendritic arbor patterning. Cell Mol Life Sci 74: 4511-453.
- Kulkarni VA, Firestein BL (2012) The dendritic tree and brain disorders. Mol Cell Neurosci. 50:10-20.
- Eiland L, McEwen BS (2012) Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus 22: 82-89.
- Wen G, Yao H, Li Y, Ding R, Ren X, et al. (2019) Regulation of Tau Protein on the Antidepressant Effects of Ketamine in the Chronic Unpredictable Mild Stress Model. Front Psychiatry 10: 287.
- Rauch SL, Shin LM, Phelps EA (2006) Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry 60: 376-382.
- Montalban E, Al-Massadi O, Sancho-Balsells A, Brito V, de Pins B, et al. (2019) Pyk2 in the amygdala modulates chronic stress sequelae via PSD-95-related micro-structural changes. Transl Psychiatry9: 3.
- Zhuang PC, Tan ZN, Jia ZY, Wang B, Grady JJ, et al. (2019) Treadmill exercise reverses depression model- induced alteration of dendritic spines in the brain areas of mood circuit. Front Behav Neurosci 13: 93.
- Varga Z, Csabai D, Miseta A, Wiborg O, Czéh B (2017) Chronic stress affects the number of GABAergic neurons in the orbitofrontal cortex of rats. Behav Brain Res 316: 104-114.
- Czéh B, Vardya I, Varga Z, Febbraro F, Csabai D, et al. (2018) Long-term stress disrupts the structural and functional integrity of GABAergic neuronal networks in the medial prefrontal cortex of rats. Front Cell Neurosci 12: 148.
- Lanius RA, Bluhm R, Lanius U, Pain C (2006) A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. J Psychiatr Res 40: 709-72.
- Ritov G, Boltyansky B, Richter-Levin G (2016) PTSD modeling in rodents shows alternating patterns of limbic activity in various types of reactions to stress. Mol Psychiatry 21: 587–58.
- Le Dorze C, Borreca A, Pignataro A, Ammassari-Teule M, Gisquet-Verrier P, et al. (2020) Emotional remodeling with oxytocin durably rescues trauma-induced behavioral and neuro-morphological changes in rats: a promising treatment for PTSD. Transl Psychiatry 10: 27.
- Bianchi M, Hagan JJ, Heidbreder CA (2005) Neuronal plasticity, stress and depression: involvement of the cytoskeletal microtubular system. Curr Drug Targets 4: 597-611.
- Segal M, Andersen P (2000) Dendritic spines are shaped by synaptic activity. Curr Opin Neurobio 5: 5832-1586.
- Zhang JY, Liu TH, Pan HQ, Zhang WH, Yin XP, et al. (2019) Chronic stress remodels synapses in amygdala in a circuit-specific manner. Biol Psychiatry 8: 189-201.
- Musazzi L, Treccani G, Mallei A, Popoli M (2013) The action of antidepressants on the glutamate system: regulation of glutamate release and glutamate receptors. Biol psychiatry 3: 1180-1188.
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, et al. (2009) The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry 14: 764-773.
- Umukoro S, Adebesin A, Agu G, Omorogbe O, Asehinde SB (2018) Antidepressant-like activity of meth jasmonate involves modulation of monoaminergic pathways in mice. Adv Med Sci 63: 36-42.


