A case is presented which highlights the importance of reconsideration of potential differential diagnoses when clinical improvement after therapeutic interventions is lacking. Attention is also directed to the fact that not always the most obvious or even prehospitally established diagnosis is the correct one. In our case report the circumstances pregnancy and seizure were directly linked to the diagnosis of eclampsia but this diagnosis had to be corrected in the later course. This case report highlights the importance of rethinking potential differential diagnoses. A complicating factor was an iatrogenic overdosage of magnesium due to imprecise and confusing drug labeling (g, mg, mmol, mval) which resulted in a cardiac arrest. Furthermore, the neuroprotective effect of magnesium and the positive impact on the outcome in children, as well as the possible cardioprotective effect of magnesium in mothers is demonstrated.
Seizures occur in less than 1 % of all pregnancies and can be caused by many different conditions [1]. Eclampsia is not the most common cause of maternal seizures in high income countries, with an incidence of approximately 0.2 to 0.9 per 1000 pregnancies in Europe [2,3]. Nevertheless, initial treatment of maternal seizures generally involves magnesium sulfate administration for prevention of recurrent eclamptic convulsions, unless other causes, e.g. pre-existent epilepsy, are already known. Magnesium sulfate has been shown in several large, well designed randomized trials as well as systematic reviews to reduce the incidence of further eclamptic seizures more effectively than phenytoin, diazepam and other anticonvulsants [4,5]. The exact mechanism by which magnesium sulfate prevents eclampsia is not entirely known. Protection of the blood brain barrier, vasodilation of cerebral arteries, reversal of inflammatory processes in the central nervous system, and N-methyl-D-aspartate (NMDA) receptor antagonism are just a few theories that have been proposed [6-8]. In addition to its anticonvulsant properties, magnesium sulfate also has a neuroprotective effect and reduces the risk of cerebral palsy in very preterm neonates (born before completed 32 weeks of gestation) [9-11]. Magnesium sulfate administration, especially in relatively high doses needed to prevent eclampsia and achieve fetal/neonatal neuroprotection, does, however, carry significant risks. Hypermagnaesemia can lead to muscle weakness, respiratory depression, hypotension and cardiac arrest. Even in the setting of randomized trials mentioned above, maternal deaths due to iatrogenic hypermagnaesemia have been reported [12]. Physicians should, therefore, be familiar with pitfalls that may lead to magnesium sulfate overdosing. In order to highlight these pitfalls we present a case of severe magnesium sulfate overdosing resulting in maternal cardiac arrest and need for peri-mortem cesarean delivery.
A 31-year-old gravida 2 para 1 was found unresponsive at home in the 31st week of until then uneventful pregnancy. At the arrival of the emergency team, tonic-clonic seizures were noted. The patient was brought in left-lateral position and seizures terminated spontaneously. At that point patient’s vital signs were taken: blood pressure 115/80 mmHg, sinus tachycardia 120/min, blood glucose 136 mg/dl, SpO2 96% without oxygen supplementation and respiratory rate 20/min. Shortly thereafter a recurrent seizure occurred and the patient was given one ampoule of Cormagnesin® 400 mg intravenously. Because the seizure persisted, 4 mg of midazolam were also administered resulting in termination of convulsions. The patient was brought to the emergency department of the nearest hospital, where she was found to be unresponsive to pain, with gaze deviation, but hemodynamically stable (blood pressure 130/70 mmHg, heart rate 115/min, room-air SpO2 97%). Fetal heart rate was assessed by ultrasound and a frequency above 100/min was documented. In the emergency department, two more episodes of tonic-clonic seizures occurred and the patient was given six more ampoules of Cormagnesin® 400 mg. Thereafter she became hypotensive and bradycardic and eventually went into asystole cardiac arrest. Cardiopulmonary resuscitation (CPR) as per European Resuscitation Council guidelines was commenced and return of spontaneous circulation (ROSC) was achieved within two minutes. Four minutes after ROSC, however, asystole recurred and CPR was started again. At this point the decision to perform a cesarean section was taken and a 1880g neonate was born within three minutes. Amniotic fluid was meconium stained and bag mask ventilation of the neonate was performed by the attending pediatrician (no chest compressions were necessary). Apgar scores at birth, 5 minutes and 10 minutes were 0, 3 and 3, respectively. Since the neonate made no spontaneous respiratory efforts, it was intubated and transferred to a neonatal intensive care unit (NICU).
After delivery of the neonate, ROSC was again achieved. The patient was hypotensive with low hemoglobin levels (6.8 g/dL). She received fluids, red-blood-cell transfusion and required vasopressors to maintain an adequate blood pressure. After the definitive surgical treatment, she was transferred to an intensive care unit, where the fist serum magnesium level was > 9.50 mg/dL (normal range 1.60-2.60 mg/dL). With subsequent lowering of serum magnesium levels, the need for vasopressors decreased and they could eventually be stopped. A CT of the head was performed (Figure 1a). In the first radiological assessment no intracranial pathology was described. The patient was extubated the same day. Maternal EEG at day seven post-partum showed a moderate, bilateral, cerebral dysfunction. On the MRI scan taken eight days after delivery, a right frontal lesion identifiable, which was read by the radiologist as cavernoma with hemorrhage or intracerebral hemorrhage due to a fall as part of a seizure event (Figure 1b). Retrospectively this lesion could have been identified in the CT examination (Figure 1a).
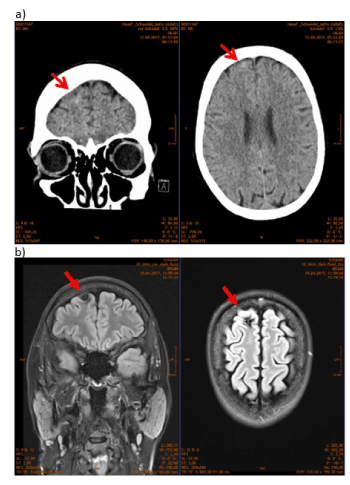
Figure 1. a) CCT scan/nativ, retrospectively a pathologic lesion can be seen (arrow). (b) MRT head FLAIR, a right frontal lesion is identifiable, which was read by the radiologist as cavernoma with hemorrhage or intracerebral hemorrhage
The neonate was extubated two hours after admission to the NICU and discharged at 42 days of life. Neonatal serum magnesium levels were not determined. At discharge from the NICU there were no signs of neurological damage on clinical examination or on cranial ultrasound. Approximately one month after discharge, however, a non-cystic, periventricular leukomalacia I° was diagnosed by MRI and a neurodevelopmental follow-up showed hyperextension tendency with otherwise unremarkable findings.
The case presented highlights some very important aspects of magnesium treatment in pregnancy. Most importantly, it illustrates how this potentially dangerous drug can be overdosed due to several inconsistencies in labeling and dosage recommendations. These may explain why albeit the problem of magnesium overdosing has been known for at least 30 years it is still reported frequently [13-17]. Most scientific societies recommend administering 4 to 6 g of magnesium sulfate to women with eclampsia to prevent further eclamptic fits [2,5,18-20]. The correct term “magnesium sulfate” is found throughout the English-language literature [21,22]. In the German-speaking area, however, often little emphasis is put on the distinction between “magnesium” and “magnesium sulfate” as a therapeutic agent, e.g. AWMF guidelines [18]. The term “magnesium” should be avoided in such guidelines, since it may prompt physicians to prescribe excessive amounts of magnesium sulfate to achieve the recommended dose of magnesium alone. It is absolutely crucial to emphasize that doses refer to magnesium sulfate unless otherwise specified. Our case clearly shows this. Magnesium sulfate was administered to the patient as a commercially available Cormagnesin® 400 mg preparation.
The “400 mg” in the drug’s name is dangerously confusing, since one ampoule of Cormagnesin® 400 mg contains 4 095 mg magnesium sulfate heptahydrate (Mg2+ SO4*7H2O), the active agent. This corresponds to 403.8 mg of elemental magnesium (Mg2+). If a physician wants to follow the guidelines of giving 4 g of “magnesium” to the patient, he or she can be easily mislead by the labeling and give 10 ampoules instead of just one. Our patients received seven ampoules of the drug which resulted in severe magnesium toxicity and cardiac arrest. Even if the issue of not enough precise guidelines using terms “magnesium sulfate” and “magnesium” as synonyms would be solved, however, the labeling of magnesium sulfate would still remain misleading. Magnesium sulfate is found as magnesium sulfate heptahydrate (Mg2+ SO4*7H2O), as already described, and this is the active agent on which dosage recommendation are based on. The 4095 mg of magnesium sulfate heptahydrate (Mg2+ SO4*7H2O) in one ampoule of Cormagnesin® 400 mg correspond to 2 000 mg of magnesium sulfate (Mg2+ SO4). This could again prompt physicians to give twice the optimal dose even if the guidelines would specifically state “magnesium sulfate”. These considerations are especially important for pre-hospital and emergency department physicians that are relatively rarely confronted with patients with severe preeclampsia/eclampsia who require magnesium sulfate treatment. We have already contacted the manufacturer of Cormagnesin® and suggested to change the drug labeling as well as the prescribing information in order to avoid complications as the one described. We also decided to present our case for the same reason to avoid such severe consequences of magnesium sulfate overdose to happen again in the future.
The second important aspect of the case which merits discussion is the importance of constructing a differential diagnosis and consider other causes of maternal seizures when seizures recur despite adequate magnesium sulfate treatment. Initial treatment with magnesium sulfate was, in our opinion, appropriate, since the emergency physician had no information on other pre-existent conditions that could lead to convulsions in this patient. This is true even in the presence of normal blood pressure, since only 50% of eclampsia is accompanied by severe hypertension and in up to 34% it can occur without proteinuria and hypertension18. However, after three episodes of recurrent seizures after magnesium sulfate administration, other causes should have been considered before giving more magnesium sulfate. These would include new onset epilepsy; a pre-existing epilepsy that was not disclosed by the patient and her family members; cerebral hemorrhagic event (spontaneous or traumatically induced); metabolic or toxicologically induced encephalopathies; and ischemic stroke.
In conclusion, the case presented emphasizes the fact that magnesium sulfate should be regarded as a potentially dangerous drug. This is especially true for medical fields in which clinicians only rarely treat critically ill obstetric patients. Efforts should be made to reduce the risks associated with magnesium sulfate treatment. Clinicians should help developing guidelines with clear dosage recommendations avoiding misleading terms, such as “magnesium” instead of “magnesium sulfate”. Moreover, they should also work closely with pharmacists to change ambiguous labeling of magnesium sulfate preparations, which are currently common worldwide.
The mother provided written permission for this case to be published. Neither the author nor one of the co-authors have a conflict of interest to declare.
- Hart LA, Sibai BM (2013) Seizures in pregnancy: epilepsy, eclampsia, and stroke. Semin Perinatol 37: 207-224. [Crossref]
- Lowe SA, Bowyer L, Lust K, McMahon LP, Morton MR, et al. (2015) The SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust N Z J Obstet Gynaecol 55: 11-16. [Crossref]
- Euro-Peristat Project with SCPE and EUROCAT. European Perinatal Health Report. The health and care of pregnant women and babies in Europe in 2010. May 2013. Available from: www.europeristat.com.
- American College of Obstetricians and Gynecologists. Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. 2013. Accessed: http://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy.
- NICE clinical guideline 107. Hypertension in pregnancy. The management of hypertensive disorders during pregnancy. Accessed from: http://www.nice.org.uk/guidance/cg107/evidence/full-guideline-134794333.
- Zhang LW, Warrington JP (2016) Magnesium sulfate prevents placental ischemiainduced increases in brain water content and cerebrospinal fluid cytokines in pregnant rats. Front Neurosci 10: 561. [Crossref]
- Johnson AC, Tremble SM, Chan SL, Moseley J, LaMarca B, et al. (2014) Magnesium sulfate treatment reverses seizure susceptibility and decreases 89 neuroinflammation in a rat model of severe preeclampsia. PLoS One 9: e113670.
- Euser AG, Cipolla MJ (2009) Magnesium sulfate for the treatment of eclampsia: A brief review. Stroke 40: 1169-1175. [Crossref]
- Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, et al. (2008) A randomised, controlled trial of magnesium sulphate for the prevention of cerebral palsy. N Engl J Med 359: 895-905. [Crossref]
- Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D (2007) Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev 18: CD004661.
- Conde-Agudelo A, Romero R (2009) Antenatal magnesium sulphate for the prevention of cerebral palsy in preterm infants less than 34 weeks' gestation: a systematic review and meta-analysis. Am J Obstet Gynecol 200: 596-609. [Crossref]
- Altman D, Carroli G, Duley L, Farrell B, Moodley J, et al. (2002) Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet 359: 1877-1890.
- Vissers RJ, Purssell R (1996) Iatrogenic magnesium overdose: two case reports. J Emerg Med 14: 187-191. [Crossref]
- Kumar K, Al Arebi A, Singh I (2013) Accidental intravenous infusion of a large dose of magnesium sulphate during labor: A case report. J Anaesthesiol Clin Pharmacol 29: 377-379. [Crossref]
- Swartjes JM, Schutte MF, Bleker OP (1992) Management of eclampsia: cardiopulmonary arrest resulting from magnesium sulphate overdose. Eur J Obstet Gynaecol Reprod Biol 47: 73-75.
- Cavell GF, Bryant C, Jheeta S (2015) Iatrogenic magnesium toxicity following intravenous infusion of magnesium sulfate: risks and strategies for prevention. BMJ Case Rep. [Crossref]
- Morisaki H, Yamamoto S, Morita Y, Kotake Y, Ochiai R, et al. (2000) Hypermagnesemia-induced cardiopulmonary arrest before induction of anaesthesia for emergency cesarean section. J Clin Anesth 12: 224-226. [Crossref]
- 015/018–S1-Leitlinie. Diagnostik und Therapie hypertensiver Schwangerschaftserkrankungen. AWMF online. Available from: https://www.awmf.org/leitlinien/detail/ll/015-018.html.German.
- American College of Obstetricians and Gynecologists. Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. 2013. Accessed from: http://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy.
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P, et al. (2014) Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can 36: 416-441. [Crossref]
- Norwitz ER, Lockwood CJ, Schachter SC, Barss VA. Eclampsia; UpToDate, November 2017. Available from: https://www.uptodate.com/contents/eclampsia.
- Norwitz ER, Lockwood CJ, Barss VA. Preeclampsia: Management und prognosis. UpToDate. Nov. 2017. Available from: https://www.uptodate.com/contents/preeclampsia-management-and-prognosis.

