Hypereosinophilic Syndrome (HES) is a condition related to helminthiasis, allergies, vasculitis, adverse reactions to drugs or malignant neoplasms. We report a case of a boy, 7 years old, presenting abdominal pain, vomiting, tachidispnea and leukocytosis with predominance of eosinophils. Bone marrow biopsy revealed intense granulocytic hypercellularity characterized by eosinophilia, as well as immature lymphoid cells in the interstitium, associated with trabecular bone infiltration. The immunohistochemical study revealed positivity for CD79a and TDT. Peripheral blood flow cytometry demonstrated 6.5% of lymphoid blasts. The diagnosis was Lymphoproliferative Disease associated with Hypereosinophilia: Acute Lymphoblastic Leukemia. The association presented is infrequent and its aggressiveness is determined by the physiological limitations imposed by the damages secondary to eosinophilia.
precursor cell lymphoblastic leukemia-lymphoma, eosinophilia, hypereosinophilic syndrome, immunohistochemistry
Hypereosinophilic syndrome (HES) is characterized by heart and central nervous system damage due to eosinophil products. HES diagnosis depends on peripheral blood and clinical features. Bone marrow is important in excluding hematopoietic malignances, as leukemia and lymphoma [1]. We report a rare case about a child with clinical presentation of HES whose bone marrow analysis indicated a diagnosis of leukemia.
Male child, 7 years old, presenting abdominal pain and vomiting for four days. Physical examination revealed severe tachydyspnea, without clinical infection signals. The blood count showed leukocytosis (194,000 cells/mm3, reference value: 4,0-11,0 cells/mm3) with a predominance of eosinophils (64% or 124,160 cells/mm3, reference value: 2,0-4,0%). Given these findings, the diagnosis of congestive heart failure, in the context of hyperosinophilia, was considered and the echocardiogram was requested. This exam showed segmental contractile alterations with preserved ejection fraction, associated with signs of mild/moderate pulmonary arterial hypertension.
In order to exclude hematopoietic malignancies, the patient was submitted a bone marrow biopsy and a bone marrow aspirate with morphological and immunophenotype evaluated by immunohistochemistry. Peripheral blood sample was also evaluated by flow cytometry.
Bone marrow biopsy revealed preserved global cellularity for age. There was intense hypercellularity of granulocytic series with maturative delay characterized by intermediate eosinophilic precursors and mature eosinophils in a lower proportion (Figures 1A and 1B). Hypercellularity of the lymphocytic series with maturation delay with predominance of immature cells in the interstitium was also noted (Figure 1C, 1D and 1E). The trabecular bone exhibited foci of infiltration.
Peripheral blood flow cytometric examination demonstrated 6.5% of lymphoid blasts. The immunohistochemical study revealed positivity for CD79a (Figure 1F) and TDT (Figure 1G) in 2% and 10% of immature cells, respectively. There was extensive positivity for myeloperoxidase due to the hypercellularity of the granulocytic series at the expense of eosinophilic precursors (Figure 1H). The clinical, flow cutometric and immunohistochemical data indicated the diagnosis of Lymphoproliferative Disease associated with Hypereosinophilia: Acute Lymphoblastic Leukemia, with B immunophenotype (B-ALL).
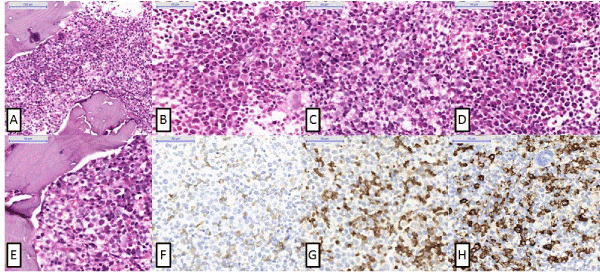
Figure 1. Figure 1-A (200x, H&E) and B (400x, H&E) show an intense hypercellularity of granulocytic series with maturative delay characterized by intermediate eosinophilic precursors and mature eosinophils in a lower proportion. C (400x, H&E), D (400x, H&E) and E (200x, H&E) show Hypercellularity of the lymphocytic series with maturation delay with predominance of immature cells in the interstitium. F (400x, CD79a) shows B-cells. In that lymphocytosis, there is almost 2% of B-cells. G (400x, TDT) confirms the immature cells. They are immature neoplastic B-cells. H (400x, myeloperoxidase) confirms that these neoplastic B-cells are among granulocytic cells which stain for myeloperoxidase.
The patient was treated with standard chemotherapy for these cases (BFM-2002, Berlin-Frankfurt Muster European Group for ALL treatment) and leukopheresis, evolving with several intercurrences secondary to immunosuppression and, especially, cardiac repercussions secondary to eosinophilia.
Eosinophilia is a condition that may be related to morbidities such as helminthiasis, allergies, vasculitis, adverse drug reactions and malignant neoplasms [1,2]. SHE occurs when there is peripheral eosinophilia greater than 1.5 x 109/L or more associated to an organ damage mediated by increased numbers of eosinophils. Hypereosinophilia is defined as the simple increase in peripheral blood eosinophil count greater than 1.5 x 109/L1.
SHE is a rare disease that occurs with sustained eosinophilia in the peripheral blood or bone marrow. The disease predominantly affects males (nine males for one woman), in the age group of 20 to 50 years [2]. Less commonly, the disease affects individuals at the extreme age [2,3]. Clinically, patients with SHS may present dyspnea, weakness, cough, myalgias, fever and cutaneous rash. The common laboratory finding is leukocytosis (above 20,000/mm3) with eosinophils predominance [3].
Tissue infiltration by eosinophils is the promoter of major organ damage due to the release of cell granules [2]. The systemic effects of sustained eosinophilia include cutaneous, pulmonary, gastrointestinal and, mainly, cardiac changes. Approximately 20% of SHE patients had cardiac damages not related to other comorbidities, and 6.0% of the subjects presented them at the first moment of the disease. Cardiac damage, as well as systemic damage, is caused by infiltration of eosinophils into cardiac fibers with degranulation of toxic mediators to tissues. Endocardial changes, restrictive cardiomyopathies and valvular insufficiencies are examples of cardiac repercussions of SHE [3]. On admission, our patient exhibited cardiac alterations, which are confirmed, later, by echocardiogram.
The occurrence of hypereosinophilia and ALL is rare, with incidence less than 1.0%. Review of the literature by Rappanoti [2] et al. published in 2010 raised 52 cases of the association reported in 44 articles. The clinical importance of the association is with regard to the treatment, since hypereosinophilia promotes cardiac changes that limit the use of certain drugs and restrict the clinical evolution of the patients [2].
Approximately 10% of SHE patients present cytogenetic alteration of FIP1L1-PDGFRA fusion [3-5]. In the case of patients with SHE and ALL, in more than half of the cases reported in the literature, there is the detection of genetic abnormalities, with the t (5;14) (q31;q32) translocation being the most commonly diagnosed. It is interesting to note that this translocation, in more than 90% of cases, is detected only in lymphoid blasts and not in eosinophils. These data support the theory that eosinophilia would be a reactive phenomenon, secondarily induced by the elevated expression of cytokine levels, such as interleukin 5, interleukin 3 and granulocyte stimulating factor, released by leukemic cells [4-7].
ALL in the context of Hypereosinophilia is a rare subtype of malignant lymphoproliferative disease whose aggressiveness is determined by the physiological limitations imposed by secondary damage to eosinophilia [5]. In the present case, it is noted that the definitive diagnosis was achieved by the correlation of morphological data with immunophenotype aspects. The patient death is associated to his cardiac repercussions secondary to eosinophilia.
- Tefferi A, Gotlib J, Pardanani A (2010) Hypereosinophilic Syndrome and Clonal Eosinophilia: Point-of-Care Diagnostic Algorithm and Treatment Update. Mayo Clin Proc 85:158-164 [Crossref]
- Rapanotti MC, Caruso R, Ammatuna E, Zaza S, Trotta L, et al. (2010) Molecular characterization of paediatric idiopathic Hypereosinophilia. Br J Haematol 151:440-446. [Crossref]
- Gotlib J. World Health Organization-defined eosinophilic disorders: 2011 update on diagnosis, risk stratification, and management. Am J Hematol 86:678-688.
- Nie YL, Jan SL, Fu LS, Chang TK, Wang JD (2010) Congestive heart failure as presentation of acute lymphoblastic leukaemia with eosinophilia. Br J Haematol 149: 633. [Crossref]
- Sutton R, Lonergan M, Tapp H, Venn NC, Haber M, et al. (2008) Two cases of hypereosinophilia and high-risk acute lymphoblastic leukemia. Leukemia 22: 1463-1465. [Crossref]
- Reiter A, Gotlib J2 (2017) Myeloid neoplasms with eosinophilia. Blood 129: 704-714. [Crossref]
- Gotib J. World Health Organization-defined eosinophilic disorders: 2015 update on diagnosis, risk stratification, and management. Am J Hematol 90:1078-1089.

