Objective: Study the effect of acute kidney injury (AKI) on long-term survival in a large cohort of neurosurgical patients who were admitted to the intensive care unit (ICU) for at least 24 hours.
Design: Retrospective cohort study.
Setting: Academic tertiary medical center.
Patients: The study consisted of 3,299 consecutive patients with no history of chronic kidney disease who were discharged after a major neurosurgical procedure at the University of Florida between 1992 and 2002. All patients were admitted to ICU postoperatively for at least twenty-four hours.
Interventions: AKI was defined by the RIFLE classification (Risk, Injury, Failure, Loss, and End stage), which requires at least a 50% increase in serum creatinine, and stratifies patients into three grades of AKI: Risk, injury, and failure. Patient survival was determined through a search of the National Social Security Death Index. Long-term survival was analyzed with a risk-adjusted Cox proportional hazards regression model.
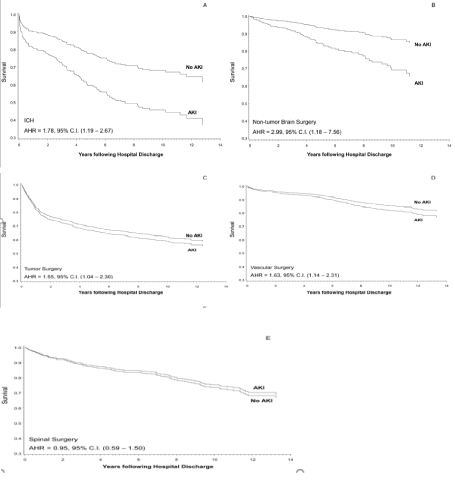
Measurements and Main Results: Among hospital survivors, 10% had an episode of AKI while in the hospital; the occurrence of AKI was dependent on the type of surgery. In the risk-adjusted model, long-term mortality was proportional to the severity of kidney injury, with an adjusted hazard ratio (AHR) of 1.15 (95% confidence interval [CI], 1.09 to 1.22) for RIFLERisk class and 1.45 (1.40-1.75) for the RIFLEFailure class compared to patients without AKI (p<0.001). The association between AKI and long-term mortality risk was dependent on the type of surgery. The survival rate was worse for patients with AKI among all surgery types, except spinal surgery.
Conclusion: Postoperative AKI of even mild severity adversely impacts the long-term outcome of hospital survivors after major neurosurgical procedures. The degree of impact on mortality is dependent on the type of surgery.
Acute kidney injury, neurosurgery, intensive care unit, long-term outcome
Acute kidney injury (AKI) during hospitalization after major surgical procedures is a risk factor for short-term mortality [1,2]. In the past, studies have focused on severe AKI, defined either as a need for hemodialysis or a substantial increase in serum creatinine (sCr), often including patients with preexisting chronic kidney disease (CKD). Using RIFLE (Risk, Injury, Failure, Loss, and End stage) AKI classification system, several studies have been able to identify the importance of small changes in sCr on patients’ outcomes [3,4]. Additionally, the effect of AKI among neurosurgical patients with aneurysmal subarachnoid hemorrhage (aSAH) was found to be an independent risk factor for short-term mortality [5,6].
Although several studies have reported a higher prevalence of RIFLE-defined AKI in various patient populations, and confirmed the association of less severe AKI with short-term mortality,[4,7,8] few studies have focused on AKI in the cohort of neurosurgical patients[9,10]. Furthermore, several studies have reported, that RIFLE-defined AKI is associated with a significant risk for long-term mortality in several different cohorts including large cohort of surgical patients as demonstrated in our previous study[11]. This association may vary with the type of surgery, and studies in more homogenous patient populations may be warranted[12] .
The goals of this study were to evaluate the long-term mortality risk associated with RIFLE-defined AKI in a large, single-center cohort of neurosurgical patients with no history of chronic kidney disease (CKD), who required at least a 24-hour admission to an intensive care unit (ICU), and to determine whether this association is dependent on the type of surgery and specific patient population.
Patient population
This study was approved by the Institutional Review Board of the University of Florida as a retrospective cohort study. 11,080 adult patients who were admitted to a surgical ICU for at least 24 hours after any type of general/gastrointestinal, vascular, CT, or neurosurgical operative procedure, and who survived to be discharged from the hospital, were identified through a search of the billing database between the years 1992 and 2002 [11]. Among this cohort, we selected 3,299 patients who underwent any type of neurosurgical procedure, with subsequent admission to a neurosurgical ICU (NeuroICU), for inclusion in this study.
Patients with a history of CKD at any stage were excluded. History of CKD was established through review of all relevant clinical notes and sCr values prior to surgery and by analysis of the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes for end-stage renal disease (ESRD) and CKD. Patients were categorized by type of operative procedure or primary condition as follows: spinal surgery (SS); vascular surgery (VS) (includes all patients with subarachnoid hemorrhage, aneurysmal surgery and arteriovenous malformation surgeries); craniotomy for tumor (CT); craniotomy for non-tumor surgery (CNT); and patients with intracerebral hemorrhage (ICH) (all ICH not related to SAH, intracerebral aneurysms or AVM; includes traumatic subdural and epidural hematoma). The need for renal replacement therapy (RRT) was recorded for each patient.
Definition of acute kidney injury
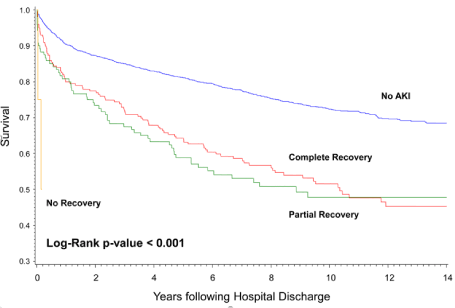
AKI is defined by RIFLE classification by comparing changes in sCr during hospitalization to baseline sCr. For baseline sCr, we used the lowest of two values: the lowest measured sCr at hospital admission or the expected sCr value (CrMDRD), calculated using the abbreviated “Modification of diet in renal disease” (MDRD) equation [13]. Patients who met the RIFLE criteria for AKI were classified as “AKI”, while those who did not were classified as “no AKI”. Patients with AKI were stratified according to the maximum RIFLE class (RIFLEmax) reached during hospital admission. RIFLE-R corresponds to a 150% increase in sCr, RIFLE-I to a 200% increase in sCr, and RIFLE-F to a 300% increase in sCr. RIFLEmax was determined by comparing the highest sCr during hospitalization with the baseline sCr. Renal outcome at the time of discharge was evaluated by comparing the discharge sCr to the baseline sCr. Complete renal recovery was recorded if the sCr returned to a level less than 50% above baseline sCr, whereas partial renal recovery was recorded when sCr was greater than 50% above baseline sCr, but with no need for RRT. No renal recovery implied a need for RRT at the time of hospital discharge.
Definition of outcomes and covariates
Comorbidities and surgical complications were identified by ICD-9-CM codes based on previously published criteria [14-16]. The billing codes used for acute renal failure (ARF) were ICD-9-CM diagnostic codes 584.XX or 997.5. Disposition upon discharge was determined from discharge summaries. Patient survival following discharge was determined through a search in 2006 of the National Social Security Death Index.
Statistical analysis
Results are expressed as means (SD) for variables with normal distribution. The Shapiro-Wilk W test and distribution plots were used to test normality of distribution. For data that did not meet normality assumptions, median and interquartile ranges were displayed (IQR), and the Kruskal-Wallis test was utilized to evaluate the independence of group levels. For categorical variables, the Pearson χ2 test or Fisher's exact test was applied as appropriate.
Survival probabilities were estimated with the product-limit method (Kaplan–Meier algorithm). Survival differences between groups were analyzed using the log-rank tests. Adjusted hazard ratios (AHR) were generated by Cox proportional hazard models, with adjustment for factors potentially associated with patient survival. These factors were chosen a priori, based on the literature on AKI in surgery patients and our clinical experience with AKI in these patients. Survival models were initiated from time of hospital discharge and followed until death or date of last follow up. Adjusted survival curves were also generated to demonstrate the impact of the individual surgery type on the degree of AKI, based on the mean level of model covariates. A double-sided P value less than 0.05 was considered statistically significant for all tests. Statistical analyses were performed with Statistica (version 8.0, Tulsa, OK) and SAS (v.9.1.3, Cary, NC). The authors had full access to the data and take responsibility for its integrity.
Among the 3,299 patients who were discharged after major neurosurgical procedure, 323 patients (10%) had an episode of AKI during hospitalization. Of the patients with AKI, only 11% had the most severe form of AKI (RIFLEmaxF), while the majority of patients had mild to moderate AKI (Table 1). The prevalence of AKI during hospitalization was dependent on the type of surgery, with the highest prevalence being among ICH patients (n=64, 17%) and the lowest among patients who underwent a non-tumor craniotomy (n=18, 6%) (Table 2). However, patients undergoing different types of surgery represented distinctive cohorts with major differences in demographic characteristics and comorbid conditions that might have constituted risk factors for AKI and other hospital complications (Table 1). Patients in ICH group were older, more likely to be of AA ethnicity and to have comorbid conditions, all of which are risk factors for AKI. Although other hospital complications were clustered in different patterns among surgical groups, mechanical ventilation, acute postoperative anemia and AKI remained uniformly the most common three complications (Table 1). Interestingly, although previous reports in literature have focused on AKI requiring RRT, only nine patients (3%) in our cohort required RRT, emphasizing the fact that severe RRT-requiring AKI is rare in this population. Only 62% of all AKI patients had complete renal recovery at the time of discharge while 37% had partial renal recovery (Table 3). On the other hand, only 35 (11%) of RIFLE-defined AKI patients had ICD-9-CM codes for acute renal failure (ARF) in the billing database.
Table 1. Demographic and Clinical Characteristics of Patients
|
No AKI
N=2976 (90%) |
AKI |
P* |
P†
|
All AKI
N=323 (10%) |
RIFLEmax R
N=222 (7%) |
RIFLEmax I
N=64 (2%) |
RIFLEmax F
N=37 (1%) |
Demographics |
|
|
|
|
|
|
|
Age 52 (16) |
51 (16) |
61 (16) |
60 (16) |
62 (16) |
63 (15) |
<0.001 |
0.58 |
Female sex (1897, 58%) |
1697 (57%) |
200 (62%) |
148 (67%) |
33 (52%) |
19 (51%) |
0.09 |
0.03 |
African-American ethnicity (326, 10%) |
289 (10%) |
37 (11%) |
16 (10%) |
15 (7%) |
6 (23%) |
0.43 |
0.38 |
Baseline serum creatinine level (mg/dL) |
0.77 (0.20) |
0.76 (0.25) |
0.73 (0.23) |
0.81 (0.28) |
0.87 (0.23) |
<0.001 |
<0.001 |
Baseline glomerular filtration rate, mL/min/1.73 m2 |
104 (25) |
100 (30) |
102 (31) |
97 (31) |
87 (23) |
<0.001 |
0.01 |
Highest serum creatinine level (mg/dL) |
0.83 (0.23) |
1.63 (1.40) |
1.16 (0.39) |
1.74 (0.61) |
4.25 (2.74) |
<0.001 |
<0.001 |
Comorbidities |
|
|
|
|
|
|
|
Hypertension (1076, 33%) |
924 (31%) |
152 (47%) |
103 (46%) |
34 (53%) |
15 (41%) |
<0.001 |
0.45 |
Diabetes mellitus (207, 6%) |
168 (6%) |
39 (12%) |
24 (11%) |
14 (22%) |
1 (3%) |
<0.001 |
0.01 |
Atrial fibrillation (123, 4%) |
87 (3%) |
36 (11%) |
17 (8%) |
8 (13%) |
11 (30%) |
<0.001 |
<0.001 |
Congestive heart failure (99, 3%) |
63 (2%) |
36 (11%) |
16 (7%) |
12 (19%) |
8 (22%) |
<0.001 |
0.003 |
Chronic pulmonary disease (295, 9%) |
255 (9%) |
40 (12%) |
27 (12%) |
7 (11%) |
6 (16%) |
0.02 |
0.73 |
Chronic liver disease (38, 1%) |
30 (1%) |
8 (2%) |
2 (1%) |
2 (3%) |
4 (11%) |
0.02 |
0.001 |
Coronary heart disease (242, 7%) |
194 (7%) |
48 (15%) |
29 (13%) |
10 (16%) |
9 (24%) |
<0.001 |
0.20 |
Malignancy (605, 18%) |
565 (19%) |
40 (12%) |
26 (12%) |
9 (14%) |
5 (14%) |
0.004 |
0.86 |
Chronic anemia (247, 7%) |
200 (7%) |
47 (15%) |
27 (12%) |
12 (19%) |
8 (22%) |
<0.001 |
0.18 |
Depression (82, 2%) |
77 (3%) |
5 (2%) |
3 (1%) |
2 (3%) |
0 (0%) |
0.25 |
0.43 |
Categorical variables are presented as number (percentages within columns). *Comparing patients without AKI to all patients with AKI. †Comparing patients within the three subgroups of AKI patients.
Table 2. AKI by type of surgery
|
No AKI
N=2976 (90%) |
AKI |
P* |
P†
|
All AKI
N=323 (10%) |
RIFLEmax R
N=222 (7%) |
RIFLEmax I
N=64 (2%) |
RIFLEmax F
N=37 (1%) |
Type of surgery |
|
|
|
|
|
<0.001 |
<0.001 |
Craniotomy, non-tumor surgery (n=312; 9%) |
294 (94%) |
18 (6%) |
11 (4%) |
6 (2%) |
1 (0%) |
|
|
Vascular surgery (n=1314, 40%) |
1192 (91%) |
122 (9%) |
88 (7%) |
21 (2%) |
13 (1%) |
|
|
Spinal surgery (n=499, 15%) |
441 (88%) |
58 (12%) |
43 (9%) |
6 (1%) |
9 (2%) |
|
|
Craniotomy, tumor (n=807, 25%) |
746 (92%) |
61 (8%) |
49 (6%) |
7 (1%) |
5 (1%) |
|
|
ICH (n=367, 11%) |
303 (83%) |
64 (17%) |
31 (8%) |
24 (7%) |
9 (2%) |
|
|
Categorical variables are presented as number (percentages within the rows). *Comparing patients without AKI to all patients with AKI. †Comparing patients within the three subgroups of AKI patients.
Kaplan-Meier plots show that patients who developed AKI had significantly worse long-term survival than patients with no AKI (p<0.001). Survival rate at five years was 82% for patients without AKI and 61% for patients with AKI. At 10 years, the survival rate drops to approximately 73% for patients without AKI and 49% for patients with AKI (Figure 1). Among patients with AKI, according to RIFLEmax class, survival rate at 10 years was: Risk 55%, Injury 38%, and Failure 33% (Figure 2).
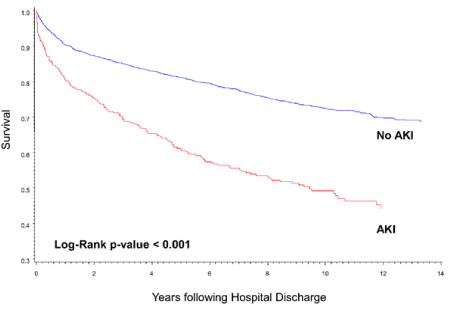
Figure 1. Long-term survival rate of patients with and without an AKI during hospitalization
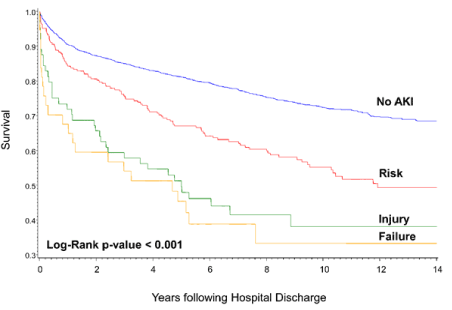
Figure 2. Long-term survival of patients stratified by AKI severity
The Cox proportional hazard model established that AKI (AHR = 1.34, 95% CI 1.11 - 1.61) is a predictor of mortality when all types of surgery are considered together. The severity of AKI in this model was also associated with an increased mortality rate. Patients with RIFLEmaxR had AHR of 1.26 (95% CI 1.01-1.56), patients with RIFLEmaxI had AHR of 1.49 (95% CI 1.06-2.10), and patients with RIFLEmaxF had AHR of 1.63 (95% CI 1.01-2.64). Moreover, male gender, older age (>61 years), chronic pulmonary disease, malignancies, chronic anemia, prolonged length of stay, and discharge to a site other than home further contributed to the increased risk for death in this model (Table 4). Among surgery types, the highest mortality was seen in the tumor craniotomy subgroup, which was used as a reference group in the Cox proportional hazard model. Compared to the reference group, all other types of surgery were associated with a significantly lower risk for long-term mortality.
Table 4. Cox proportional hazard model for patient mortality following hospital discharge
Parameter
(reference level) |
Level |
All patients
(n=3299) |
Vascular surgery
(n=1314, 40%) |
Craniotomy, tumor
(n=807, 25%) |
Spinal surgery
(n=499, 15%) |
ICH
(n=367, 11%) |
Craniotomy, non-tumor surgery
(n=312, 9%) |
AHR (95%CI)
P value |
AHR (95%CI)
P value |
AHR (95%CI)
P value |
AHR (95%CI)
P value |
AHR (95%CI)
P value |
AHR (95%CI)
P value |
AKI (No AKI) |
|
1.34 (1.11 - 1.61)
0.002 |
1.27 (0.88 -1.84)
0.20 |
1.65 (1.12 – 2.43)
0.01 |
0.92 (0.58 – 1.46)
0.71 |
2.00 (1.32 – 3.04)
0.001 |
2.88 (0.94 – 8.80)
0.06 |
Type of surgery
(Craniotomy, tumor surgery) |
Vascular surgery |
0.48 (0.40 – 0.58)
<0.001 |
|
|
|
|
|
|
Spinal surgery |
0.64 (0.52 – 0.78)
<0.001 |
|
|
|
|
|
|
Craniotomy, non-tumor surgery |
0.73 (0.55 – 0.97)
0.03
|
|
|
|
|
|
|
ICH |
0.77 (0.62 – 0.97)
0.03 |
|
|
|
|
|
Demographics |
|
|
|
|
|
|
|
Age Group
(18-45) years) |
46-60 years |
1.97 (1.60 – 2.43)
<0.001 |
1.74 (1.11 – 2.70)
0.01 |
1.81 (1.31 – 2.50)
<0.001 |
1.75 (1.03 – 2.98)
0.04 |
3.20 (1.54 – 6.65)
0.002 |
2.91 (1.38 – 6.14)
0.005 |
|
61-70 years |
3.17 (2.55 – 3.93)
<0.001 |
3.24 (2.07 – 5.08)
<0.001 |
3.00 (2.13 – 4.21)
<0.001 |
2.89 (1.65 – 5.06)
<0.001 |
3.23 (1.49 – 7.01)
0.003 |
11.94 (4.94 – 28.83)
<0.001 |
|
71+ years |
5.60 (4.48 – 7.01)
<0.001 |
7.59 (4.73 – 12.17)
<0.001 |
4.07 (2.73 – 6.06)
<0.001 |
4.14 (2.33 – 7.34)
<0.001 |
12.08 (6.27 – 23.28)
<0.001 |
17.89 (5.78 – 55.41)
<0.001 |
Gender
(Male) |
Female |
0.62 (0.61 – 0.79)
<0.001 |
0.64 (0.49 – 0.85)
0.002 |
0.58 (0.46 – 0.73)
<0.001 |
0.72 (0.51 – 1.02)
0.06 |
0.80 (0.55 – 1.17)
0.25 |
1.20 (0.64 – 2.25)
0.57 |
Ethnicity (Caucasian) |
African American |
0.91 (0.72 – 1.16)
0.43 |
0.83 (0.50 – 1.40)
0.49 |
1.04 (0.66 – 1.64)
0.86 |
0.65 (0.34-1.24)
0.19 |
0.94 (0.60 – 1.47)
0.79 |
1.11 (0.38 – 3.24)
0.85 |
|
Other* |
0.75 (0.56 – 1.02)
0.06 |
0.64 (0.38 – 1.09)
0.10 |
0.84 (0.46 – 1.55)
0.58 |
1.23 (0.61 – 2.49)
0.56 |
0.66 (0.31 – 1.42)
0.29 |
0.50 (0.07 – 3.41)
0.48 |
Comorbidities |
|
|
|
|
|
|
|
Diabetes Mellitus (None) |
Yes |
1.0 (0.79 – 1.26)
0.99 |
1.00 (0.60 – 1.67)
0.99 |
1.13 (0.73 – 1.75)
0.58 |
0.944 (0.56 – 1.58)
0.83 |
0.89 (0.51 – 1.56)
0.68 |
1.52 (0.40 – 5.82)
0.54 |
Congestive Heart Failure
(None) |
Yes |
1.11 (0.83 – 1.49)
0.47 |
0.94 (0.55 -1.62)
0.83
|
1.29 (0.59 – 2.84)
0.52 |
1.40 (0.74 – 2.68)
0.30 |
1.34 (0.72 – 2.49)
0.36 |
1.19 (0.14 – 10.37)
0.87 |
Chronic Pulmonary Disease (None) |
Yes |
1.46 (1.21 – 1.78)
<0.001 |
1.28 (0.90 -1.82)
0.18 |
1.75 (1.22 – 2.50)
0.002 |
1.60 (0.99 – 2.59)
0.05 |
0.88 (0.50 – 1.57)
0.67 |
1.62 (0.59 – 4.46)
0.35 |
Hypertension (None) |
Yes |
1.09 (0.94 – 1.25)
0.26 |
1.65 (1.26 – 2.15)
<0.001
|
0.95 (0.72 – 1.24)
0.69 |
1.56 (1.08 – 2.24)
0.02 |
0.79 (0.55 – 1.14)
0.21 |
0.40 (0.29 – 1.42)
0.27 |
Atrial Fibrillation (None) |
Yes |
1.10 (0.85 – 1.44)
0.49 |
1.09 (0.63 – 1.87)
0.77 |
1.38 (0.72 – 2.66)
0.34 |
0.93 (0.51 – 1.70)
0.81 |
1.34 (0.83 – 2.17)
0.23 |
0.72 (0.13 – 4.04)
0.71 |
Coronary Heart Disease
(None) |
Yes |
1.10 (0.90 - 1.36)
0.37 |
1.69 (1.18 – 2.41)
0.004 |
0.74 (0.48 – 1.14)
0.17 |
1.42 (0.87 – 2.31)
0.16 |
0.97 (0.56 – 1.68)
0.90 |
0.78 (0.19 – 3.16)
0.73 |
Liver Disease (None) |
Yes |
1.83 (1.14 - 2.93)
0.01
|
2.18 (0.72 – 6.55)
0.17 |
0.89 (0.12 – 6.82)
0.91 |
1.67 (0.63 – 4.43)
0.31 |
1.70 (0.74 – 3.93)
0.21 |
1.60 (0.26 – 9.94)
0.61 |
Malignancies (None) |
Yes |
3.92 (3.38 - 4.55)
<0.001 |
1.54 (1.02 – 2.32)
0.04 |
7.40 (5.60 – 9.79)
<0.001 |
4.40 (3.00 – 6.44)
<0.001 |
1.22 (0.69 – 2.18)
0.49 |
7.09 (3.54 – 14.20)
<0.001 |
Chronic Anemia (None) |
Yes |
1.46 (1.18 - 1.79)
<0.001 |
1.16 (0.75 – 1.80)
0.50 |
0.85 (0.52 - 1.39)
0.51 |
2.27 (1.48 – 3.49)
<0.001 |
1.40 (0.83 – 2.37)
0.21 |
1.42 (0.58 – 3.52)
0.45 |
Depression
(None) |
Yes |
1.52 (1.04 - 2.22)
0.03 |
1.75 (0.86 – 3.56)
0.12 |
3.08 (1.55 – 6.12)
0.001 |
0.74 (0.18 – 3.13)
0.68 |
2.47 (0.84 – 7.29)
0.10 |
3.26 (1.05 – 10.15)
0.04 |
Complications |
|
|
|
|
|
|
|
Mechanical ventilation > 96 hrs (None) |
Yes |
1.07 (0.87 – 1.32)
0.49 |
0.98 (0.67 – 1.45)
0.93 |
1.35 (0.85 – 2.14)
0.21 |
1.00 (0.60 – 1.64)
0.99 |
1.58 (0.99 – 2.50)
0.05 |
0.49 (0.14 – 1.70)
0.26 |
Tracheostomy (None) |
Yes |
1.26 (0.98 - 1.64)
0.08 |
3.49 (2.22 – 5.47)
<0.001 |
1.28 (0.65 – 2.52)
0.47 |
1.33 (0.63 – 2.83)
0.46 |
0.64 (0.39 – 1.07)
0.09 |
0.45 (0.06 – 3.23)
0.43 |
Acute anemia (None) |
Yes |
0.98 (0.82 -1.18)
0.81 |
1.01 (0.74 – 1.40)
0.93 |
1.02 (0.71 – 1.47)
0.92 |
0.96 (0.62 – 1.49)
0.86 |
0.96 (0.58 – 1.60)
0.88 |
0.45 (0.13 – 1.61)
0.22 |
Coagulopathy (None) |
Yes |
0.91 (0.64 - 1.29)
0.58 |
0.54 (0.26 – 1.14)
0.11 |
1.49 (0.69 – 3.21)
0.31 |
1.37 (0.58 – 3.25)
0.47 |
0.60 (0.29 – 1.24)
0.17 |
4.13 (0.70 – 24.27)
0.12 |
Sepsis
(None) |
Yes |
1.24 (0.90 – 1.69)
0.20 |
0.75 (0.42 – 1.31)
0.31 |
3.41 (1.42 – 8.19)
0.006 |
0.86 (0.33 – 2.25)
0.76 |
1.72 (0.98 – 3.03)
0.06 |
1.35 (0.21 – 8.71)
0.76 |
Discharge site (Home) |
Other† |
1.39 (1.17 – 1.66)
<0.001 |
1.37 (0.98 – 1.93)
0.07
|
1.87 (1.34 – 2.62)
<0.001 |
0.84 (0.54 – 1.30)
0.43 |
1.78 (1.20 – 2.65)
0.004 |
2.80 (1.14 – 6.85)
0.02 |
Hospital LOS
(0-7 days) |
8-11 days |
1.02 (0.84 – 1.24)
0.85 |
0.92 (0.58 – 1.46)
0.72 |
1.00 (0.75 – 1.34)
0.99
|
0.84 (0.52 – 1.38)
0.49 |
1.18 (0.65 – 2.14)
0.60 |
1.38 (0.62 – 3.07)
0.43 |
|
12-19 days |
1.12 (0.91 – 1.37)
0.29 |
1.26 (0.85 – 1.87)
0.25 |
0.86 (0.59 – 1.26)
0.44 |
1.35 (0.85 – 2.15)
0.20 |
1.21 (0.68 – 2.15)
0.51 |
1.25 (0.49 – 3.15)
0.64 |
|
20+ days |
1.53 (1.20 – 1.94)
0.0006 |
1.26 (0.79 – 2.01)
0.33 |
1.04 (0.62 – 1.75)
0.88 |
2.41 (1.41 – 4.11)
0.001 |
1.09 (0.58 – 2.07)
0.78 |
3.24 (1.26 – 8.30)
0.01 |
*Other includes Asian, Hispanic, and unknown. †Other includes discharge to in-patient rehabilitation facility, nursing home and other acute care facilities.