Background: Levosimendan, an inotrope and vasodilator, is a second-line treatment for cardiogenic shock. One of its main advantages is its prolonged effect compared to Dobutamine.
Case presentation: Our reports show two cases of successful treatment with levosimendan involving patients presenting in a state of cardiogenic shock with electrical storm, which had never been described before. Levosimendan infusion, with no initial bolus dose, enabled us to correct the low cardiac output in both cases, with no secondary rhythm disturbances. Apart from the absence of any pro-arrhythmic effect, infusion of Levosimendan allowed a double angioplasty to be performed under optimal haemodynamic conditions in the first case; and provided us sufficient time to reflect on implantable cardioverter defibrillator implantation with resynchronization due to its conversion into active metabolites in the second case.
Conclusion: Through the neutral effects of levosimendan on heart rhythm and the absence of Myocardial oxygen consumption (MvO2) increasing, these two cases show the feasibility and safety of levosimendan treatment in this indication; and emphasise levosimendan’s lack of pro-arrhythmic effect when used without a bolus dose.
levosimendan, ventricular tachycardia, cardiogenic shock
Levosimendan is an inotropic and vasodilator agent known for many years. One of its main advantages is its prolonged effect compared to Dobutamine. However, it is still maybe underused in the intensive care unit. This paper discusses two clinical cases of patients admitted to the cardiology intensive care unit with cardiogenic shock accompanied by severe arrhythmia and treated by Levosimendan. In these two cases, we will discuss the absence of a pro-arrhythmic effect.
Case number 1: A 63-year-old male patient was initially admitted with advanced congestive heart failure. He was obese (BMI 42 kg/m²), a former heavy smoker with dyspnoea on the slightest exertion of several weeks' duration, significant anasarca on admission, 75% oxygen saturation in ambient air and blood pressure of 90/60 mmHg. The electrocardiogram revealed sinus tachycardia at 136 BPM accompanied by numerous non-sustained ventricular tachycardias. The cardiac ultrasound revealed severe bi-ventricular dysfunction with a dramatic drop in left ventricular ejection fraction (LVEF) to 15%, global hypokinesia, left ventricular end-diastolic diameter of 62 mm, cardiac index of 1.7 l/min/m², E/E’ ratio of 15, grade III mitral insufficiency, bi-ventricular asynchronism, tricuspid S wave of 7 cm/s and bilateral pleural effusion (Figures 1 and 2). Test results: creatinine clearance 80 ml/min, BNP 2000 pg/ml, negative troponins, hepatic transaminases were normal. Coronary angiography was performed on D3, after diuretic treatment (loss of 10 kg) and revealed significant stenosis in the proximal and mid portions of the left anterior descending artery (LAD) with significant stenosis of the right coronary artery, medium segment. The clinical course was marked by sustained, refractory ventricular tachycardias on D4 despite high doses of anti-arrhythmic combination therapy comprising Cordarone (600 mg/24 hours) and Xylocaine (1 g/24 hours), oliguria and a low cardiac output (cardiac index of 1.5 l/min/m²). A decision to carry out complete coronary revascularisation was taken after infusion of Levosimendan (0.2 gamma/kg/min over 24 hours). Hemodynamic tolerance was excellent, cardiac output was corrected and the ventricular tachycardias disappeared. Angioplasties of the right coronary artery and LAD were performed on D1 following infusion of Levosimendan (active stents). Treatment for heart failure was gradually introduced comprising Ramipril and Bisoprolol 1.25 mg, with unfortunately further rhythmic deterioration and recurrence of sustained ventricular tachycardias 7 days after infusion of Levosimendan. A decision to implant a defibrillator with resynchronisation was taken after a second course of Levosimendan at the same dose level (D 14). Following resynchronisation, the LVEF increased to 30%. The clinical course was favourable, and the patient was discharged on D 30 [note ICD (implantable cardioverter defibrillator) shock on D20]. The six-month follow-up was good. The patient presented grade II dyspnoea according to the NYHA (New York Heart Association) classification and LVEF had increased to 40%.
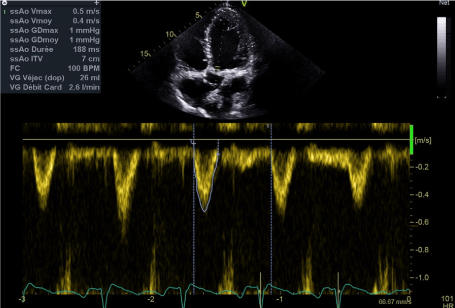
Figure 1. Sub-aortic at the admission, first patient
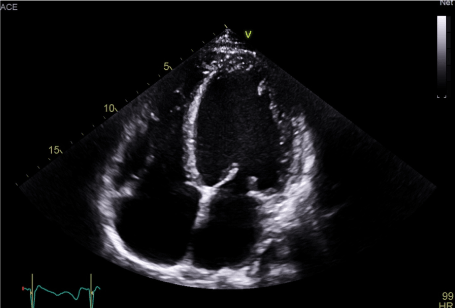
Figure 2. TEE at the admission, first patient
Case number 2: The second case involves a 67-year-old male patient who was initially treated for elective removal of chronic occlusion of the right coronary artery due to stable angina with inferior wall ischemia on stress echocardiography. His cardiovascular risk factors are as follows: type II, insulin-dependent diabetes, heavy smoker, ischemic cardiopathy with LAD angioplasty in 2016 and intact LVEF. The RCA angioplasty procedure was carried out via double radial access with anterograde strategy. During the procedure, the patient suddenly presented refractory cardiac arrest (ventricular fibrillation) associated with iatrogenic occlusion of the acute marginal branch. The patient was intubated and 11 electric shocks were administered overall in conjunction with cardio-thoracic resuscitation and infusion of adrenaline (5 mg as bolus injection). The arrhythmia disappeared following repermeabilisation of the acute marginal branch. The procedure was completed, and the patient was transferred to the intensive care unit with intra-aortic balloon and continuous adrenaline infusion (5 mg/h). A severe low left ventricular function was noted on admission: LVEF 15% with evidence of myocardial stunning, subaortic velocity time integral 8 cm/sec, grade 2 mitral insufficiency, systolic pulmonary arterial pressures 40 mmHg and dilated inferior vena cava. Treatment with noradrenaline and dobutamine was administered with persistent signs of low cardiac output according to the echo (cardiac index 1.9 l/min/m²). The patient received one course of Levosimendan (0.2 gamma/kg/min over 24 hours) with correct haemodynamic tolerance following continuation of vasopressor treatment and crystalloid fluid loading on D0 and D1 following i.v. infusion. No arrhythmia was reported. The clinical course improved rapidly with extubation on D1, normalisation of left ventricular function and discharge on D15.
Levosimendan is an “inodilator” with positive inotropic function and additional phopshodiesterase III inhibiting properties, which is working by increasing the calcium sensitivity of contractile proteins [1]. Its action on myosin is indirect binding to calcium-saturated cardiac troponin C and enhancing the myofilament sensitivity to calcium [1]. Levosimendan improves myocardial contraction, increases stroke volume, without increasing oxygen consumption (MvO2). On the other hand, by opening the ATP-sensitive potassium channels, it induces vasodilation, including coronary arteries [2].
Following the results of the LIDO study [3], it is now recommended as second-line therapy in the management of cardiogenic shock and is a treatment of choice in situations of refractory cardiogenic shock with circulatory assistance [4]. However, to our knowledge, the case we reported is the first instance where Levosimendan has been used to correct severe arrhythmia. The therapeutic management of acute heart failure involves traditionally dobutamine which is an adrenoceptor agonist with a strong beta-adrenergic effect and is the inotrope of first choice [5]. However, dobutamine has a strong chronotropic effect which is dose-dependent and is well-known to be associated to frequent tachycardia [6].
Based on the cases outlined, we are able to discuss the clinical absence of any pro-arrhythmic effect of Levosimendan use. Indeed, with the first patient, sustained ventricular tachycardia totally disappeared following i.v. infusion of Levosimendan. The tachycardia was probably the consequence of low cardiac output secondary to advanced, hypokinetic dilated cardiomyopathy. In case number 2, no arrhythmia recurred despite the initial ventricular tachycardia storm. The aim is not to redefine Levosimendan as an anti-arrhythmic agent, but we believe it is important to emphasise the absence of any pro-arrhythmic effect particularly following continuous slow, i.v. infusion without bolus. On the other hand, it is clear that levosimendan can be proarrhythmic when it is used with a bolus at initiation of treatment, as shown by the results of the SURVIVE randomized trial [7]. Bolus use or loading dose are no longer recommended [4]. The levosimendan infusion, with no initial bolus dose, enabled us to correct the low cardiac output in both cases, with no secondary rhythm disturbances. Unlike catecholamines, levosimendan does not increase myocardial oxygen consumption (MvO2) and does not cause tachycardia, which explains its lack of arrhythmogenic effect, which is particularly prevalent in ischaemic cardiomyopathy [8]. In a meta-analysis focused on patients with Heart Failure Complicating Acute Coronary Syndrome, tachycardia was not more prevalent in the Levosimendan group versus placebo [9]. The feasibility and safety in use of Levosimendan in this indication should also be raised.
Lastly, the prolonged inotropic effect of Levosimendan due to its conversion into active metabolites with a half-life of more than 7 to 9 days provided sufficient time to reflect on ICD implantation with resynchronization [10]. In our cases, Levosimendan could be described as providing a "Bridge to medical reflection" or "bridge to recovery", thus avoiding recourse to circulatory support and its well-known risks.
To conclude, in the light of both these clinical cases, we consider Levosimendan to be a safe and appropriate inotropic agent for the management of cardiogenic shock with severe arrhythmia. To our knowledge, this indication has never been described before. Additional investigations will probably be required to corroborate our case and prove the absence of any pro-arrhythmic side effect of Levosimendan.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
We gratefully acknowledge the valuable contribution of the Department of Cardiac Intensive Care Unit and the department of interventional cardiology.
The authors Alain Rougé and Jacques Monségu declare that they have no competing or conflicts of interests, no financial relationship with a business, organization or group specified below, regarding the subjects discussed in the paper.
The authors declare that there is no funding regarding the publication of this article.
- Pollesello P, Papp Z, Papp JG (2016) Calcium sensitizers: What have we learned over the last 25 years? Int J Cardiol 203: 543-548. [Crossref]
- Ikonomidis I, Parissis JT, Paraskevaidis I, Kourea K, Bistola V, et al. (2007) Effects of levosimendan on coronary artery flow and cardiac performance in patients with advanced heart failure. Eur J Heart Fail 9: 1172-1177. [Crossref]
- Follath F, Cleland JGF, Just H, Papp JGY, Scholz H, et al. (2002) Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet 360: 196-202. [Crossref]
- Herpain A, Bouchez S, Girardis M, Guarracino F, Knotzer J, et al. (2019) Use of Levosimendan in Intensive Care Unit Settings: An Opinion Paper. J Cardiovasc Pharmacol 73: 3-14. [Crossref]
- Thiele H, Ohman EM, Desch S, Eitel I, de Waha S (2015) Management of cardiogenic shock. Eur Heart J 36: 1223-1230. [Crossref]
- Hoke RS, Müller-Werdan U, Lautenschläger C, Werdan K, Ebelt H (2012) Heart rate as an independent risk factor in patients with multiple organ dysfunction: a prospective, observational study. Clin Res Cardiol 101: 139-147. [Crossref]
- Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, et al. (2007) Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA 297: 1883-1891. [Crossref]
- Ukkonen H, Saraste M, Akkila J, Knuuti MJ, Lehikoinen P, et al. (1997) Myocardial efficiency during calcium sensitization with levosimendan: a noninvasive study with positron emission tomography and echocardiography in healthy volunteers. Clin Pharmacol Ther 61: 596-607. [Crossref]
- Shang G, Yang X, Song D, Ti Y, Shang Y, et al. (2017) Effects of Levosimendan on Patients with Heart Failure Complicating Acute Coronary Syndrome: A Meta-Analysis of Randomized Controlled Trials. Am J Cardiovasc Drugs 6: 453-4663. [Crossref]
- Erdei N, Papp Z, Pollesello P, Edes I, Bagi Z (2006) The levosimendan metabolite OR-1896 elicits vasodilation by activating the K(ATP) and BK(Ca) channels in rat isolated arterioles. Br J Pharmacol 148: 696-702. [Crossref]


