Background: Working memory is a system that can store and process information of the current task in the process of solving cognitive tasks. It also plays an important role in leaning motor skills. The memory system of the "cortex - basal ganglia - thalamic loop" model suggests a need for the participation of the prefrontal lobe (PL) and basal ganglia (BG). The PL is critical in modulation of attention and working memory. And the BG is a major hub on the working memory loop. We investigated differential characteristic of working memory and learning ability caused by a unilateral basal ganglia lesion (BGL) and a prefrontal lobe lesion (PLL) to provide a strategy for cognitive rehabilitation.
Materials and method: In the current study, we divided 27 patients into a PLL group (n=12) and a BGL group (n=15). All subjects were evaluated with Mini-mental state examination (MMSE), Wechsler Memory Scale-Revised (WMS-R), Trail Making Test (TMT), Tower of Hanoi (TOH) and Wisconsin card sorting test (WCST). Then, all patients were trained by a process of “testing-learning-retesting” with TOH and WCST. Subsequently, their learning performance was re-evaluated.
Results: We found a marked decrease in the working memory of PLL and BGL. However, after training, BGL scores were significantly improved, while PLL were not.
Conclusions: This indicated that unilateral BGL retained a degree of learning ability, and thus early cognitive training has the advantage of improving working memory and learning ability in patients with BGL.
prefrontal lobe lesion, basal ganglia lesion, working memory, learning ability, cognitive training
In 1974, Baddeley and Hitch proposed the concept of a working memory based studies of short-term memory impairment [1]. It is important to appreciate that the memory system refers to the ability to temporarily maintain and manipulate information. It is a combination of the traditional fields of attention, concentration, and short-term memory [2]. The memory system is the basis of many higher cognitive functions including learning, language understanding, reasoning and judgment. Thus, it can be used as a necessary means of occupational therapy.
Working memory has traditionally been divided into components that process phonologic information or spatial information and an executive system that allocates attention resources [2]. The central executive system is at the core of a working memory. Numerous studies have shown that working memory required the participation of cortical networks and sub-cortical areas, especially the prefrontal cortex to perform a specific task [3,4].
The learning process is constituted by the composition of the following three basic aspects: 1) directional links (i.e., storage systems); 2) action links (i.e., retrieval system); and 3) feedback links (i.e., back system). The first two areas constitute the working memory, while the latter aspect can be considered corrective actions that are used for retrieving the results of inspection, regulation and recognition.
More recent studies have suggested that working memory and error detection are important in motor skill learning [5,6]. Neurocomputational models of basal ganglia function has shown us that this model can select the action or make the frontal cortical express a reward or to a correct feedback, reducing the occurrence of errors or negative feedback [7]. The frontal lobe is the most advanced part of the cerebrum that includes the primary motor area, premotor cortex and the prefrontal cortex. Fiber links exist between the prefrontal area and other areas such as the striatum, the amygdala, the temporal lobe, and the occipital and parietal lobe, so the prefrontal cortex participates in a variety of sensory information processing, attention, memory, thinking and emotional responses [8,9]. Behavioral studies have also confirmed that the prefrontal cortex and attention and memory are closely related, including working memory, source memory, and sequential organization, which inhibit the release of proactive capacity, metamemory, and problem-solving ability [8,9]. Some studies have found that a visual working memory can be impaired if prefrontal cortex is damaged [10,11]. The prefrontal cortex plays an important role in encode, update and maintain the working memory task context [12].
Basal ganglia are composed of an input structure and an output structure, the former of which, includes the caudate nucleus, putamen and nucleus accumbens, and the latter of which, includes the globus pallidus, subthalamic nucleus, and the substantia nigra reticular. Neuroanatomy studies have found neural circuitry between the basal ganglia and the frontal lobe, which participate in the formation of the brain's higher cognitive functions [13]. There is a high density of afferent projections to the frontal lobe, so the frontal lobe is an important functional fronto-striatal interaction [14,15]. Taylor proposed the Working Memory System of a "cortex - basal ganglia - thalamic loop" model [16]. This model was composed of: (1) short loops: cortex - thalamus - cortex; (2) a long loop: cortex - striatum - within the globus pallidus - thalamus - cortex; (3) the first indirect loop: cortex - (subthalamic nucleus - external globus pallidus) - within the globus pallidus - thalamus - cortex; and (4) a second indirect Loop: cortex - striatum - (external globus pallidus - the subthalamic nucleus) - within the globus pallidus - thalamus – cortex. However, most current studies on cognitive function related to the basal ganglia were derived from basal ganglia degenerative diseases [17,18]. For instance, Parkinson’s disease patients in the off-medication state display strong impairment in the rule-switching paradigm due to their incapacity to apply a different rule either from perseveration or from learned irrelevance [19]. Their attention flexibility is reduced and can’t switch freely between the two tasks [20].
The basal ganglia degenerative diseases generally affect both sides and many different degrees of damage are found within the brain cortex; thus, it is difficult to determine the real factors that lead to cognitive impairment. Neuroimaging studies suggested the impairment of working memory may be caused by a wider range of cortex or a connectivity deficiency, rather than a focused deficit in the prefrontal cortex [21]. Some research has shown that the prefrontal cortex is critical for top-down intra-hemispheric modulation of visual working memory with the basal ganglia involved in global support of the visual working memory processes [22]. Thus, we speculated that unilateral damage derived from the basal ganglia and prefrontal lobe might show similar impairments in working memory and learning ability, which could be easily restored in the basal ganglia lesion than in the prefrontal lobe lesion by proper cognitive training.
The objective of this study was to investigate the differential characteristics of working memory and learning ability caused by unilateral basal ganglia and frontal lobe damage in order to provide a cognitive rehabilitation strategy.
The research had been approved by the Institutional Review Board (IRB) of The First Affiliated Hospital of Nanjing Medical University, and all clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. Informed and written consent had also been obtained from the participants.
Participants
Twenty-seven patients with brain injuries (16 males and 11 females) from the First Affiliated Hospital of Nanjing Medical University and the Affiliated Hospital of Nantong University took part in this study. According to the injury sites, all patients were assigned to the group with the prefrontal lobe lesion (PLL, n=12, 6 in the left, 6 in the right) and the group with the basal ganglia lesion (BGL, n=15, 9 in the left, 6 in the right). The patient eligibility criteria included: (1) a vascular lesion (bleeding or infarction) in the basal ganglia and the prefrontal lobe that was identified by brain computerized tomography (CT) or magnetic resonance imaging (MRI) scanning; (2) Mini-mental state examination (MMSE) ≥24 without pronounced cognitive or language problems. In addition, patient exclusion criteria included the presence of a history of mental disease.
Ten healthy volunteers were recruited as a control group. Volunteer eligibility criteria included: (1) no brain damage on CT or MRI scanning; (2) no obvious cognitive or language problems (i.e., an MMSE ≥24); and (3) no history of cardiac or cerebral vascular, endocrinal or mental diseases. The demographic data of all experimental groups are shown in Figure 1 and Table 1.
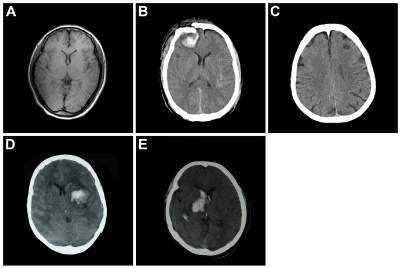
Figure 1. Brain CT and MRI scanning.
A: normal (MR); B: hemorrhage of the left PF (MR); C: infarction of the right PF (CT); D: hemorrhage of the left BG (CT); and E: hemorrhage of the right BG (CT).
Table 1. General information of subjects included in experiments.
|
Controls |
PLL |
BGL |
Gender (male/female) |
5/5 |
6/6 |
10/5 |
Age (year) |
53.5 ± 16.87 |
48.13 ± 13.89 |
55.12 ± 13.75 |
Education level (year) |
12.8 ± 2.97 |
10.15 ± 2.96 |
12.08 ± 3.54 |
Course (day) |
|
32.34 ± 13.56 |
65.00 ± 26.58 |
Location of lesion |
|
9 PLH
3 PLI |
6 BGH
9 BGI |
Values are means ± SD. PLL: Prefrontal Lobe Lesion; BGL: Basal Ganglia Lesion; PLH: Prefrontal Lobe Hemorrhage; PLI: Prefrontal Lobe Infarction; BGH: Basal Ganglia Hemorrhage; BGI: Basal Ganglia Infarction.
Assessment tasks
MMSE: MMSE was used to examine the degree and field of impairment in cognitive function that comprised orientation (i.e., the when and where), calculation, memory (i.e., temporal memory and recall), language (i.e., name, repeat, reading, writing, construction, and three-step instruction), as the inclusion criteria for patients [23].
Wechsler Memory Scale-Revised (WMS-R): WMS-R was used to examine auditory, visual, verbal and spatial working memory that comprised figural memory, logical memory, visual paired associates, verbal paired associates, visual reproduction, digit span, visual memory span [24].
Trail Making Test (TMT): The TMT was used to examine switching speed and executive function. In TMT-A, the subjects connected a line from 1 to 25 as fast and accurately as possible. In TMT-B, the subjects connected alternately a line between numbers and letters ((i.e., 1-A-2-B-3-C). The score is the time spent to complete the task. The test is stopped if the subjects can’t complete it in 300 seconds or five errors are made, in which of the situation, a score of 300 seconds and five errors are recorded [25,26].
Tower of Hanoi (TOH): The TOH is a device with three pillars and five different sizes of the discs (ABCDE). These discs are stacked on three pillars according to a start state (Figure 2A), the subjects are asked to make the discs stack on descending order on middle pillar (Figure 2B). The subjects are told the three rules for the task: (1) only one disc can be moved at a time; (2) any disc not being currently moved must remain on a pillars; (3) a smaller disc must be placed on a larger disc. Three response measurements of accuracy, speed, and scores (scores = accuracy·speed·difficulty index (DI)·2000, DI=1.47) are used to assess executive functions of planning, order and problem resolving [27].
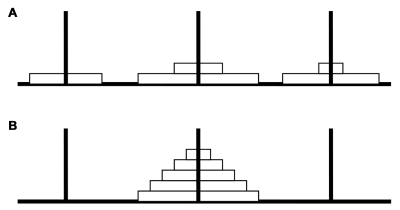
Figure 2. TOH sketch.
The five disks are stacked on three pegs (A). By moving only one disk at a time, the subject must rearrange the stack of 5 disks in descending order on the middle peg (B).
Wisconsin card sorting test (WCST): The WCST materials consist of four stimulus cards and a deck of 64 response cards. The set of four stimulus cards being comprised of one red triangle, two green stars, three yellow crosses, and four blue circles reflected three stimulus parameters: Color, Form, and Number. The 64 response cards also displayed characteristics of varying forms, color and numbers of figures. Each response card could be matched to a stimulus card on one, or a combination of the three stimulus parameters. The response cards were numbered from 1 to 64, with one of those numbers on the lower left corner of the reverse side to ensure a standard order of presentation. The WCST record page was used to record the subjects’ performance and to enter scoring dimensions that were used for calculating the WCST scores. Response measurements contained Percent Errors (PE), Percent Preservative Responses (PPR), Percent Conceptual Level Responses (PCLR), Numbers of Categories Completed (NCC), and Trails to Complete the First Category (TCFC). WCST was used to evaluate learning, conceptual classification of working memory, formation and shifting of concept, and capacity of planning, judging and organization [28].
Procedures
Baseline assessment: All subjects were given access to a quiet room with appropriate temperature and soft light. The history taking and the necessary physical examination were performed before neuropsychological assessments. General information such as name, age, gender, education level, race, address and hands-dominant (i.e., right or left) were recorded. All of the subjects were right hand-dominant. All subjects received MMSE, WMS, TMT, TOH and WCST tests before training.
Training process: We designed the training process with “testing-learning-retesting” of TOH and WCST to evaluate the effects of the prefrontal lobe and basal ganglia on executive function (Figure 3).
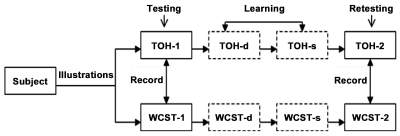
Figure 3. Learning process of “Testing-Learning-Retesting” with TOH and WCST.
TOH-1 and WCST-1: in which the subjects were directly instructed to operate TOH and WCST and to first record their scores. TOH-d and WCST-d: in which the operating essentials of TOH and WCST were introduced and operational processes were demonstrated to the subjects. TOH-s and WCST-s: in which subjects were required to operate TOH and WCST again after studying the procedures.TOH-2 and WCST-2: the subject was requested to test TOH and WCST again and to finally record their scores.
In TOH, a start state is given in which five discs are stacked on three pillars (Figure 2A). First, the subjects were told the rules of the test and operated themselves to make the discs stack on descending order on middle pillar (Figure 2B). At this time, three response measurements of accuracy, speed, and scores were recorded in each trial, named as TOH-1 during testing. Second, the five discs were stacked on the same state. We moved the discs to Figure 2B through seven steps and demonstrated to the subjects how to move the disks, which was named TOH-d. Then the subjects were requested to operate TOH again. We immediately corrected mistakes in each step if the subjects made an error. We named the learning TOH-s. Finally, the subjects were requested to test TOH again and to record their scores, which were named as TOH-2 during retesting.
In WCST, the four stimulus cards with the following characteristics were in front of the subjects (Figure 4). We handed the subjects the 64 response cards and asked them to match each consecutive card with one of the four stimulus cards according to a certain sorting rules. The subjects were told whether the match was right or wrong and never told the right classification rules. When the subjects had six consecutive correct matches, we changed the sorting principle without warning and asked the subjects develop a new sorting strategy according to our feedback. The WCST switched among three possible sorting principles (i.e., Color, Form, and Number), while we recorded the subjects responses as WCST-1. In addition, we then decked the four stimulus cards and the 64 response cards as an identical standard order of presentation. We explained the rules again and had two investigators demonstrate how to classify and match, which was named as WCST-d. The subjects were requested to operate WCST with the same order of category rules again. If the subjects had made six consecutive “wrong” matches to the sorting principle, we would tell the subjects that we had changed to Color, Form, or Number and then instruct the subjects to match, which was named as WCST-s. Finally, the subjects were requested to test WCST again and to record their scores, which was named as WCST -2 during retesting.
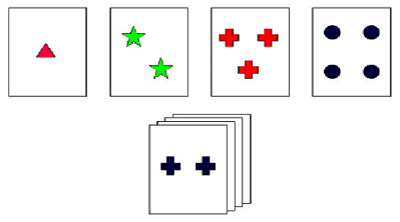
Figure 4. WCST sketch.
The four stimulus cards with the following characteristics are placed before the subject. The subject randomly matches each consecutive card from the 64 response cards with one of four stimulus cards according to color, shape, or number of symbols. The subject is told whether the response is correct or not and is never told the correct sorting principle. Then without warning, the sorting principle changes after six consecutive “correct” responses.
Statistics
All data were determined by the SPSS version 16 software package. All values were represented as mean ± standard deviation. Significant differences among three groups were determined by one-way ANOVA. Test of homogeneity of variance applied Bonferroni’s method of multiple comparisons, but the heterogeneity of variance used Tamhane's method. Independent Samples T-test was used to compare the mean values of TOH and WCST before and after training between these two groups. Independent Samples T-test was also used to compare the mean values of difference before and after training in TOH and WCST between these two groups. The changes in TOH and WCST before and after training in the same group were analyzed by paired Student’s t test. The criterion for statistical significance was an alpha value of p < 0.05.
General information and MMSE of subjects in three groups
Demographic data such as gender, age and education showed no significant differences among the three groups (Table 1). The scores of total and items of calculation and recall were significantly lower in these two groups with PLL and BGL as compared the control group. There were no significant differences in all items of MMSE between groups with PLL and BGL (Table 2).
Table 2. Baseline test of MMSE in three groups
MMSE |
Control |
PLL |
BGL |
Orientation |
10.00 ± 0.00 |
9.5 ± 0.52 |
10.00 ± 0.00 |
Temporal memory |
3.00 ± 0.00 |
2.58 ± 0.51 |
2.73 ± 0.46 |
Calculation |
5.00 ± 0.00 |
3.33 ± 1.15* |
4.13 ± 0.52* |
Recall |
2.70 ± 0.48 |
0.92 ± 0.67* |
1.4 ± 0.51* |
Language |
9.00 ± 0.00 |
8.83 ± 0.39 |
8.73 ± 0.46 |
Values are means ± SD; *p<0.05 versus values in control by Bonferoni post-hoc test. PLL: Prefrontal Lobe Lesion; BGL: Basal Ganglia Lesion
Baseline tests of WMS-R and TMT in three groups
All items of WMS-R were significantly lower in these two groups with frontal lesions and basal ganglia lesions than in the control group, and there were no significant differences between these two groups with PLL and BGL. The completion times of TMT-A and TMT-B in these two groups with PLL and BGL were significantly greater than their control group counterparts, although there were no significant differences between these two groups with PLL and BGL (Table 3).
Table 3. Base-line tests of WMS-R and TMT in three groups
|
Control |
PLL |
BGL |
WMS-R |
|
|
|
FM |
8.00 ± 0.94 |
4.00 ± 2.00* |
5.67 ± 1.83* |
LM |
36.80 ± 3.49 |
14.5 ± 4.30* |
17.2 ± 7.68* |
ViPA |
14.0 ± 1.49 |
5.67 ± 3.55* |
5.67 ± 4.27* |
VePA |
20.50 ± 1.96 |
12.17 ±4.20* |
13.40 ± 6.08* |
VR |
40.00 ± 1.49 |
24.17 ± 9.53* |
32.46 ± 7.33* |
DS |
19.90 ± 2.02 |
10.33 ± 3.47* |
13.4 ± 3.52* |
VS |
21.70 ± 2.75 |
10.25 ± 2.63* |
13.13± 3.37* |
TMT |
|
|
|
TMT-A (s) |
31.50 ± 10.60 |
125.92 ± 77.78* |
101.4 ± 53.62* |
TMT-B (s) |
92.00 ± 24.74 |
436.25 ± 133.42* |
348.87 ± 172.14* |
Values are means ± SD; *p<0.05 versus values in control by Bonferoni post-hoc test. S: Seconds; FM: Figural Memory; LM: Logical Memory; ViPA: Visual Paired Associates; VePA: Verbal Paired Associates; VR: Visual Reproduction; DS: Digit Span; VS: Visual Memory Span; TMT: Trail Making Test.
Baseline tests of TOH and WCST in three groups
The scores of total and the items of accuracy and speed in TOH were significantly lower in these two groups with PLL and BGL than control group, but were not significantly different between groups with PLL and BGL. In WCST, Percent Errors (PE), Percent Preservative Responses (PPR) and Trails to Complete First Category (TCFC) in these two groups with PLL and BGL increased significantly, and Percent Conceptual Level Responses (PCLR) and Number of Categories Completed (NCC) in groups with PLL and BGL decreased significantly when compared with the control group. TCFC in the group with PLL increased significantly when compared with the BGL groups and the control (Table 4).
Table 4. Base-line tests of TOH and WCST in three groups
|
Control |
PLL |
BGL |
TOH |
|
|
|
accuracy |
0.932 ± 0.094 |
0.593 ± 0.212* |
0.664 ± 0.164* |
speed |
0.885 ± 0.026 |
0.536 ± 0.239* |
0.627 ± 0.219* |
scores |
2456.9 ± 238.2 |
982.7 ± 581.4* |
1268.4 ± 582.3* |
WCST |
|
|
|
PE |
0.203 ± 0.048 |
0.581 ± 0.156* |
0.568 ± 0.117* |
PPR |
0.127 ± 0.020 |
0.366 ± 0.109* |
0.367 ± 0.11* |
PCLR |
0.781 ± 0.052 |
0.352 ± 0.157* |
0.352 ± 0.157* |
NCC |
5.70 ± 0.48 |
2.17 ± 1.80* |
2.53 ± 1.30* |
TCFC |
7.00 ± 0.94 |
24.00 ± 13.7* |
11.6 ± 5.53*# |
Values are means ± SD. *p<0.05 versus values in control by Bonferoni post-hoc test. #p<0.05 versus values in PLL by Bonferoni post-hoc test. PLL: Prefrontal Lobe Lesion; BGL: Basal Ganglia Lesion. TOH scores = accuracy•speed•1.47•consistent (consistent=2000). PE: Percent Errors; PPR: Percent Preservative Responses; PCLR: Percent Conceptual Level Responses; NCC: Number of Categories Completed; TCFC: Trails to Complete First Category.
Comparison of TOH and WCST before and after training in these two groups with PLL and BGL
There was no significant difference in TOH and WCST before and after training in the group with PLL except for scores of TOH. In the group with BGL, accuracy and the TOH scores after training increased significantly in comparison with the group with BGL before training and the group with PLL after training. The speed of TOH after training increased significantly in comparison with the group with BGL before training. PE, PPR and TCFC of WCST after training in the BGL group were significantly lower than was determined before training in the same group and after training in the PLL group. PCLR and NCC of WCST after training were significantly higher than was found before training in the same group and after training in the PLL group. The difference in Post-training and Pre-training of HOT and WCST multiple indicators in BGL group improved significantly compared with PLL group (Table 5).
Table 5. Comparison of TOH and WCST in the two groups with brain injuries between pre-training and post-training
|
PLL |
BGL |
Pre-training |
Post-training |
Post-Pre |
Pre-training |
Post-training |
Post-Pre |
TOH |
|
|
|
|
|
|
accuracy |
0.593 ± 0.212 |
0.645 ± 0.192 |
0.053±0.097 |
0.664 ± 0.164 |
0.889± 0.112*# |
0.224 ± 0.193△ |
speed |
0.536 ± 0.239 |
0.619 ± 0.218 |
0.083±0.163 |
0.627 ± 0.219 |
0.768 ± 0.152* |
0.151 ± 0.139 |
scores |
982.7± 581.4 |
1183.58 ± 571.68* |
200.83± 288.1 |
1268.4 ± 582.3 |
2012.13 ± 494.87*# |
748.53 ± 501.22△ |
WCST |
|
|
|
|
|
|
PE |
0.581 ± 0.156 |
0.561 ± 0.198 |
-0.02±0.131 |
0.568 ± 0.117 |
0.363 ± 0.141*# |
-0.204 ± 0.144△ |
PPR |
0.366 ± 0.109 |
0.402 ± 0.218 |
0.038±0.119 |
0.367 ± 0.11 |
0.227± 0.087*# |
-0.143 ± 0.107△ |
PCLR |
0.352 ± 0.157 |
0.377 ± 0.199 |
0.023±0.074 |
0.352± 0.157 |
0.591 ± 0.19*# |
0.238 ± 0.217△ |
NCC |
2.17 ± 1.80 |
2.42 ± 1.98 |
0.25±0.62 |
2.53 ± 1.30 |
4.00 ± 1.73*# |
1.47 ± 1.55△ |
TCFC |
24.00 ± 13.7 |
19.75 ± 15.57 |
-4.25±13.07 |
11.6 ± 5.53 |
7.47 ± 2.64*# |
-4.00 ± 6.02 |
Values are mean ± SD. * p<0.05 versus values in respective pre-training of PLL and BGL by paired Student’s t test. #p<0.05 versus values in post-training of PLL by Independent-Sample T-tests. △p<0.05 versus values in post-pre of PLL by Independent-Sample T-tests.
PLL: Prefrontal Lobe Lesion; BGL: Basal Ganglia Lesion; TOH scores= accuracy•speed•1.47•consistent (consistent=2000); PE: Percent Errors; PPR: Percent Preservative Responses; PCLR: Percent Conceptual Level Responses; NCC: Number of Categories Completed; TCFC: Trails to Complete First Category.
Subjects with prefrontal lobe lesion had impaired abilities in terms of speed of resolving problems, attention, memory, formation and switching of conception, and planning and problem resolving, which will severely affect their quality of life in terms of their occupation and in society. The study found that working memory was regulated by phasic dopamine gating signals and frontal lobe played a key role in the encoding, updating, maintaining stages of working memory [12]. Garlinghouse et al., found that the left and right frontal lobe volumes was smaller, working memory was poorer in daily life [29].
In accord with previous studies, the results derived from MMSE, WMS-R and TMT indicated that subjects with prefrontal lesions had significant dysfunction in calculation, temporal memory, and short-term memory, and attention ability such as concentration, conversion, distribution and control [30,31]. Other studies have shown that subjects with prefrontal lesions had significant decreases in first correct moves and times for completion of the Tower of London (TOL) than did controls and higher percent errors in three different dimensions of attention shifting tasks [32]. In our present study, scores of TOH and WCST in subjects with frontal lobe lesions were significantly lower than was found in controls. It was difficult to concentrate the attention of the subjects with frontal lobe lesions, which couldn’t find the rules and conversion the rules.
According to the “Cortex - basal ganglia - thulamus” circle model of a working memory system [16], we assumed that basal ganglia damage could block the information flow from the prefrontal lobe. Therefore basal ganglia lesions and prefrontal lobe lesions have similar symptoms of cognitive dysfunction, which is called “Remote Effects”. Animal study have shown that the basal ganglia could quickly learn task-relevant rules and send preprocessed information to the prefrontal cortex for subsequent selection and further processing [33]. Neurodegenerative disease studies showed that the destruction of the frontal lobe or the pathway between the frontal and subcortical lobe could result in working memory impairment, such as that found in Alzheimer's disease, multiple sclerosis, and Parkinson's disease [18,34].
Our present study found that there were no statistical differences in all items of TOH and the mjority of items for WCST except the trails to complete the first category between these two groups with BGL and PLL, which suggested the importance of the connecting role of basal ganglia on working memory. In addition, fMRI study of verbal working memory has found that there was a reciprocal action between the basal ganglia and the ventrolateral prefrontal cortex in the stage of encoding and maintenance, and the dorsal lateral prefrontal cortex, and the anterior cingulate cortex during the extraction phase [35].
In regard to set-shifting of fronto-striatal activation, Monchi et al., reported a significant increased activity of the ventrolateral prefrontal cortex and caudate nucleus during the reception of negative feedback in fMRI [36]. This observation indicated that a shift to a new response set was required, while the putamen was significantly active with the posterior PFC and premotor cortex during matching following a negative feedback, which suggested involvement in the execution of novel actions.
In our present study, the trails to complete the first category of WCST in the group with PLL were significantly higher than in the control group and the group with BGL. The trails to complete the first category is the number of successful completions of the first classification project, which is typically used for judging the degree of the initial concept of brain information to the outside world. These experimental results suggested that patients with frontal lobe injury were significantly damaged during the initial conceptualizing stages of formation, which is distinct from the basal ganglia damage.
The frontal lobe can integrate with the external information conducted by the posterior cerebral cortex (i.e., temporal lobe, parietal lobe, and occipital lobe) and internal information conducted by the limbic system. Frontal lobe damage will result in the same performance as temporo-parietal lobe injury, which is one of primarily serious learning obstacles associated with a particular module and reasoning ability obstacles. This means that there will be an inability to learn sequence arrangement and organization, and that an impaired working memory might interfere with learning ability [37].
Our present study found that TOH and WCST in subjects with frontal lobe lesions had no significant difference before and after training, suggesting that damage to the frontal lobe leads to injured learning ability. Since damage to the frontal lobe decreases proactive inhibition release, the frontal lobe still stores previous error messages, and cannot effectively integrate or update new information, resulting in reduced effectiveness of learning.
Basal ganglia is a middle path for a variety of information inputs and outputs, some research has shown that it plays an important role in the classification of learning [38,39]. In addition, the dorsal striatum was responsible for that function by contributing to various evaluation processes leading to the chosen outcome in contingency learning [40]. In the present study, subjects with basal ganglia lesions improved significantly most of the scores in TOH and WCST after training, which indicated that basal ganglia injury (hemorrhage or infarction) still retained a certain learning ability. Previous studies of basal ganglia degenerative diseases like PD, HD and AD found that patients present classification learning defects [41]. Some researchers have found that the dopamine pathway plays a significant role in implicit learning [42]. Also, advanced neurodegenerative disease is not restricted to the basal ganglia, which is likely to spread to other areas of the brain, especially the cerebral cortex. Since subjects in the present study were limited to local damage of the unilateral basal ganglion (hemorrhage or infarction), then presumably the unaffected side of the basal ganglia retained some learning ability. Wiedl et al., found indicator scores in WCST and TOH after training were associated with difficulty and the greater the difficulty, the higher the correlation [43].
In our present study, learning scores of TOH and WCST training were significantly better in subjects with basal ganglia lesions than in subjects with prefrontal lobe lesions, which suggested that TOH and WCST training should be considered a tool of cognitive rehabilitation training. A limited scope of this study arises from the fact that training and testing was performed on the same tasks, therefore transfer effects of cognitive training are not examined. It is formally possible that the type of rehabilitation proposed herein is task specific. This might imply that the conclusion is that unilateral basal ganglia patients can be retrained on each specific basic skill as shown by the trainability of both these tasks in this study.
2021 Copyright OAT. All rights reserv
Basal ganglia lesions and frontal lobe lesions may decrease the working memory. Working memory defects caused by frontal lobe lesions may be more serious and more widespread. Unilateral basal ganglia lesions still retain a certain learning ability. These rehabilitation strategies such as cognitive training and learning could make patients with injured basal ganglia obtain certain basic skills, and finally enabling them to return to their families and society at large. The fewer samples of this study did not divide the frontal lobe lesion into the left and right sides as compared to the unilateral basal ganglia lesion. We hope that we can identify more direct evidence to demonstrate a role for the basal ganglia by collecting more samples and using fMRI or Transcranial Magnetic Stimulation (TMS).
This study was supported by the National Nature Science Foundation of China (81171854). The study also received funding from the Jiangsu Social Development Support Project (Grant no. BE2012675) in China. Support from the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions is also appreciated. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the final manuscript.
All authors declared that they have no conflict of interest.
This study was supported by the National Nature Science Foundation of China (81171854). The study also received funding from the Jiangsu Social Development Support Project (Grant no. BE2012675) in China. Support from the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions is also appreciated.
- McGettigan C, Warren JE, Eisner F, Marshall CR, Shanmugalingam P, et al. (2011) Neural correlates of sublexical processing in phonological working memory. J Cogn Neurosci 23: 961-977. [Crossref]
- Budson AE, Price BH (2005) Memory dysfunction. N Engl J Med 352: 692-699. [Crossref]
- Y. Jiang, J. V. Haxby, A. Martin, L. G. Ungerleider, and R. Parasuraman, Complementary neural mechanisms for tracking items in human working memory, Science 287: 643-646, 2000. [Crossref]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE (2000) The prefrontal cortex: response selection or maintenance within working memory? Science 288: 1656-1660. [Crossref]
- Anguera JA, Seidler RD, Gehring WJ (2009) Changes in performance monitoring during sensorimotor adaptation. J Neurophysiol 102: 1868-1879. [Crossref]
- Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD (2010) Contributions of spatial working memory to visuomotor learning. J Cogn Neurosci 22: 1917-1930. [Crossref]
- Cohen MX, Frank MJ (2009) Neurocomputational models of basal ganglia function in learning, memory and choice. Behav Brain Res 199: 141-156. [Crossref]
- Petrides M (1994) Frontal lobes and memory: evidence from investigations of the effects of cortical excisions in nonhuman primates, Handbook of Neuropsychology 9: 59-81.
- Shimamura A (1995) Memory and frontal lobe function. Journal of Cognitive Neuroscience 803-813.
- Rossi AF1, Bichot NP, Desimone R, Ungerleider LG (2007) Top down attentional deficits in macaques with lesions of lateral prefrontal cortex. J Neurosci 27: 11306-11314. [Crossref]
- Tsuchida A1, Fellows LK (2009) Lesion evidence that two distinct regions within prefrontal cortex are critical for n-back performance in humans. J Cogn Neurosci 21: 2263-2275. [Crossref]
- D'Ardenne K1, Eshel N, Luka J, Lenartowicz A, Nystrom LE, et al. (2012) Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proc Natl Acad Sci U S A 109: 19900-19909. [Crossref]
- Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357-381. [Crossref]
- Selemon LD, Goldman-Rakic PS (1988) Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behaviour. J Neurosci 8: 4049-4068. [Crossref]
- Yeterian EH, Pandya DN (1991) Prefrontostriatal connections in relation to cortical architectonic organization in rhesus monkeys. J Comp Neurol 312: 43-67. [Crossref]
- Taylor JG, Taylor NR (2000) Analysis of recurrent cortico-basal ganglia-thalamic loops for working memory. Biol Cybern 82: 415-432. [Crossref]
- Ashby FG, Noble S, Filoteo JV, Waldron EM, Ell SW (2003) Category learning deficits in Parkinson's disease. Neuropsychology 17: 115-124. [Crossref]
- Lewis SJ, Cools R, Robbins TW, Dove A, Barker RA, et al. (2003) Using executive heterogeneity to explore the nature of working memory deficits in Parkinson's disease. Neuropsychologia 41: 645-654. [Crossref]
- Owen AM, Roberts AC, Hodges JR, Summers BA, Polkey CE, et al. (1993) Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson's disease. Brain 116: 1159-1175. [Crossref]
- Cools R, Barker RA, Sahakian BJ, Robbins TW (2003) L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson's disease. Neuropsychologia 41: 1431-1441. [Crossref]
- Barendse EM, Hendriks MP, Jansen JF, Backes WH, Hofman PA, et al. (2013) Working memory deficits in high-functioning adolescents with autism spectrum disorders: neuropsychological and neuroimaging correlates. J Neurodev Disord 5: 14. [Crossref]
- Voytek B, Knight RT (2010) Prefrontal cortex and basal ganglia contributions to visual working memory. Proc Natl Acad Sci U S A 107: 18167-18172. [Crossref]
- Li G (1989) Mini-Mental State Examination test in different populations studied. Chinese Mental Health Journal 3: 148.
- Wechsler David (1987) Manual for the Wechsler Memory Scale-Revised. San Antonio: The Psychological Corporation [Crossref]
- Reitan R (1958) Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual Motor Skills 8: 271-276.
- Sink KM, Espeland MA, Rushing J, Castro CM, Church TS, et al. (2014) The LIFE Cognition Study: design and baseline characteristics, Clin Interv Aging 9: 1425-1436. [Crossref]
- Goel V, Grafman J (1995) Are the frontal lobes implicated in planning functions? Interpreting data from the Tower of Hanoi. Neuropsychologia 33: 623-642. [Crossref]
- Grant DA, Berg EA (1948) A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J Exp Psychol 38: 404-411. [Crossref]
- Garlinghouse MA, Roth RM, Isquith PK, Flashman LA, Saykin AJ (2010) Subjective rating of working memory is associated with frontal lobe volume in schizophrenia. Schizophr Res 120: 71-75. [Crossref]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, et al. (1995) The neural basis of the central executive system of working memory. Nature 378: 279-281. [Crossref]
- Prabhakaran V, Narayanan K, Zhao Z, Gabrieli JD (2000) Integration of diverse information in working memory within the frontal lobe. Nat Neurosci 3: 85-90. [Crossref]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, et al. (2002) Decision-making processes following damage to the prefrontal cortex. Brain 125: 624-639. [Crossref]
- Pasupathy A, Miller KE (2005) Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature 433: 873-876.
- Moretti R, Torre P, Antonello RM, Cazzato G, Bava A (2002) Use of galantamine to treat vascular dementia. Lancet 360: 1512-1513. [Crossref]
- Chang C, Crottaz-Herbette S, Menon V (2007) Temporal dynamics of basal ganglia response and connectivity during verbal working memory. Neuroimage 34: 1253-1269. [Crossref]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A (2001) Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci 21: 7733-7741. [Crossref]
- Lezak MD, Howieson, E. Bigler, Tranel D (2004) Neuropsychological Assessment: Oxiford University Press. [Crossref]
- Ashby FG, Maddox WT, Bohil CJ (2002) Observational versus feedback training in rule-based and information-integration category learning, Mem Cognit 30: 666-677. [Crossref]
- Ashby FG, O'Brien JB (2005) Category learning and multiple memory systems. Trends Cogn Sci 9: 83-89. [Crossref]
- Provost JS, Hanganu A, Monchi O (2015) Neuroimaging studies of the striatum in cognition Part I: healthy individuals. Front Syst Neurosci 9: 140. [Crossref]
- Shohamy D, Myers CE, Onlaor S, Gluck MA (2004) Role of the basal ganglia in category learning: how do patients with Parkinson's disease learn? Behav Neurosci 118: 676-686. [Crossref]
- Swainson R, Rogers RD, Sahakian BJ, Summers BA, Polkey CE, et al. (2000) Probabilistic learning and reversal deficits in patients with Parkinson's disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication, Neuropsychologia 38: 596-612. [Crossref]
- Wiedl KH, Schottke H, Green MF, Nuechterlein KH (2004) Dynamic testing in schizophrenia: does training change the construct validity of a test? Schizophr Bull 30: 703-711. [Crossref]




