There is controversy over what findings should be considered symptoms of a cytomegalovirus infection. Congenital infection was established through dried blood spot in a baby with lenticulostriated vasculopathy. Decision of treat was difficult due to the lack of a consensus about lenticulostriated vasculopathy representing a sign of symptomatic congenital infection.
congenital infection, cytomegalovirus, hearing loss, infant, lenticulostriated vasculopathy
Treatment of symptomatic congenital Cytomegalovirus (CMV) infection has been proven effective in reducing the risk of hearing loss [1]. However, defining a symptomatic infection can sometimes be challenging, as there is controversy as to whether some findings should be considered symptoms of a CMV infection or not. This fact is essential when determining the therapeutic approach to the infant.
We present the case of a preterm infant born at 28 weeks, with no major comorbidities during admission. Brain ultrasound scans performed at birth, 48 hours, 7 days, 15 days and 30 days of life were normal (Figure 1) but at 36 weeks’ Postmenstrual Age (PMA) a moderate Lenticulostriated Vasculopathy (LSV) was found (Figure 2). CMV screening was not performed at birth. Due to the ultrasonography finding, a polymerase chain reaction (PCR) for CMV was performed in urine. It was positive and PCR for CMV in blood resulted in 5476 copies/ml. Dried blood spot PCR assay for CMV confirmed the diagnosis of congenital CMV infection (the blood sample had been extracted at 48 hours of life). The infant did not show any clinical or analytical manifestation of CMV infection during admission. MRI studies at 36 weeks PMA and at 44 weeks PMA were normal. PCR for CMV in cerebrospinal fluid was negative and an ophthalmological study and auditory evoked potentials were also normal. A decision not to initiate treatment was made by consensus of both the medical staff and the patient’s parents. The infant is now 18 months old and the neurological development, including auditory evoked potentials, is normal.
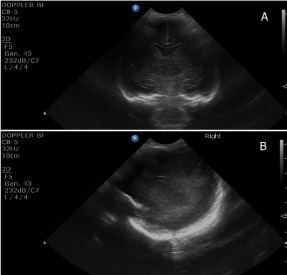
Figure 1. Normal coronal (A) and parasagittal (B) cranial ultrasound scans performed at 7 days of life of a preterm infant born at 28 weeks of gestational age.
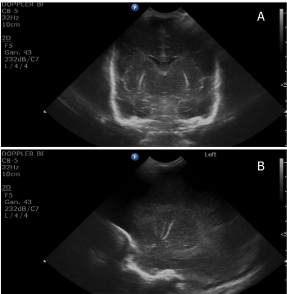
Figure 2. Normal coronal (A) and parasagittal (B) cranial ultrasound scans performed at 7 days of life of a preterm infant born at 28 weeks of gestational age.
This clinical case illustrates two challenges in congenital CMV infection. The first one is determining whether the aetiology of a CMV infection is congenital or acquired, when a urine specimen has not been tested for CMV within the first two weeks of life [2]. Establishing a correct diagnosis is of paramount importance, since we know that acquired as opposed to congenital CMV infection, does not seem to imply an increased risk of hearing loss in the long term, and hence it does not require antiviral treatment [3,4]. In any case, this is usually a solvable issue, although using dried blood spot can yield false negative results [2].
The second challenge, to our knowledge not yet solved in medical literature, is the question of whether LSV should be considered a sign of symptomatic congenital infection [5,6]. There is a lack of consensus regarding whether an isolated LSV should be considered a criterion of a symptomatic infection, given that LSV can also be present in uninfected preterm and term infants and in very heterogeneous pathological conditions [5]. LSV has been described in sick preterm babies and most often with a concurrent CMV infection [7]. In such cases, LSV has been reported to appear later than in term infants (mean age at diagnosis 1 month) and sometimes this finding disappears when babies reach term age [7]. Interestingly, our patient showed LSV nearly at term age, but not in previous routine ultrasound scans. He did not show any common medical problems during admission that could justify LSV, hence this finding was probably related to congenital CMV. This point is crucial, as a recent retrospective study concluded that isolated LSV is an indicator of an increased risk of developing hearing loss, and that antiviral treatment reduces this risk significantly [5,6].
This clinical case reminds clinicians that making the diagnosis of congenital infection using the patient’s dried blood spots is feasible, and illustrates the difficulty of distinguishing between a symptomatic and an asymptomatic infection in cases where the only manifestation is a non-specific condition such as LSV. The decision to treat the patient with ganciclovir to reduce the risk of hearing loss will depend on this consideration. Antiviral treatment is not risk-free (neutropenia being its most feared side effect), requires close follow up and its costs are not negligible. Therefore, more prospective research is needed to settle whether isolated LSV implies that the infection must be considered symptomatic, to establish the associated risk of hearing loss, and to assess the benefits and risks of antiviral treatment in this neonatal subpopulation.
- Kimberlin DW, Lin CY, Sanchez PJ, Demmler GJ, Dankner W, et al. (2003) Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr143: 16-25. [Crossref]
- Boppana SB, Ross SA, Novak Z, Shimamura M, Tolan RW, et al. (2010) Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA 303: 1375-1382. [Crossref]
- Swanson EC, Schleiss MR (2013) Congenital cytomegalovirus infection: new prospects for prevention and therapy. Pediatr Clin North Am 60: 335-349. [Crossref]
- Nijman J, van Zanten BG, de Waard AK, Koopman-Esseboom C, de Vries LS, et al. (2012) Hearing in preterm infants with postnatally acquired cytomegalovirus infection. Pediatr Infect Dis J 31: 1082-1084. [Crossref]
- Amir J, Schwarz M, Levy I, Haimi-Cohen Y, Pardo J (2011) Is lenticulostriated vasculopathy a sign of central nervous system insult in infants with congenital CMV infection? Arch Dis Child 96: 846-850. [Crossref]
- Bilavsky E, Schwarz M, Pardo J, Attias J, Levy I, et al. (2015) Lenticulostriated vasculopathy is a high-risk marker for hearing loss in congenital cytomegalovirus infections. Acta Paediatr 104: e388-94. [Crossref]
- Chamnanvanakij S, Rogers CG, Luppino C, Broyles SR, Hickman J, et al. (2000) Linear hyperechogenicity within the basal ganglia and thalamus of preterm infants. Pediatr Neurol 23: 129-133. [Crossref]


