Left heart failure (LHF) is a complex syndrome affecting cardiac function and/or structure with significant morbidity and mortality. LHF has had extensive research on epidemiology, clinical manifestation, diagnosis and clinical management but most of the early research did not distinguish LHF from other forms of heart failure such as right ventricular and bi-ventricular failure. Further, the definition, diagnosis and clinical management guidelines focus on symptomatic (overt) heart failure, with little research evidence on asymptomatic patients with LV dysfunction. Thus, the present review article seeks to combine current research evidence on LHF to advance the understanding of its clinical definition, manifestation, prognosis, pathophysiology, presentation, diagnosis and management.
left heart failure, left-sided heart failure, left ventricular failure
Heart failure (HF) defines a complex syndrome that impairs cardiac structure and/or function leading to the inability of the heart to supply sufficient oxygen to meet metabolic demands of tissues [1]. It is a global pandemic estimated to affect 26 million people worldwide [2]. Although mortality rates associated with cardiovascular diseases (CVD) as a whole have been in decline, HF is the only major CVD whose prevalence is rising (especially among geriatric population) with largely unchanged or even worsening early post-discharge mortality and re-admission rates [3,4]. HF imposes a substantial public health and economic burden estimated at $108 billion per annum globally, which will continue to rise because of increased longevity and a rapidly expanding and industrialized global population [5]. The health care industry must develop effective strategies to management this substantial public health and economic burden. Strategies may include adopting evidence-based approaches to prevent HF and implementing new treatment guidelines and protocols with proven efficacy into large-scale clinical practice [6]. Successful implementation of effective HF strategies will warrant a comprehensive understanding of the clinical status, etiopathogenesis, diagnosis and etiology-specific clinical management approaches. This literature review and meta-analysis seek to aggregate published scholarly and practitioner reports on the clinical status, diagnosis and management of HF, with an emphasis on the left heart failure (LHF), which has been the traditional research focus in the field of HF.
Definition
Historically, clinical research has associated HF with left heart (or ventricular) failure. Most clinical trials on HF published after 1990 selected patients based on left ventricular ejection fraction (LVEF) values (< 40%) obtained using cardiac imaging modalities – echocardiography, radionuclide or cardiac magnetic resonance. Thus, the conventional definition of HF referred to LHF and the two terms were interchangeably used. More recently, the 2016 updated European Society of Cardiology (ECS) guidelines for diagnosis and management of acute and chronic heart failure provided a more comprehensive definition taking into account changes in LV morphology and function, and the attendant clinical signs and symptoms. The guidelines define HF as a clinical syndrome characterized by typical symptoms of breathlessness, ankle swelling and fatigue accompanied by signs of elevated jugular venous pressure, pulmonary crackles and peripheral edema occurring in the setting of structural and/or functional cardiac abnormalities or elevated intra-cardiac pressures at rest or during stress [7]. The American Heart Association/American College of Cardiology (AHA/ACC) guidelines similarly define HF as a complex clinical syndrome in the setting of structural and/or cardiac disorders that impair ventricular ability to fill or to eject blood [8,9]. However, the current definitions restrict themselves to symptomatic stages of HF yet the pre-clinical stage may manifest with asymptomatic systolic or diastolic LV dysfunction, which are precursors of LHF. Recognition of the pre-clinical stage is important since they are associated with poor outcomes and commencing treatment at this stage may significantly improve therapeutic efficacy in asymptomatic patients [7,10].
Classification
The 2016 ESC guidelines classify LHF based on LVEF values; time-course (progression) of the disease; or symptomatic severity.
By left ventricular ejection fraction: The measurement of LVEF was the original approach used to describe HF. Mathematically, EF refers to the stroke volume (the end-diastolic volume minus the end-systolic volume) divided by the end-diastolic volume. In LHF with systolic dysfunction (depressed contraction and emptying of the LV), an increase in end-diastolic volume maintains stroke volume because the heart ejects a smaller fraction of a larger volume. The more the EF becomes depressed, the greater the end-diastolic and the end-systolic volumes [10]. However, LVEF values and normal ranges are largely dependent on the method use for imaging and analysis, and operator efficiency. Classification by LVEF values categorizes LHF into three. (a) LHF with preserved (or normal) ejection fraction (EF ≥ 50%); (b) LHF with reduced ejection fraction (original classification) (EF < 40%); and (c) a new category LHF with mid-range or mildly reduced EF (40-49%) [7,11]. Classification of patients based on LVEF is clinically important for evaluating prognosis (the more depressed the LVEF the poorer the prognosis); for indicating underlying etiologies and co-morbidities; and for assessing response to therapy [12].
By time course of the syndrome: Classification by time course of the disease describes a symptomatic cardiac syndrome graded according to the New York Heart Association (NYHA) functional classification I to IV while recognizing that treatment can render LHF asymptomatic (Table 1).
Table 1. New York heart association functional classification
|
Class |
Description (Symptom Severity/Physical Activity) |
|
Class I |
No limitation of physical activity. Ordinary physical activity does not cause undue breathlessness, fatigue, or palpitations. |
|
Class II |
Slight limitation of physical activity. Comfortable at rest, but ordinary physical activity results in undue breathlessness, fatigue, or palpitations. |
|
Class III |
Marked limitation of physical activity. Comfortable at rest, but less than ordinary physical activity results in undue breathlessness, fatigue, or palpitations. |
|
Class IV |
Unable to carry on any physical activity without discomfort. Symptoms at rest can be present. If any physical activity is undertaken, discomfort is increased. |
Adapted from the 2012 ESC Guidelines for diagnosis and treatment of acute/chronic heart failure [12]
For patients who have never exhibited typical signs or symptoms of LHF but have LV dysfunction or any other underlying cardiac abnormalities, the syndrome is termed asymptomatic LHF. For patients who have had LHF for a long period, the syndrome is termed chronic HF. Patients under therapy with generally unchanged clinical signs and symptom of LHF for at least one month are termed as stable. However, if chronic stable LHF deteriorates, the condition is termed as decompensated LHF, which may occur acutely and often lead to hospitalization. De novo refers to a new onset of HF that presents acutely often as a consequence of acute myocardial infarction or sub-acutely (gradual) as in the case of dilated cardiomyopathy that gradually progresses for weeks or months before manifestation of symptoms [7]. Finally, the term congestive HF is sometimes used to describe an acute or chronic HF with demonstrable clinical evidence of volume overload. Classification of LHF based on clinical course is important to indicate the stage or severity of illness [1,12].
By symptomatic severity: The NYHA functional classification provides a measure of severity of HF symptoms. The 2016 AHA/ACC guidelines divide LHF into four stages (A-D) based on the presence or the absence of clinical signs and symptoms (Table 2).
Table 2: Stages of left heart failure based on signs and symptoms [7]
|
Stage |
Clinical Description based present/absent of signs and symptoms |
|
A |
At high risk for HF but without structural heart disease or symptoms of HF |
|
B |
Structural heart disease but without signs or symptoms of HF |
|
C |
Structural heart disease with prior or current symptoms of HF |
|
D |
Refractory HF requiring specialized interventions |
On the other hand, the NYHA classification enables objective selection of patients in almost all randomized clinical trials in HF and description of response to therapy (identification of patients who may benefit from therapy). The NYHA class I are HF asymptomatic while those in class II, III and IV have mild, moderate and severe symptoms respectively [12]. The term “advanced HF” sometimes is used to describe patients with severe symptoms, recurrent decompensation and severe cardiac dysfunction [16]. Severity of symptoms however has a weak correlation with LV function but has a clear relationship with survival although mild symptoms may also have a relatively high absolute risk of hospitalization or death [13-15]. Symptoms may deteriorate rapidly suggesting an elevated risk of hospitalization and death, and the need to seek prompt medication attention and treatment, or severe symptoms may improve rapidly with treatment. Improvement in symptoms is a major therapeutic target. The other important targets are to reduce morbidity (hospitalization) and mortality.
Epidemiology
Left heart failure was initially identified as an emerging epidemic in 1997 [17]. Since then the prevalence of HF has increased steadily to reach over 26 million people worldwide [2]. The increased prevalence may reflect increased incidence, survival or a combination of the two factors [18]. There is considerable geographic variation in the prevalence and incidence of HF depending on different definitions, etiologies and clinical characteristics observed among patients [20] (Figure 1).
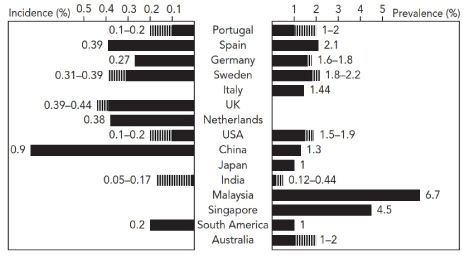
Figure 1. Prevalence and incidence of heart failure worldwide [19]
The incidence and prevalence of HF reveals significant geographical variation with incidence ranging from a low of 0.05-0.17% in India to a high of 0.9% in China and prevalence from 0.12-0.44% in India and 6.7% in Malaysia. Adapted from Savarese & Lund, 2017, p. 8)
In 2017, the prevalence of HF in the U.S was 5.7 million and projected to rise to 8 million by 2030 translating into a 46% increase in prevalence. In Southwestern Europe, the Epidemiology of Heart Failure and Learning (EPICA) study in the late 1990s reported a prevalence of 1.36% in 25-49 year olds rising to 12.67% and 16.40% in the 60-69 and > 80 years groups respectively [20]. In Germany, HF prevalence was 1.6% (women) and 1.8% (men) [21]; in Sweden, HF prevalence was 1.8% [22]; and 1.44% in Italy with increasing rates with ageing population [23]. Asia has a higher prevalence of between 1.3% and 6.7% [24], 1.3% in China [25] and 1.0% in Japan [26]. In Sub-Saharan Africa, there are no epidemiology studies into prevalence and incidence [27]. In Southeast Asia, Malaysia has the highest HF prevalence of 6.7% and 4.5% in Singapore, while in South America and Australia, the prevalence ranges between 1% and 2% similar to that reported in developed countries [28,29]. The increasing prevalence of HF observed worldwide may have no links with an increase in HF incidence, which data on temporal trends suggest is stabilizing or even decreasing. However, an ageing population and improved HF survival due to increased efficacy of diagnosis and treatment could explain better the increasing prevalence [30,31].
Prognosis
Left heart failure, whether diagnosed during hospitalization (active treatment) or in asymptomatic patients, is a life-threatening condition with an ominous prognosis, and significant mortality and hospital re-admissions [3]. In the U.S, the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) study reported 60-90 day mortality of 9.8% and re-hospitalization of 29.9% [32]. The Get With The Guidelines (GWTG) registry reported one-year mortality of 37.5% and re-hospitalization of 30.9% [33]. In Europe, the EuroHeart Failure Survey comparing prognosis between HF with depressed and preserved ejection fraction reports depressed LVEF has a higher mortality (12%) than preserved LVEF (10%) but with the same hospital re-admission rates [34]. The subsequent EuroHeart Failure Survey II examining patients hospitalized for HF found hospital in-mortality of 6.4% [35]. The multi-center European Society of Cardiology Heart Failure Long-Term (ESC-HF-LT) registry enrolling 12,440 acute and chronic HF patients reported a one-year mortality rate of 23.6% and 6.4%, and hospital re-admission of 36% and 14.5% for acute and chronic HF respectively [36].
The high mortality and re-hospitalization rates of HF underscore the importance of evaluating prognostic predictors. The estimation of prognosis for mortality and re-hospitalization in HF patients will assist in the decision on the appropriate type and timing of therapy, response to therapy, and planning of health and social services and resources [3,10]. The 2016 ESC guidelines lists several prognostic predictors of death and/or hospitalization in HF patients (Table 3). However, their clinical applicability is limited and a precise risk stratification in HF patients remains a clinical challenge.
Table 3: Prognostic predictors of death and hospitalization in left heart failure
|
Predictors of an Ominous Prognosis
|
Description of Specific Predictors of an Ominous Prognosis (Death and/or Hospitalization)
|
|
Demographic data |
Older age, male sex, low socio-economic status. |
|
Severity of heart failure |
Advanced NYHA Class, longer HF duration, reduced peak oxygen consumption, high VE-VCO2 slope, Cheyne–Stoke ventilation, short 6-minute walking distance, reduced muscle strength, poor quality of life. |
|
Clinical status |
High resting heart rate, low blood pressure, clinical features of fluid overload (both pulmonary congestion and peripheral edema, jugular venous dilation, hepatomegaly), edema, jugular venous dilatation, hepatomegaly), clinical features of peripheral hypoperfusion, body wasting, frailty. |
|
Myocardial remodeling and severity of heart dysfunction |
Depressed LVEF, LV dilatation, severe diastolic LV dysfunction, elevated LV filling pressure, mitral regurgitation, aortic stenosis, LV hypertrophy, left atrial dilatation, RV dysfunction, pulmonary hypertension, dysynchrony, vast area of hypo/akinesia, wide QRS complex, presumed inflammation or infiltration on CMR, inducible ischemia and poor viability on imaging |
|
Biomarkers of neurohormonal activation |
Low sodium, high natriuretic peptides, high plasma renin activity, high aldosterone and catecholamine, high endothelin-1, high adrenomedullin, high vasopressin |
|
Other biomarkers |
Markers of renal function, inflammation markers, cardiac stress markers, cardiac damage markers, metabolic markers, collagen markers, markers of organ damage/dysfunction. |
|
Genetic testing |
Certain mutations in inherited cardiomyopathies associated with high-risk of sudden cardiac death or rapid HF progression |
|
Cardiovascular co-morbidities |
Atrial fibrillation, ventricular arrhythmia, non-revascularizable coronary artery disease, previous stroke/TIA, peripheral artery disease. |
|
Non-cardiovascular co-morbidities |
Diabetes, anemia, iron deficiency, COPD, renal failure, liver dysfunction, sleep apnea, cognitive impairment, depression |
|
Non-adherence |
Non-adherence with recommended HF treatment |
|
Clinical events |
HF hospitalization, aborted cardiac arrest, ICD shocks. |
CMR: Cardiac Magnetic Resonance; COPD: Chronic Obstructive Pulmonary Disease; HF: Heart Failure; ICD: Implantable Cardioverter Defibrillator; LV: Left Ventricular; LVEF: Left Ventricular Ejection Fraction; NYHA: New York Heart Association; QRS: Q, R, and S waves RV: Right Ventricular; TIA: Transient Ischemic Attack; VE-VCO2: Ventilatory Equivalent Ratio for Carbon Dioxide. Adapted from the 2012 ESC Guidelines for diagnosis and treatment of acute/chronic heart failure [12]
Left heart failure is a clinical syndrome with heterogeneous etiology and pathophysiology [37]. There is no expert consensus on a single classification system for HF etiology with many overlapping causes. Many HF patients may present with various pathologies – cardiac and extra cardiac – that conspire to cause HF. Identification of these pathologies should be included in the diagnosis algorithm of LHF. The 2016 ESC guidelines report the etiology of HF is diverse within and among world regions. It subsumes the numerous etiologies of LHF into three broad categories, namely (a) myocardial injury; (b) abnormal loading conditions; and (c) arrhythmias (Table 4).
Table 4: Etiology of left heart failure [12]
|
Etiology
|
Condition(s)
|
Specific Examples
|
|
Myocardial Injury |
Ischemic heart disease |
Myocardial scar/stunning/hibernation, epicardial CAD, abnormal coronary microcirculation, endothelial dysfunction. |
|
|
Toxic damage |
Substance abuse (alcohol, cocaine, amphetamine); heavy metals (copper, iron, lead); medication (cytostatic, immunomodulating, antidepressant, antiarrhythmic, non-steroidal, anti-inflammatory) and radiation. |
|
|
Immune-mediated and inflammatory damage |
Infection-related (bacteria, fungi, protozoa parasites and viruses (HIV/AIDS); and non-infection related (lymphocytic/giant cell myocarditis, autoimmune diseases. |
|
|
Infiltration |
Malignancy (direct infiltration and metastases) or non-malignancy related (amyloidosis, sarcoidosis, haemochromatosis, glycogen storage diseases, lysosomal storage disease. |
|
|
Metabolic disturbances |
Hormonal (thyroid disease, acromegaly, Addison disease, diabetes, metabolic syndrome, phaeochromocytoma, pathologies related to pregnancy and peripartum); and nutritional (deficiency in thiamine, L-carnitine, selenium, iron, phosphates, calcium, complex malnutrition and obesity) |
|
|
Genetic abnormalities |
Cardiomyopathies – dilated, hypertrophic, restrictive, left ventricular non-compaction, arrhythmogenic right ventricular dysplasia; muscular dystrophies and laminopathies |
|
Abnormal loading conditions |
Hypertension |
|
|
|
Valve and myocardium structural defects |
Acquired (mitral, aortic, tricuspid and pulmonary valve diseases.) and congenital (atrial and ventricular septum defects) |
|
|
Pericardial and endomyocardial pathologies |
Pericardial (constrictive pericarditis
Pericardial effusion) and endomyocardial (hypereosinophilic syndrome, endomyocardial fibrosis, endocardial firbroelastosis) |
|
|
High output states |
Severe anemia, sepsis, thyrotoxicosis, Paget’s disease, arteriovenous fistula, pregnancy |
|
|
Volume overload |
Renal failure, iatrogenic fluid overload |
|
Arrhythmias |
Tachyarrhythmias |
Atrial ventricular arrhythmias |
|
|
Bradyarrhythmias |
Sinus node dysfunction, conduction disorders |
The pathophysiology of LHF is not precisely understood. However, MI-induced LV remodeling has been well demonstrated as a key pathophysiologic mechanism in the development of LHF. It defines a process by which mechanical, neurohormonal and genetic factors regulate the LV size, shape and function. LV remodeling may be physiological and adaptive during normal growth or pathological secondary to myocardial infarction (MI), cardiomyopathy, hypertension or valvular disease [38]. Myocardial injury such as MI may lead to maladaptive changes in the cardiomyocytes and extracellular matrix (ECM). Acute loss of myocardium leads to an abrupt increase in loading conditions inducing a pathological pattern of LV remodeling involving the infarcted myocardium and remote non-infarcted myocardium. Cardiomyocytes necrosis and the consequent increased loading conditions trigger a cascade of biochemical intracellular signaling processes, which initiate and modulates reparative changes including LV dilatation, hypertrophy and the formation of discrete collagen scar. Hypertrophy occurs as an adaptive response during post-infarction remodeling to offset increased load, attenuate progressive dilatation and stabilizes contractile function [38].
Left ventricular remodeling may continue for weeks or even months until the tensile strength of the collagen scar counter-balances the distending forces. The balance depends on the size, location and transmurality of the infarct, the degree of myocardial stunning, patency of infarct-related artery and local tropical factors [39,40]. Post MI remodeling of the LV may be divided into an early remodeling (within 72 hours) and late remodeling (beyond 72 hours). Early remodeling involves the expansion of the myocardial infarct resulting into early ventricular rupture of aneurysm formation. Late remodeling involves the LV globally associated with time-dependent dilatation, distortion of LV shape and mural hypertrophy [38]. If left untreated, LV systolic dysfunction progressively worsens over time characterized with increasing dilatation of the LV and declining LVEF despite some patients being initially asymptomatic [13,41].
Two important mechanisms underlie the pathophysiological changes associated with or consequent to LV remodeling. The first mechanism is recurrent myocardial infarction resulting into additional cardiomyocyte necrosis and the second mechanism is systemic responses usually occurring secondary to declining systolic function (LVEF) particularly neuro-hormonal activation [10,41]. Important neurohormonal systems activated in HF are the renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system (SNS). The two systemic responses cause additional myocardial injury, have deleterious effect on blood vessels, kidneys, muscles, bone marrow, lungs and liver, and lead to a pathophysiological vicious cycle responsible for many clinical features of LHF including myocardial electrical instability. Interruption of these two neurohormonal responses is the basis of much of the effectiveness of LHF treatment [10,13,41]. Clinically, changes due to activation of the RAAS and SNS systems are associated with the development of HF symptoms and poor prognosis over time leading to diminished quality of life, declining functional capacity, hospital re-admission and premature death secondary to pump failure or ventricular arrhythmias [10]. Additionally, in LHF patients, limited cardiac reserve is dependent on several mechanisms including atrial contraction, synchronized contraction of the LV and normal interdependence of LV and RV. Any event affecting any of these mechanisms such as the development of atrial fibrillation (AF) or conduction abnormalities such as left bundle branch block (LBBB) or imposing additional hemodynamic load can result into acute decompensation [13].
Signs and symptoms
Patients with LHF presents with many non-specific symptoms that do not help in discriminating between HF and other similar cardiac syndromes. Symptoms of LHF could be categorized into typical and less typical symptoms, whereas signs could be categorized into specific and less specific signs [42-45] (Table 5).
Table 5: Symptoms of left heart failure
|
Symptoms
|
Signs
|
|
Typical
|
Less Typical
|
More Specific
|
Less Specific
|
|
Breathlessness |
Nocturnal cough |
Elevated jugular venous pressure |
Weight gain > 2kg/week |
|
Orthopnea |
Wheezing |
Hepatojugular reflux |
Weight loss in advanced HF |
|
Paroxysmal nocturnal dyspnea |
Bloated feeling |
Third heart sound |
Tissue wasting |
|
Reduced exercise tolerance |
Loss of appetite |
Laterally displaced apical impulse |
Cardiac murmur |
|
Fatigue, tiredness |
Confusion |
|
Peripheral edema |
|
Ankle swelling |
Depression |
|
Pulmonary crepitation |
|
|
Palpitations |
|
Tachycardia |
|
|
Dizziness |
|
Irregular pulse |
|
|
Syncope |
|
Tachypnea |
|
|
Bendopnea |
|
Hepatomegaly |
|
|
|
|
Ascites |
|
|
|
|
Cold extremities |
|
|
|
|
Oliguria |
Despite LHF having numerous signs and symptoms, their precise use in clinical practice challenging. Symptoms and signs resulting from fluid retention usually resolves quickly with diuretic treatment. Signs such as elevated jugular venous pressure and displacement of the apical impulse may be more specific but are challenging to detect and have poor reproducibility [45,46]. In obese individuals, geriatric and those with chronic lung disease, identification of symptoms and signs maybe difficult [47-50]. In addition, young LHF patients usually have different causes, clinical presentation and outcomes compared to older patients [50]. However, symptoms and signs of LHF should be assessed at each clinic visit with particular attention on evidence of congestive HF because they provide valuable diagnostic clues and prognostic information. Symptoms and signs are not only important in raising suspicion for LHF but also in monitoring response to treatment and stability over time. The persistence of symptoms despite therapy suggests the need for additional therapy while the worsening of symptoms warrants prompt medical attention [7].
Diagnosis
Diagnosis of LHF lacks a single universally acceptable diagnostic test usually requiring a series of physical and imaging tests. The 2016 ESC guidelines aggregates current research and practitioner (expert) evidence to provide an algorithm for the clinical diagnosis of LHF in non-acute settings. The algorithm recommends a series of four diagnostic tests: (a) detailed clinical history; (b) physical examination; (c) echocardiography cardiac imaging; and (d) laboratory tests (Figure 2).
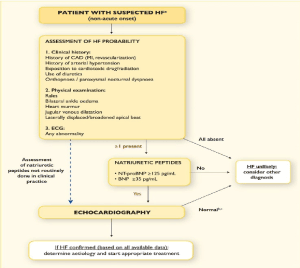
Figure 2.Diagnostic algorithm for heart failure of non-acute onset
Patients presenting with symptoms/signs of LHF for the first time in a non-urgent primary care, diagnosis should begin with obtaining patient history, presenting symptoms, physical examination and resting ECG. If all are normal LHF is less likely but if at least one is abnormal plasma BPN should be measured and echocardiography done to confirm LHF. Adapted from the 2016 ESC Guidelines for diagnosis and treatment of chronic heart failure [7]
Clinical history: Obtaining a detailed history of the patient is crucial to ascertain the presence or absence of possible causes of cardiac injury [51]. Diagnosis of LHF is less likely in an individual with no relevant medical history suggesting the absence of possible causes of LHF compared to patients with significant risk factors particularly MI [43,51]. The assessment of detailed history should focus on identifying known risk factors for LHF such as previous diagnosis of HF, diabetes, hypertension, valvular heart disease, advanced age and obesity, which may predict volume overload typical in congestive HF [52,53]. In non-acute settings, detailed patient history is the recommended first diagnostic step to assess the probability of LHF based on identifiable risk factors such as CAD, arterial hypertension, diuretic use, or orthopnea. In addition, knowledge of precipitating conditions may have therapeutic and prognostic implications. Detailed patient history should include identifying and assessing symptomatology [54].
Presenting symptoms: The assessment of presenting symptoms should complement findings from assessment of detailed history. Characteristic symptoms such as dyspnea, fatigue and signs of volume overload including peripheral edema and pulmonary rales are usually typical consequences of systemic and pulmonary congestion in the setting of elevated LV filling pressures [54]. However, some symptoms such as peripheral edema, exertional dyspnea and fatigue are not very helpful in differential diagnosis since they are non-specific. Orthopnea and paroxysmal nocturnal dyspnea are more typical of heart failure but less common and less sensitive. Signs such as audible third sound, displacement of apical impulse and elevated jugular pressures are specific but identifying them is challenging and their reproducibility is poor [45,55]. Other signs such as fluid retention may resolve following diuretic therapy [7].
Physical examination: After the assessment of patient history and symptomatology, physical examination should be performed to obtain additional diagnostic clues. Physical examination should include observation of patient, palpitations, cardiac auscultation, evaluation of vital signs, heart and lungs as well as jugular veins [45,56-58]. Further physical examination may include evaluation of abdomen and extremities, in particular jugular venous dilatation, pulmonary crepitation (rakes) and peripheral edema [51,53]. Abnormal findings including pulmonary crepitation, jugular venous dilatation, hepatojugular reflux and peripheral edema suggest volume overload and increase the possibility of a diagnosis of LHF [56,59]. On the other hand, the absence of these indicators does not rule out the diagnosis of LHF [56].
Laboratory assessment: When the assessment of detailed history, symptomatology or physical examination yields any abnormal findings suggestive of cardiac problem, and echocardiography is not immediately available, laboratory tests should be undertaken. Plasma concentration of natriuretic peptides (NPs) should be measured. Elevated levels of NPs assist in the establishment of initial working diagnosis, identifying patients requiring further cardiac investigation. Patients with normal plasma NPs levels are unlikely to have LHF. Values of NPs below the cut-off points help to identify patients who do not require echocardiography. The cut-off points (upper limit) in non-acute settings are 35 pg/mL for B-type natriuretic peptide (BNP) and 125 pg/mL for N-terminal pro-BNP (NT-proBNP). In acute settings higher cut-off points are used (BNP = 100 pg/mL; NT-proBNP = 300 pg/mL) [7]. These cut-off points have high negative predictive values (94-98%) but very low positive predictive values (44-57%) demonstrating that the measurement of plasma NPs should be used to rule out LHF rather than establishing diagnosis [60-64]. The low positive predictive values arises because numerous cardiac and extra cardiac can cause elevated NPs and weaken their diagnostic effectiveness in LHF. Conditions such as atrial fibrillation (AF), age of the patients, renal failure are key factors interfering with the interpretation of plasma NP measurement but in obese patients NPs levels may be relatively very low [65].
In addition to NPs assessment, twelve (12)-lead electrocardiogram (ECG) may be considered to increase the likelihood of LHF diagnosis. ECG is recommended to assess hearth rhythm, heart rate, QRS morphology and QRS duration [54]. It provides additional diagnostic clues and information about etiology such as MI and indications for treatment [7,10]. Patients with completely normal ECG are unlikely to have LHF (sensitivity = 89%) but has a low specificity indicating ECG is more useful to rule out diagnosis of LHF than to establish diagnosis [45,59,66].
Cardiac imaging: The role of cardiac imaging in the diagnosis of LHF is well established. The gold standard cardiac imaging modality in LHF is echocardiography because of accuracy, safety, cost-effectiveness and wide availability [7,10]. Beyond myocardial abnormalities, echocardiography documents other impairments (abnormality of the valves, pericardium, endocardium, heart rhythm, or conduction) [54]. Echocardiography enables the evaluation of cardiac chamber volumes, ventricular systolic and diastolic function, ventricular wall thickness, valve function and pulmonary hypertension. In non-acute settings, echocardiography in patients following assessment of NPs levels above the cut-off points. In the absence of NPs assessment, echocardiography is recommended for patients with de novo acute HF. In high-risk patients with hemodynamic instability (especially those with cardiogenic shock) and patients suspected with acute life-threatening structural and functional cardiac abnormalities [7].
Echocardiography provides objective quantifiable assessment of alterations in cardiac structure and/or function. It refers to all cardiac ultrasound imaging modalities such as 2D and 3D echocardiography, pulsed and continuous wave Doppler, Color flow Doppler, tissue Doppler imaging (DTI) contrast echocardiography and deformation imaging (strain and strain rate). Transthoracic echocardiography (TTE) is the preferred modality for the assessment of systolic and diastolic function of both the LV and RV [54]. Diagnosis of LHF has traditionally been based on LVEF < 40% [7,10]. Other key structural alterations include left atrial volume index (LAVI) > 34mL/m2 or left ventricular mass index (LVMI) ≥ 115 g/m2 for males and ≥ 95 g/m2 for females [67-69]. Key functional alterations include E/e’ ≥ 13 and mean e’ septal and lateral wall < 9 cm/s [70-71]. Surrogate (indirect) echocardiographic markers for diagnosis of LHF include longitudinal strain (to assess systolic dysfunction) or tricuspid regurgitation velocity (TRV: to assess pulmonary artery pressures) [69,72]. Other cardiac test such as chest x-ray, exercise testing, invasive hemodynamic assessment and endomyocardial biopsy may be performed during the diagnosis process. Lung ultrasound is a useful test in selected patients to assess pulmonary congestion [7,73].
Meta analysis of diagnosis methods
The clinical diagnosis of LV systolic dysfunction is difficult and misdiagnosis often contributes to inappropriate and sub-optimal treatment of LHF patients. Recently, the ESC and the ACC/AHA published comprehensive management guidelines and treatment protocols for heart failure in clinical practice. The two guidelines recommend assessment of LV systolic function based on echocardiography-defined LVEF as the gold standard for confirmatory diagnosis of LHF. In case echocardiography is not immediately available, the measurement of plasma concentration of natriuretic peptides (NPs) should be considered. However, existing studies investigating plasma NPs suggest high negative and low positive predictive value of NPs suggesting it does not establish diagnosis rather assists in the exclusion of LHF. Thus, the present meta-analysis combines findings from previous studies to establish the diagnostic accuracy of NPs in the diagnosis of LHF.
Search and inclusion strategy: Comprehensive search for relevant studies investigating the accuracy of the concentration of plasma natriuretic peptides (NPs) in the diagnosis of LHF was achieved through online search in three electronic databases Medline, EMBASE and PubMed. Broad-based search terms included a combination of a range of text words and MeSH terms concerning suspected LV systolic function, diagnostic tests performed (assessment of plasma NPs) and the diagnostic process. Additional studies were retrieved from citation search of studies obtained from the online search, review article and manual library search. Pre-determined inclusion and exclusion criteria was applied to the abstracts or full text of qualifying studies pertinent to the review topic. Inclusion criteria were as follows. (a) study population included individuals suspected with LV systolic dysfunction with or without comorbidities; (b) sturdy intervention included diagnostic tests with at least assessment of one plasma NPs – BNP and NT-proBNP; (c) gold standard reference to the diagnosis of LV systolic dysfunction (echocardiography); (d) study design (prospective/retrospective clinical trials); and (e) outcome measures (specificity, sensitivity, negative/positive predictive values). Exclusion criteria were: (a) studies with only abstracts; (b) conference papers (not final, subject to revision); and (c) studies with difficulty in extracting the pertinent data. Two reviewers screened all the qualifying studies against the inclusion/exclusion criteria as well as participated in data abstraction. In case of discrepancy, resolution as through consensus. Abstracted data from the included studies were as follows: first author and reference, publication year, patient sample, NPs used (BNP or NT‐proBNP), NPs cut-off point, sensitivity, specificity, negative predictive value, and positive predictive value (Table 6).
Table 6: Characteristics of studies included in meta-analysis
|
1st Author [Ref #]
|
Year
|
Patient Population
|
Natriuretic Peptide
|
Cut-Off (pg/mL)
|
Sensitivity (%)
|
Specificity (%)
|
Negative Predictive Value (%)
|
Positive Predictive Value (%)
|
|
Davis et al. [74] |
1994 |
52 |
BNP |
22 |
93 |
90 |
NR |
NR |
|
Cowie et al. [75] |
1997 |
29 |
BNP |
22.2 |
97 |
84 |
98 |
70 |
|
Landray et al. [76] |
2000 |
126 |
BNP |
17.9 |
88 |
34 |
NR |
NR |
|
Dao et al. [77] |
2001 |
97 |
BNP |
80 |
98 |
92 |
98 |
90 |
|
Logeart et al. [78] |
2002 |
115 |
BNP |
80 |
97 |
27 |
93 |
76 |
|
Maisel et al. [79] |
2002 |
744 |
BNP |
100 |
90 |
76 |
NR |
NR |
|
Villacorta et al. [80] |
2002 |
70 |
BNP |
200 |
100 |
97 |
100 |
97 |
|
Sim et al. [81] |
2003 |
83 |
BNP |
19 |
100 |
49 |
98 |
47 |
|
Barcarse et al. [82] |
2004 |
98 |
BNP |
100 |
65 |
88 |
100 |
NR |
|
Bayes‐Genis et al. [83] |
2004 |
100 |
NT‐proBNP |
125 |
98 |
46 |
100 |
89 |
|
Dokainish et al. [84] |
2004 |
122 |
BNP |
300 |
88 |
60 |
NR |
NR |
|
Knudsen et al. [85] |
2004 |
155 |
BNP |
50 |
100 |
37 |
100 |
52 |
|
Kruger et al. [86] |
2004 |
73 |
BNP |
94 |
89 |
58 |
NR |
NR |
|
Gustafsson et al. [87] |
2005 |
367 |
NT-proBNP |
125 |
97 |
46 |
99 |
15 |
|
Januzzi et al. [88] |
2005 |
600 |
NT‐proBNP |
300 |
NR |
NR |
99 |
NR |
|
Mueller et al. [89] |
2005 |
251 |
NT‐proBNP |
125 |
94 |
46 |
87 |
68 |
|
|
|
|
BNP |
100 |
96 |
61 |
93 |
75 |
Study characteristics and outcomes: The combined online search and review of bibliographies yielded 2,234 potential relevant citations and abstracts. Of these 2,234, only 115 required detailed scrutiny of whole text to reach an inclusion decision. Ultimately, sixteen (16) studies that met the inclusion/exclusion criteria were included in this meta-analysis [74-89]. Thirteen studies [74-82,84-86,89] investigated BNP while four [83,87-89] investigated NT‐proBNP. Only one study [89] investigated both BNP and NT‐proBNP. Sensitivity and specificity was provided in all studies except one [88]. Altogether, the 16 studies had a combined patient population of 3,082. Eleven (11) studies reported negative predictive values [75,77,78,80-83 85,87-89], while nine studies reported positive predictive values [75,77,78,80,81,83,85,87,89]. Cut-off values had a wide variation (17.9 to 300 pg/mL) because of the inclusion of both primary care (non-acute) and acute care settings that have significantly different cut-off points. Acute settings have significantly higher cut-off points for both BNP and NT‐proBNP.
The combined findings from 3,082 patients reveal that the diagnosis of LV systolic dysfunction using plasma NPs has a very high sensitivity (weighted mean = 92.9%; SD = 8.88; range 65-100%) but a relatively lower specificity (weighted mean = 62.0%; SD = 23.69; range 27-97%). The negative predictive values were significantly high (weighted mean = 97.5%; SD 4.0; range 87-100) but the positive predictive values was significantly low (weighted mean = 67.11; SD = 25.81; range 15-97). The cut-off values of plasma BNP for negative prediction (weighted mean = 26 pg/mL for non-acute settings and weighted mean = 131.75 pg/mL for acute settings). Cut-off values for NT‐proBNP were 125 pg/mL for non-acute settings and 300 pg/mL for acute settings. The findings reveal cut-off values for BNP and NT‐proBNP differ significantly for non-acute and acute settings, with acute settings having significantly higher cut-off values.
The most recent ESC and the ACC guidelines recommend in the event of the lack of immediate availability of echocardiography, the assessment of the concentration of plasma NPs and/or ECG could provide important clues for the diagnosis or the exclusion of LHF in patients suspected with LV systolic dysfunction [7]. The assessment of plasma NPs are also important for selecting patients for echocardiography. The present meta-analysis sought to determine the accuracy of plasma NPs in the differential diagnosis of LHF. The findings reveal that in patients with symptoms suspected to be LHF, plasma NPs concentration is a useful marker to exclude diagnosis of LHF as well as a useful marker to suggest likelihood of LHF warranting the need for further clinical assessment. The mean cut-off points for BNP were 26 pg/mL and 131.75 pg/mL for non-acute and acute settings, which were lower than cut-off points for NT‐proBNP 125 pg/mL and 300 pg/mL for acute and non-acute settings respectively. The cut-off points for negative predictive value and positive predictive value differed but optimal values suggest higher sensitivity but lower specificity altogether suggesting plasma NPs values are valuable for the exclusion of LHF rather than to establish diagnosis.
The present findings are consistent with the guidelines of ESC for the clinical management of LHF as well as findings of previous meta-analyses and clinical trials. The ESC guidelines recommend 35 pg/mL and 125 pg/mL as optimal cut-off points for the highest sensitivity of negative predictive value (exclusion threshold) for BNP in non-acute and acute settings respectively, and 100 pg/mL and 300 pg/mL as optimal cut-off points for negative predictive values of NT‐proBNP [7]. The National Institute of Clinical Excellence (NICE) 2010 partial update to 2003 about diagnosis and management of chronic HF recommends both ECG and plasma NPs have a relatively high sensitivity but comparatively low specificity. High sensitivity rules out a diagnosis of LHF while low specificity may encourage indiscriminate and inefficient testing strategies [90]. In a previous meta-analysis on diagnostic accuracy and population screening using plasma NPs of LV systolic dysfunction, although both BNP and NT-proBNP are indicators for LV systolic dysfunction, BNP is a better indicator. However, the performance of both BNP and NT-proBNP decrease with age. While BNP has a good correlation with echocardiographic parameters, it has a better correlation with clinical status [91]. Another meta-analysis on the diagnostic value of plasma NPs and ECG finds both are useful in excluding the diagnosis of LV systolic dysfunction (good sensitivity) and thus select patients for echocardiography to prevent the misuse of resources on many tests [92]. Although cut-off points have been suggested for plasma NPs for negative prediction of LV systolic dysfunction, it is not clear what BNP cut-off points are optimal for detecting clinical LHF. In summary, the assessment of plasma NPs, especially BNP, has high sensitivity for exclusion of LHF diagnosis and selecting patients for echocardiography. Accuracy in the selection of appropriate patients reduces resource usage and cost on performing several other diagnostic tests.
Research-based evidence on the management of LHF is well documented. The 2016 ESC [7] and the 2013 ACC/AHA guidelines [90] provide detailed recommendations for LHF management in clinical practice. The ESC guidelines provide clinical management approaches for LHF in general, while the guidelines by the ACC/AHA are specific to the four stages of LHF (Stages A to D: Table 2). However, both guidelines emphasize on prevention strategies for at risk population and medical therapy as the mainstay of LHF treatment for symptomatic patients [7,93].
Asymptomatic patients: Preventive strategies target to delay or prevent the development of symptoms (asymptomatic LHF or Stage A in ACC/AHA recommendations), the development of overt LHF or the prevent death before the onset of symptoms by using interventions aimed at modifying risk factors. The common risk factors target for treatment or management include hypertension [93-96], tobacco use [97], modest alcohol consumption (relationship between alcohol and the risk of de novo LHF is U-shaped) [98-100], physical activity [101], obesity and diabetes mellitus [102-107], atherosclerotic disease [108-109], chemotherapy regimens [110-112], and cocaine and amphetamines [92]. In addition to management of risk factors, in asymptomatic patients with long-standing reduced LVEF irrespective of etiology, ACE-inhibitors can reduces the risk of LHF requiring hospitalization or mineralocorticoid receptor antagonist (MRAs) [113-114]. In patients with asymptomatic LV systolic dysfunction (LVEF < 30%) secondary to ischemia and ≥ 40 days following acute MI, implantable cardioverter-defibrillator (ICD) is recommended to improve survival [115]. Table 7 summarizes details of preventive strategies and their therapeutic target while Table 8 summarizes therapeutic interventions to delay or prevent the onset of overt heart failure as per the ESC guidelines [7].
Table 7: Interventions for preventing the development left heart failure
|
Interventions |
Therapeutic Target |
|
Treatment of hypertension |
To prevent or delay the onset of HF and prolong life. |
|
Treatment with statins |
In patients with or at high-risk of CAD with or without LV systolic dysfunction to prevent or delay the onset of HF and prolong life |
|
Counselling and treatment for smoking cessation and alcohol intake reduction |
To prevent or delay the onset of HF. |
|
Treating other risk factors such as obesity, dysglycemia |
To prevent or delay the onset of HF. |
Table 8: Preventive therapy for the development of left heart failure
|
Preventive Therapy |
Indications (patients with…) |
Therapeutic Target |
|
Empagliflozin |
Type 2 Diabetes |
Prevent/delay onset of LHF and prolong life |
|
ACE-Inhibitors |
Asymptomatic LV systolic dysfunction and a history of MI
Stable CAD with or without LV systolic dysfunction |
Prevent/delay onset of LHF |
|
Beta-blocker |
Asymptomatic LV systolic dysfunction and a history of MI |
Prevent/delay onset of LHF |
|
ICD |
Asymptomatic LV systolic dysfunction (LVEF ≤ 30%) of ischemic origin 40 days post MI.
Asymptomatic non-ischemic dilated cardiomyopathy (LVEF ≤30%) receiving optical medical therapy. |
Prevent sudden death and prolong life. |
ACE: Angiotensin-Converting Enzyme; CAD: Coronary Artery Disease; HF: Heart Failure; ICD: Implantable Cardioverter-Defibrillator; LV: Left Ventricular; LVEF: Left Ventricular Ejection Fraction; MI: Myocardial Infarction
Symptomatic patients: The 2016 ESC guidelines proposes an algorithm for clinical management of LHF. Treatment varies depending on symptoms, and involves medical and/or non-medical therapy (Figure 3).
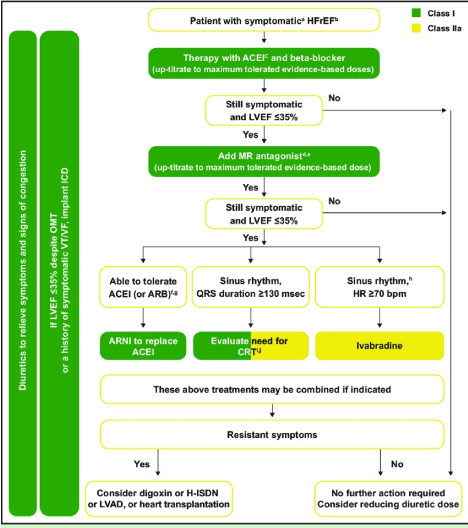
Figure 3.The 2016 ESC recommended treatment algorithm for left heart failure
Therapeutic algorithm for symptomatic HFrEF patients. Recommended treatment should begin with ACE-I and beta blocker to maximum tolerated evidence-based levels. If still symptomatic MR antagonist should be added. If still symptomatic and LVEF < 36%, ARNI should replace ACE-I, CRT for those with QRS duration > 130 msec or Ivabradine for those with sinus rhythm and heart rate > 71 beats per minute. Finally, for those with resistance (or persistent) symptoms consider digoxin or cardiac transplantation. (Green indicates class I recommendation while yellow class IIa recommendations) Adapted from the 2016 ESC Guidelines for diagnosis and treatment of chronic heart failure [7].
Medical Therapy: Medical therapy is the mainstay of the treatment of LHF. The therapeutic goal is to improve clinical status, functional capacity, quality of life and reduce mortality [7,93]. The ESC clinical management algorithm (Figure 3) recommends treatment strategy for medical therapy and devices in LHF patients with reduced LVEF. The main medication indicated for treatment of LHF are three neuro-hormonal antagonists – ACE-inhibitors, MRAs and beta-blockers. The three antagonists have been shown to reduce morbidity and improve survival in LHF patients, and thus recommended for the treatment of every LHF patients with reduced LVEF (< 40%) unless contra-indicated or not tolerated [7]. ACE-Inhibitors are usually the first-line medication. Although beta-blockers have not been evaluated in congested and decompensated LHF patients, they have been shown to complement ACE-inhibitors, and could be started together once the diagnosis of LHF has been confirmed. Mineralocorticoid receptor antagonist (MRA) are recommended in all symptomatic patients despite treatment with ACE-inhibitor and beta-blocker to reduce mortality and hospitalization. MRAs require close monitoring in patients with renal dysfunction or having serum potassium levels > 5.0 mmol/L [7,93]. Other medications include diuretics (to manage signs and symptoms of congestion), Angiotensin receptor neprilysin inhibitor, Ivabradine, and Angiotensin II type I receptor blockers (ARB: indicated as an alternative to ACE-inhibitors for intolerant patients) and a combination of hydralazine and isosorbide dinitrate [7].
Non-medical therapy: Non-medical or device therapy are usually indicated for primary and secondary prevention of sudden cardiac death (SCD) [93]. The most frequently indicated device therapy in LHF patients are ICD and Cardiac Resynchronization Therapy (CRT). ICD are effective in preventing electrical disturbances such as bradycardia and ventricular arrhythmias, which cause a significant percentage of sudden death among LHF patients [93]. ICD is contra-indicated within 40 days post MI, NYHA Class IV with severe symptoms refractory to medication, unless they are candidates for CRT. Left ventricular assist device (LVAD) or heart transplantation. A wearable ICD may be indicated for LHF patients with at risk of SCD for a limited period or as a bridge to an implanted device [7]. The second device therapy, CRT, improves cardiac performance in selected patients, improves symptoms and well-being and reduces morbidity and mortality [116-117]. It is recommended for symptomatic LHF patients with sinus rhythm with QRS duration ≥ 150 msec, LEVF ≤ 35%, NYHA Class III-IV, and LHF patients indicated for ventricular pacing and high degree AV block but contra-indicated in LHF patient with QRS duration of < 130 msec [117-121].
Meta-analysis of clinical management methods: Despite having favorable outcomes on short-term surrogate clinical markers, clinical management methods for LHF generally have a deleterious effect on long-term outcomes. Regulatory bodies and the ESC and ACC/AHA clinical practice guidelines rely on mortality and morbidity data to approve or recommend therapeutic interventions for LHF. Preventing LHF-associated hospitalization and improving functional capacity are other important benefits for consideration if mortality is rules out [122-125]. The aim of this meta-analysis is to combine findings from large-scale clinical trials on therapeutic interventions for LHF patients with depressed LV systolic function (LVEF< 45%) to determine treatment efficacy based on mortality, morbidity and hospitalization outcomes.
Search strategy and inclusion criteria: A comprehensive online and library search was undertaken for a literature-based systematic review and meta-analysis of large-scale clinical trials published to the end of 2017. Online databases PubMed, EMBASE and Medline were searched for relevant clinical trials investigating the efficacy of the current treatment guidelines and protocols of LHF. A combination of broad-based key words were used to ensure all relevant clinical trials have been identified. They key words included left heart failure, left ventricular failure, medication and all-cause mortality. Additional studies were obtained from screening of bibliographies of articles obtained from the online search, review articles, and conference abstracts. The inclusion criteria included studies (a) large-scale randomized parallel group controlled trial (treatment and placebo); (b) recruited LHF patients with reduced LVEF (< 40%); (c) had a long follow-up period of at least six months; and (d) report clinical outcomes – all-cause mortality (death from any cause) and/or hospitalization. Articles were not excluded based on publication year and language. Studies with only abstracts, conference papers, and secondary research articles were excluded. Abstracted data from each included studies included first author, year of publication, patient population, inclusion criteria used for patient recruitment, primary medication used, mean follow-up period and reduction in all-cause mortality and/or hospitalization. (Table 9).
Table 9: Characteristics of studies included in meta-analysis
|
1st Author [Ref #]
|
Year
|
Patient Population
|
Inclusion Criteria
|
Primary Medication
|
Mean Follow-up (Months)
|
Reduction in all-cause Mortality/ Hospitalization (%)
|
pValue
|
|
Swedberg et al. [126] |
1988 |
127 |
CHF, NYHA-lV, Cardiomegaly on Chest X-ray. |
ACE-I (Enalapril) |
6.3 |
40 |
0.001 |
|
Pitt et al. [127] |
1991 |
822 |
LVEF≤35%, NYHA III–IV |
MRA (Spironolactone) |
24.0 |
30 |
0.001 |
|
Packer et al. [128] |
1996 |
1596 |
LVEF ≤ 30%; NYHA II–IV |
ACE-I (Lisinopril) |
45.6 |
8 |
0.130 |
|
Dargie et al. [129] |
1999 |
1991 |
LVEF ≤ 40%; NYHA II–IV |
β-blocker (Metoprolol) |
12.0 |
34 |
0.001 |
|
Merit-HF Study Group [130] |
1999 |
1156 |
LVEF ≤ 25%; NYHA II–IV |
β-blocker (Carvedilol) |
10.8 |
35 |
0.001 |
|
Packer et al. [131] |
2001 |
1285 |
LVEF ≤ 35%; NYHA I–IV |
ACE-I (Enalapril) |
42.0 |
16 |
0.004 |
|
Packer et al. [132] |
2002 |
1327 |
LVEF ≤ 35%; NYHA III–IV |
β-blocker (Bisoprolol) |
15.6 |
34 |
0.001 |
|
Flather et al. [133] |
2005 |
1067 |
LVEF ≤ 35%; Age ≥ 70;
HF hospitalization |
β-blocker (Nebivolol) |
21.6 |
14 |
0.04 |
|
Willenheimer et al. [134] |
2005 |
3268 |
LVEF ≤35%, NYHA II–IV, HF Hospitalization < 12 months |
If-channel blocker (Ivabradine) |
22.8 |
18 |
0.001 |
|
Zannad et al. [135] |
2011 |
1364 |
NYHA II, LVEF ≤30% or LVEF
30–35% with QRS >130 ms |
MRA (Eplerinone) |
21.6 |
37 |
0.001 |
|
Faris et al. [136] |
2012 |
1013 |
LVEF ≤40%, NYHA II–IV |
ARB (Candesartan) |
33.6 |
23 |
0.001 |
|
Kotecha et al. [137] |
2014 |
1276 |
LVEF ≤40%, NYHA II–IV, |
ARB (Candesartan) |
40.8 |
15 |
0.01 |
ACE-I: Angiotensin-Converting Enzyme - Inhibitor; CHF: Congestive Heart Failure; NYHA: New York Heart Association
Study characteristics and outcomes: Combined electronic and bibliographic searching for relevant literature identified 1102 potential publications, of which twelve (12) appeared to satisfy the inclusion/exclusion criteria, were retrieved and included in the present meta-analysis [126-137]. The 12 studies had a combined patient population of 16,292 in which the outcomes of medical therapy (all-cause mortality and/or hospitalization) was compared against a placebo group. Three studies investigated ACE-inhibitors (Enalapril or Lisinopril) [126,129,131]; four investigated beta-blockers (Carvedilol, Bisoprolol, Metoprolol and Nebivolol) [129,130,133,133]; two studied MRA (Spironolactone Eplerinone) [127,135], two studied ARB (Ivabradine and Candesartan) [136,137] and one studied I-f channel blocker (Ivabradine) [134]. Altogether, in a mean follow-up period of 25.33 months (SD = 10.87), medical therapy using ACE-inhibitors, beta-blockers, ARB and/or I-f channel blocker significantly reduced all-cause mortality (mortality from any cause) and/or hospitalization by a weighted mean of 24.73% (SD = 13.05). However, although echocardiographic-define LVEF is the preferred reference standard for recruiting patients in clinical trials, some studies use NHYA functional class and clinical symptoms such as breathlessness, dyspnea and HF hospitalization with the past 12 months.
Discussion: The main therapeutic goals in the treatment of patients with LHF has been to improve clinical status, function al capacity and quality of life, prevent hospitalization and reduce mortality. Medical therapy using neuro-hormonal antagonists (ACE-inhibitors, MRAs and beta-blockers) is a common clinical practice [92]. Medical therapy for LHF has had favorable clinical effect on short-term surrogate markers but on the long-term cause a detrimental effect on patient outcomes [122]. Further, due to the lack of long-term markers for LHF treatment, the ESC, ACC/AHA and NICE guidelines rely on mortality and hospitalization data to for recommending and approving medication for LHF treatment [123,124]. The present meta-analysis finds medication therapy for managing LV systolic dysfunction such as ACE-inhibitors, ARBs, beta-blocker and i-f channel blockers produce a protective effect against mortality and hospitalization to suggest treatment efficacy. However, the mean follow-up period in the present meta-analysis (25.33 months) was not sufficiently long to evaluate long-term outcomes.
The positive therapeutic effect of neuro-hormonal antagonists has been demonstrated elsewhere in patients with LHF. Unless contra-indicate or not-tolerated neuro-hormonal antagonists are recommended for all LHF patients [138]. Although the present findings reveal ARB are effective in reducing all-cause mortality, current evidence on LHF patients with reduced LVEF (<40%) are inconclusive and thus ARBs are restricted to LHF patients intolerant to ACE-inhibitors or on ACE-inhibitor treatment but intolerant to MRAs [3,10]. Neuro-hormonal antagonists are also indicated for patients with asymptomatic LV systolic dysfunction or with prior myocardial infarction to reduce the risk of developing LHF, hospitalization and death [7]. In addition to increasing survival (reduce mortality), iIvabradine, an i-f channel blocker also is useful to elevate heart rate and improve outcomes and recommended for used on patient-to patient basis [10].
Efforts to improve the effectiveness of neuro-hormonal antagonists in LHF treatment, they should be used in conjunction with diuretics in patients presenting with clinical signs and symptoms of congestion. Diuretics relive symptoms of congestion and improve exercise capacity in patients with signs and symptoms of congestive heart failure but their long-term effect on mortality and hospitalization lacks evidence-based clinical trials. Ongoing research on LHF medication such as neprilysin (NEP) inhibitor indicated promises of begin superior to ACE-inhibitors in reducing HF-related mortality and hospitalization [138]. The new medication is indicated to replace ACE-inhibitors in acute care or ambulatory HF with reduced LVEF who remain symptomatic despite optimal medical therapy [136,137]. In sum, medication is an effective therapy for LHF patients since it significantly reduced all-cause mortality and hospitalization.
Until recently, the term heart failure (HF) has been synonymous with left heart failure (LHF), denoting a complex clinical syndrome resulting from structural and/or functional cardiac disorders. Although its incidence is stabilizing or even reducing, its prevalence is steadily increasing due to advancements in HF treatment and increased survival. Its prognosis is poor, marked with high early post-discharge mortality and hospitalization. Common clinical symptoms are breathlessness, ankle swelling and fatigue accompanied by signs of elevated jugular venous pressure, pulmonary crackles and peripheral edema. Diagnosis of LHF consists of a series of tests including assessment of detailed patient history and symptomatology, measurement of plasma natriuretic peptides and echocardiography cardiac imaging, which is considered the gold standard for assessing LV systolic dysfunction. Natriuretic peptides (BNP) plays an important role in excluding LHF diagnosis as well as in selecting patients for echocardiography. Other important complementary tests include cheat x-rays, electrocardiogram, exercise testing, invasive hemodynamic assessment and endomyocardial biopsy. Lung ultrasound is recommended for patients suspected with pulmonary congestion. Clinical management focuses on preventive strategies that modify risk factors for asymptomatic patients, medical therapy for symptomatic patients with LV systolic dysfunction and overt heart failure to prevent death and hospitalization and improve exercise capacity. Device therapy (implantable cardioverter-defibrillator and/or cardiac resynchronization therapy) indicated for patients with worsening symptoms for primary and/or secondary prevention of sudden cardiac death.
- Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ., Ponikowski P, et al. (2008) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 29: 2388-2442.[Crossref]
- Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, et al. (2014) The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 63: 1123-1133. [Crossref]
- McMurray JJ, Stewart S (2000) Epidemiology, aetiology, and prognosis of heart failure. Heart 83: 596-602. [Crossref]
- Mendez GF, Cowie MR (2001) The epidemiological features of heart failure in developing countries: a review of the literature. Int J Cardiol 80: 213-219. [Crossref]
- Cook C, Cole G, Asaria P, Jabbour R, Francis DP (2014) The annual global economic burden of heart failure. Int J Cardiol 171: 368-376. [Crossref]
- O'connell JB (2000) The economic burden of heart failure. Clin Cardiol 23: III6-III10. [Crossref]
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, et al. (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37: 2129-2200. [Crossref]
- Hunt SA (2005) ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: A report of the american college of cardiology/american heart association task force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure). J Am Coll Cardiol 46:e1–82. [Crossref]
- Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, et al. (2009) 2009 focused update. ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines: Developed in collaboration with the international society for heart and lung transplantation. Circ 119:1977–2016. [Crossref]
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, et al. (2012) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur J Heart Fail 14: 803-869. [Crossref]
- Chaplin S (2016) New guidelines from the European Society of Cardiology. Prescriber 27: 51-54. [Crossref]
- Butler J, Fonarow GC, Zile MR, Lam CS, Roessig L, et al. (2014) Developing therapies for heart failure with preserved ejection fraction: current state and future directions. JACC: Heart Failure 2: 97-112. [Crossref]
- McMurray JJ (2010) Systolic heart failure. N Engl J Med 362: 228-238. [Crossref]
- Chen J, Normand SLT, Wang Y, Krumholz HM (2011) National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. Jama 306: 1669-1678. [Crossref]
- Dunlay SM, Redfield MM, Weston SA, Therneau TM, Long KH, et al. (2009) Hospitalizations after heart failure diagnosis: a community perspective. J Am Coll Cardiol 54: 1695-1702. [Crossref]
- Metra M, Ponikowski P, Dickstein K, McMurray JJ, Gavazzi A, et al. (2007) Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 9: 684-694. [Crossref]
- Braunwald E (1997) Cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med 337: 1360-1369. [Crossref]
- Roger VL (2013) Epidemiology of heart failure. Circ Res 113: 646-659. [Crossref]
- Savares G, Lund LH (2017) Global Public Health Burden of Heart Failure. Cardiac Failure Review 3: 7-11. [Crossref]
- Ceia F, Fonseca C, Mota T, Morais H, Matias F, et al. (2002) Prevalence of chronic heart failure in Southwestern Europe: the EPICA study. Eur J Heart Fail 4: 531-539. [Crossref]
- Ohlmeier C, Mikolajczyk R, Frick J, Prutz F, Haverkamp W, et al. (2015) Incidence, prevalence and 1-year all-cause mortality of heart failure in Germany: a study based on electronic healthcare data of more than six million persons. Clin Res Cardiol 104: 688-696. [Crossref]
- Zarrinkoub R, Wettermark B, Wändell P, Mejhert M, Szulkin R, et al. (2013) The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail 15: 995-1002. [Crossref]
- Buja A, Solinas G, Visca M, Federico B, Gini R, et al. (2016) Prevalence of heart failure and adherence to process indicators: Which socio-demographic determinants are involved? Int J Environ Res Public Health 13: 238. [Crossref]
- Sakata Y, Shimokawa H (2013) Epidemiology of heart failure in Asia. Circ J 77: 2209-2217. [Crossref]
- Hu SS, Kong LZ, Gao RL, Zhu ML, Wen WA, et al. (2012) Outline of the report on cardiovascular disease in China, 2010. Biomed Environ Sci 25: 251-256. [Crossref]
- Yang YN, Ma YT, Liu F, Huang D, Li XM, et al. (2010) Incidence and distributing feature of chronic heart failure in adult population of Xinjiang. Zhonghua Xin Xue Guan Bing Za Zhi 38: 460-464. [Crossref]
- Lam CS (2015) Heart failure in Southeast Asia: facts and numbers. ESC heart failure 2: 46-49. [Crossref]
- Ciapponi A, Alcaraz A, Calderon M, Matta MG, Chaparro M, et al. (2016) Burden of heart failure in Latin America: a systematic review and meta-analysis. Revista Española de Cardiología (English Edition), 69: 1051-1060. [Crossref]
- Sahle BW, Owen AJ, Mutowo MP, Krum H, Reid CM (2016) Prevalence of heart failure in Australia: a systematic review. BMC Cardiovasc Disord: 32. [Crossref]
- Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, et al. (2002) Long-term trends in the incidence of and survival with heart failure. N Engl J Med 347: 1397-1402. [Crossref]
- Najafi F, Jamrozik K, Dobson AJ (2009) Understanding the ‘epidemic of heart failure’: a systematic review of trends in determinants of heart failure. Eur J Heart Fail 11: 472-479. [Crossref]
- Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, et al. (2007) Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol 50: 768-777. [Crossref]
- Cheng RK, Cox M, Neely, ML, Heidenreich PA, Bhatt DL, et al. (2014) Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J 168: 721-730. [Crossref]
- Lenzen MJ, Scholte WJM, Boersma E, Vantrimpont PJMJ, Follath F, et al. (2004) Differences between patients with a preserved and a depressed left ventricular function: a report from the EuroHeart Failure Survey. Eur Heart J 25: 1214-1220. [Crossref]
- Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, et al. (2006) EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J 27: 2725-2736. [Crossref]
- Crespo-Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, et al. (2016) Heart Failure Association (HFA) of the European Society of Cardiology (ESC). European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail 18: 613-625. [Crossref]
- Johnson FL (2014) Pathophysiology and etiology of heart failure. Cardiol Clin 32: 9-19. [Crossref]
- Sutton MGSJ, Sharpe N (2000) Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circ 101: 2981-2988. [Crossref]
- Pfeffer MA, Braunwald E (1990) Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circ 81: 1161-1172. [Crossref]
- Warren SE, Royal HD, Markis JE, Grossman W, McKay R (1988) Time course of left ventricular dilation after myocardial infarction: influence of infarct-related artery and success of coronary thrombolysis. J Am Coll Cardiol 11: 12-19. [Crossref]
- Shah AM, Mann DL (2011) In search of new therapeutic targets and strategies for heart failure: recent advances in basic science. The Lancet 378: 704-712. [Crossref]
- Davie AP, Francis CM, Caruana L, Sutherland GR, McMurray JJ (1997) Assessing diagnosis in heart failure: which features are any use? QJM: monthly journal of the Association of Physicians 90: 335-339. [Crossref]
- Mant J, Doust J, Roalfe A, Barton P, Cowie MR, et al. (2009) Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. Health Technology Assessment 13. [Crossref]
- Oudejans I, Mosterd A, Bloemen JA, Valk MJ, Velzen E, et al. (2011) Clinical evaluation of geriatric outpatients with suspected heart failure: value of symptoms, signs, and additional tests. Eur J Heart Fail 13: 518-527. [Crossref]
- Kelder JC, Cramer MJ, van Wijngaarden J, van Tooren R, Mosterd A, et al. (2011) The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure: Clinical perspective. Circ 124: 2865-2873. [Crossref]
- Boonman‐de Winter LJ, Rutten FH, Cramer MJ, Landman MJ, Zuithoff N, et al. (2015) Efficiently screening heart failure in patients with type 2 diabetes. Eur J Heart Fail 17: 187-195. [Crossref]
- Daniels LB, Clopton P, Bhalla V, Krishnaswamy P, Nowak RM, et al. (2006) How obesity affects the cut-points for B-type natriuretic peptide in the diagnosis of acute heart failure: results from the Breathing Not Properly Multinational Study. Am Heart J 151: 999-1005. [Crossref]
- Hawkins NM, Petrie MC, Jhund PS, Chalmers GW, Dunn FG, et al (2009) Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail 11: 130-139. [Crossref]
- Rutten FH, Cramer MJM, Grobbee DE, Sachs AP, Kirkels JH, et al. (2005) Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J 26: 1887-1894. [Crossref]
- Wong CM, Hawkins NM, Jhund PS, MacDonald MR, Solomon SD, et al. (2013) Clinical characteristics and outcomes of young and very young adults with heart failure: the CHARM programme (Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity). J Am Coll Cardiol 62: 1845-1854. [Crossref]
- Nardone DA (2006) Medical history, physical examination, and routine tests are useful for diagnosing heart failure in dyspnea. BMJ Evidence-Based Medicine 11: 72. [Crossref]
- Wong GC, Ayas NT (2007) Clinical approaches to the diagnosis of acute heart failure. Curr Opin Cardiol 22: 207-213. [Crossref]
- Peacock WF, Soto KM (2010) Current techniques of fluid status assessment. In Fluid Overload 164: 128-142. Karger Publishers. [Crossref]
- Vitale C, Spoletini I (2017) Clinical Diagnosis in Heart Failure. International Cardiovascular Forum Journal 10: 12-15. [Crossref]
- Van Riet EE, Hoes AW, Limburg A, Landman MA, van der Hoeven H, Rutten FH (2014) Prevalence of unrecognized heart failure in older persons with shortness of breath on exertion. Eur J Heart Fail 16: 772-777. [Crossref]
- Kakouros NS, Kakouros SN (2015) Clinical assessment in acute heart failure. Hellenic Journal of Cardiology 56: 285-301. [Crossref]
- Leier CV, Chatterjee K (2007) The physical examination in heart failure—Part I. Congestive Heart Failure 13: 41-47. [Crossref]
- Leier CV, Chatterjee K (2007) The physical examination in heart failure—Part II. Congestive Heart Failure 13: 99-0103. [Crossref]
- Caldentey G, Khairy P, Roy D, Leduc H, Talajic M, et al. (2014) Prognostic value of the physical examination in patients with heart failure and atrial fibrillation: insights from the AF-CHF trial (atrial fibrillation and chronic heart failure). JACC Heart Fail 2: 15-23. [Crossref]
- Roberts E, Ludman AJ, Dworzynski K, Al-Mohammad A, Cowie MR, et al. (2015) The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta-analysis in the acute care setting. BMJ 350: h910. [Crossref]
- Zaphiriou A, Robb S, Murray‐Thomas T, Mendez G, Fox K, et al. (2005) The diagnostic accuracy of plasma BNP and NTproBNP in patients referred from primary care with suspected heart failure: results of the UK natriuretic peptide study. Eur J Heart Fail 7: 537-541. [Crossref]
- Krishnaswamy P, Lubien E, Clopton P, Koon J, Kazanegra R, et al. (2001) Utility of B-natriuretic peptide levels in identifying patients with left ventricular systolic or diastolic dysfunction. Am J Med 111: 274-279. [Crossref]
- Kelder JC, Cramer MJ, Verweij WM, Grobbee DE, Hoes AW (2011) Clinical utility of three B-type natriuretic peptide assays for the initial diagnostic assessment of new slow-onset heart failure. J Card Fail 17: 729-734. [Crossref]
- Fuat A, Murphy JJ, Hungin APS, Curr J, Mehrzad AA, et al. (2006) The diagnostic accuracy and utility of a B-type natriuretic peptide test in a community population of patients with suspected heart failure. Br J Gen Pract 56: 327-333. [Crossref]
- Madamanchi C, Alhosaini H, Sumida A, Runge MS (2014) Obesity and natriuretic peptides, BNP and NT-proBNP: mechanisms and diagnostic implications for heart failure. Int J Cardiol 176: 611-617. [Crossref]
- Davie AP, Francis CM, Love MP, Caruana L, Starkey IR, et al (1996) Value of the electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ 312: 222. [Crossref]
- Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, et al. (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur heart J 28: 2539-2550. [Crossref]
- Dokainish H, Nguyen JS, Bobek J, Goswami R, Lakkis NM (2011) Assessment of the American Society of Echocardiography-European Association of Echocardiography guidelines for diastolic function in patients with depressed ejection fraction: an echocardiographic and invasive haemodynamic study. Eur J Echocardiogr 12: 857-864. [Crossref]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28: 1-39. [Crossref]
- Cohen GI, Pietrolungo JF, Thomas JD, Klein AL (1996) A practical guide to assessment of ventricular diastolic function using Doppler echocardiography. J Am Coll Cardiol 27: 1753-1760. [Crossref]
- Gilman G, Nelson TA, Hansen WH, Khandheria BK, Ommen SR (2007) Diastolic function: a sonographer’s approach to the essential echocardiographic measurements of left ventricular diastolic function. J Am Soc Echocardiogr 20: 199-209. [Crossref]
- Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, et al. (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 10: 165-193. [Crossref]
- Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, et al. (2012) International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 38: 577-591. [Crossref]
- Davis M, Espiner EA, Yandle T, Richards G, Town I, et al. (1994) Plasma brain natriuretic peptide in assessment of acute dyspnoea. The Lancet 343: 440-444. [Crossref]
- Cowie MR, Struthers AD, Wood DA, Coats AJ, Thompson SG, et al. (1997) Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. The Lancet 350: 1349-1353. [Crossref]
- Landray MJ, Lehman R, Arnold I (2000) Measuring brain natriuretic peptide in suspected left ventricular systolic dysfunction in general practice: cross-sectional study. BMJ 320: 985-986. [Crossref]
- Dao Q, Krishnaswamy P, Kazanegr R, Harrison A, Amirnovin R, et al. (2001) Utility of B-type natriuretic peptide in the diagnosis of congestive heart failure in an urgent-care setting. J Am Coll Cardiol 37: 379-385. [Crossref]
- Logeart D, Saudubray C, Beyne P, Thabut G, Ennezat PV, et al. (2002) Comparative value of Doppler echocardiography and B-type natriuretic peptide assay in the etiologic diagnosis of acute dyspnea. J Am Coll Cardiol 40: 1794-1800. [Crossref]
- Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, et al. (2002) Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 347: 161-167. [Crossref]
- Villacorta H, Duarte A, Duarte NM, Carrano A, Mesquita ET, et al. (2002) The role of B-type natriuretic peptide in the diagnosis of congestive heart failure in patients presenting to an emergency department with dyspnea. Arquivos brasileiros de cardiologia 79: 569-572. [Crossref]
- Sim V, Hampton D, Phillips C, Lo SN, Vasishta S, et al. (2003) The use of brain natriuretic peptide as a screening test for left ventricular systolic dysfunction-cost-effectiveness in relation to open access echocardiography. Family Practice 20: 570-574. [Crossref]
- Barcarse E, Kazanegra R, Chen A, Chiu A, Clopton P, et al. (2004) Combination of B‐Type Natriuretic Peptide Levels and Non‐Invasive Hemodynamic Parameters in Diagnosing Congestive Heart Failure in the Emergency Department. Congestive Heart Failure 10: 171-176. [Crossref]
- Bayes‐Genis A, Santalo‐Bel M, Zapico‐Muniz, Lopez L, Cotes C, et al. (2004) N‐terminal probrain natriuretic peptide (NT‐proBNP) in the emergency diagnosis and in‐hospital monitoring of patients with dyspnoea and ventricular dysfunction. Eur J Heart Fail 6: 301-308. [Crossref]
- Dokainish H, Zoghbi WA, Lakkis NM, Quinones MA, Nagueh SF (2004) Comparative accuracy of B-type natriuretic peptide and tissue Doppler echocardiography in the diagnosis of congestive heart failure. Am J Cardiol 93: 1130-1135. [Crossref]
- Knudsen CW, Riis JS, Finsen AV, Eikvar L, Muller C, et al. (2004) Diagnostic value of a rapid test for B‐type natriuretic peptide in patients presenting with acute dyspnoe: effect of age and gender. Eur J Heart Fail 6: 55-62. [Crossref]
- Kruger S, Filzmaier K, Graf J, Kunz D, Stickel T, et al. (2004). QRS prolongation on surface ECG and brain natriuretic peptide as indicators of left ventricular systolic dysfunction. J. Intern. Med 255: 206-212. [Crossref]
- Gustafsson F, Steensgaard-Hansen F, Badskjær J, Poulsen AH, Corell P, et al. (2005) Diagnostic and prognostic performance of N-terminal ProBNP in primary care patients with suspected heart failure. J Card Fail 11: S15-S20. [Crossref]
- Januzzi JL, Camargo CA, Anwaruddin S, Baggish AL, Chen AA, et al. (2005) The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol 95: 948-954. [Crossref]
- Mueller T, Gegenhuber A, Poel W, Haltmayer M (2005) Diagnostic accuracy of B type natriuretic peptide and amino terminal proBNP in the emergency diagnosis of heart failure. Heart 91: 606-61290. [Crossref]
- National CGCU (2010) Chronic Heart Failure: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care: Partial Update. [Crossref]
- Ewald B, Ewald D, Thakkinstian A, Attia J (2008) Meta‐analysis of B type natriuretic peptide and N‐terminal pro B natriuretic peptide in the diagnosis of clinical heart failure and population screening for left ventricular systolic dysfunction. Intern Med J 38: 101-113. [Crossref]
- Davenport C, Cheng EYL, Kwok YTT, Lai AHO, Wakabayashi T, et al. (2006) Assessing the diagnostic test accuracy of natriuretic peptides and ECG in the diagnosis of left ventricular systolic dysfunction: a systematic review and meta-analysis. Br J Gen Pract 56: 48-56. [Crossref]
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, et al. (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62: e147-e239. [Crossref]
- Kostis JB, Davis BR, Cutler J, Grimm RH, Berge KG, et al. (1997) Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. Jama, 278: 212-216. [Crossref]
- Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, et al. (2008) Treatment of hypertension in patients 80 years of age or older. N Engl J Med 358: 1887-1898. [Crossref]
- Sciarretta S, Palano F, Tocci G, Baldini R, Volp M (2011) Antihypertensive treatment and development of heart failure in hypertension: a Bayesian network meta-analysis of studies in patients with hypertension and high cardiovascular risk. Arch Intern Med 171: 384-394. [Crossref]
- SPRINT Research Group. (2015). A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 373: 2103-2116. [Crossref]
- Suskin N, Sheth T, Negassa A, Yusuf S (2001) Relationship of current and past smoking to mortality and morbidity in patients with left ventricular dysfunction. J Am Coll Cardiol 37: 1677-1682. [Crossref]
- Larsson SC, Orsini N, Wolk A (2015) Alcohol consumption and risk of heart failure: a dose–response meta‐analysis of prospective studies. Eur J Heart Fail 17: 367-373. [Crossref]
- Dorans KS, Mostofsky E, Levitan EB, Hakansson N, Wolk A, et al. (2015) Alcohol and incident heart failure among middle-aged and elderly men: the cohort of Swedish men. Circ-Heart Fail CIRCHEARTFAILURE-114. [Crossref]
- Gonçalves A, Claggett B, Jhund PS, Rosamond W, Deswal A, et al. (2015) Alcohol consumption and risk of heart failure: the Atherosclerosis Risk in Communities Study. Eur Heart J 36: 939-945. [Crossref]
- Pandey A, Garg S, Khunger M, Darden D, Ayer C, et al. (2015) Dose response relationship between physical activity and risk of heart failure: a meta-analysis. Circ CIRCULATIONAHA-115. [Crossref]
- Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, et al. (2002) Obesity and the risk of heart failure. N Engl J Med 347: 305-313. [Crossref]
- Kenchaiah S, Sesso HD, Gaziano JM (2009) Body mass index and vigorous physical activity and the risk of heart failure among men. Circ 119: 44-52. [Crossref]
- Lee DS, Massaro JM, Wang TJ, Kannel WB, Benjamin EJ, et al. (2007) Antecedent blood pressure, body mass index, and the risk of incident heart failure in later life. Hypertens 50: 869-876. [Crossref]
- Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, et al. (2008) Incident Heart Failure Prediction in the ElderlyCLINICAL PERSPECTIVE: The Health ABC Heart Failure Score. Circ-Heart Fail 1: 125-133. [Crossref]
- Lind M, Bounias I, Olsson M, Gudbjörnsdottir S, Svensson AM, Rosengren A (2011) Glycaemic control and incidence of heart failure in 20 985 patients with type 1 diabetes: an observational study. The Lancet 378: 140-146. [Crossref]
- Kalogeropoulos A, Georgiopoulou V, Harris TB, Kritchevsky SB, Bauer DC, et al. (2009) Glycemic status and incident heart failure in elderly without history of diabetes mellitus: the health, aging, and body composition study. J Card Fail 15: 593-599. [Crossref]
- Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, et al. (2008) Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol 52: 1769-1781. [Crossref]
- Taylor F, Ward K, Moore TH, Burke M, Davey G, et al. (2011) Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 1. [Crossref]
- Yusuf SW, Ilias-Khan NA, Durand JB (2011). Chemotherapy-induced cardiomyopathy. Expert Rev Cardiovasc Ther 9: 231-243. [Crossref]
- Choueiri TK, Mayer EL, Je Y, Rosenberg JE, Nguyen PL, et al. (2011) Congestive heart failure risk in patients with breast cancer treated with bevacizumab. J Clin Oncol 29: 632-638. [Crossref]
- Du XL, Xia R, Burau K, Liu CC (2011) Cardiac risk associated with the receipt of anthracycline and trastuzumab in a large nationwide cohort of older women with breast cancer, 1998–2005. Medical oncology 28: 80-90. [Crossref]
- Jong P, Yusuf S, Rousseau MF, Ahn SA, Bangdiwala SI (2003) Effect of enalapril on 12-year survival and life expectancy in patients with left ventricular systolic dysfunction: a follow-up study. The Lancet 361: 1843-1848. [Crossref]
- Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown Jr EJ, et al. (1992) Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the Survival and Ventricular Enlargement Trial. N Engl J Med 327: 669-677. [Crossref]
- Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, et al. (2002) Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 346: 877-883. [Crossref]
- Sohaib SA, Finegold JA, Nijjer SS, Hossain R, Linde C, et al. (2015) Opportunity to increase life span in narrow QRS cardiac resynchronization therapy recipients by deactivating ventricular pacing: evidence from randomized controlled trials. JACC: Heart Failure 3: 327-336. [Crossref]
- Cleland JG, Abraham WT, Linde C, Gold MR, Young JB, et al. (2013) An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J 34: 3547-3556. [Crossref]
- Gage RM, Burns KV, Bank AJ (2014) Echocardiographic and clinical response to cardiac resynchronization therapy in heart failure patients with and without previous right ventricular pacing. Eur J Heart Fail 16: 1199-1205. [Crossref]
- Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, et al. (2013) Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 369: 1395-1405. [Crossref]
- Steffel J, Robertson M, Singh JP, Abraham WT, Bax JJ, et al. (2015) The effect of QRS duration on cardiac resynchronization therapy in patients with a narrow QRS complex: a subgroup analysis of the EchoCRT trial. Eu Heart J 36: 1983-1989. [Crossref]
- Zusterzeel R, Selzman KA, Sanders WE, Canos DA, O’Callaghan KM, et al. (2014) Cardiac resynchronization therapy in women: US Food and Drug Administration meta-analysis of patient-level data. JAMA Intern Med 174: 1340-1348. [Crossref]
- Stewart S, Jenkins A, Buchan S, McGuire A, Capewell S, McMurray JJ (2002) The current cost of heart failure to the National Health Service in the UK. Eur J Heart Fail 4: 361-371. [Crossref]
- Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, et al. (2014). The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 63: 1123-1133. [Crossref]
- Gheorghiade M, Shah AN, Vaduganathan M, Butler J, Bonow RO, et al. (2013) Recognizing hospitalized heart failure as an entity and developing new therapies to improve outcomes: academics', clinicians', industry's, regulators', and payers' perspectives. Heart Failure Clinics 9: 285-290. [Crossref]
- Swedberg K, Kjekshus J, CONSENSUS Trial Study Group (1988) Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol 62: 60A-66A. [Crossref]
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, et al. (1999) The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 341: 709-717. [Crossref]
- Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, et al. (1996) The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 334: 1349-1355. [Crossref]
- Dargie HJ (1999) The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 353: 9-13. [Crossref]
- Merit-HF Study Group. (1999). Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in-congestive heart failure (MERIT-HF). The Lancet 353: 2001-2007. [Crossref]
- Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, et al. (2001) Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 344: 1651-1658. [Crossref]
- Packer M, Fowler MB, Roecke EB, Coats AJ, Katus HA, et al. (2002) Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circ 106: 2194-2199. [Crossref]
- Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, et al. (2005) Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 26: 215-225. [Crossref]
- Willenheimer R, van Veldhuisen DJ, Silke B, Erdmann E, Follath F, et al. (2005) Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence: results of the randomized Cardiac Insufficiency Bisoprolol Study (CIBIS) III. Circ 112: 2426-2435. [Crossref]
- Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, et al. (2011) Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 364: 11-21. [Crossref]
- Faris RF, Flather M, Purcell H, Poole-Wilson PA, Coats AJ (2012) Diuretics for heart failure. Cochrane Database Syst Rev 2. [Crossref]
- Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, et al. (2014) Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. The Lancet 384: 2235-2243. [Crossref]



