The prevalence of left bundle branch block (LBBB) is significantly higher in the heart failure (HF) population compared to the general population. LBBB is more often associated with structural heart disease especially dilated cardiomyopathy (DCM) of a non-ischemic origin. In isolation, LBBB is not a cause of specific clinical concern and does not affect prognosis. However, in the presence of structural heart disease, LBBB is an independent predictor of cardiovascular mortality and HF events suggesting that long-standing LBBB may be the cause of HF. In addition, guideline directed medical treatment (GDMT) in DCM patients with LBBB is less effective or associated with worsening symptoms. Conversely, cardiac resynchronization therapy (CRT) has shown beneficial outcomes in DCM patients with LBBB. It reverses LV dysfunction and protects against HF events, which suggests the possibility of a specific type of LBBB-induced CM characterized by LV dilatation and depressed LV systolic function in the absence of any other known aetiology. Despite the evidence, current medical literature does not consider LBBB-induced CM as a clinical entity but a complication of CM or structural heart disease. Since LBBB-induced CM requires early treatment with CRT instead of after three months of GDMT, diagnosis of LBBB-induced CM is important to guide the choice of optimal treatment (CRT). The present review summarizes the current published evidence on LBBB-induced CM and identifies gaps in knowledge that may benefit from additional research.
cardiac resynchronization therapy, left bundle branch block-cardiomyopathy, left ventricular dyssynchrony
The presence of left bundle branch block (LBBB) on the standard 12-lead electrocardiogram (ECG) poses multiple important and puzzling questions to the health care provider. LBBB provides important prognostic information but also poses serious challenges to the performance and interpretation of diagnostic tests. There is a realistic need to consider LBBB as a cardiac clinical entity rather than simply an ECG finding [1]. Its presence often suggests an ominous prognosis in acute cases such as acute myocardial infarction and chronic conditions such as heart failure (HF). LBBB also occurs frequently in patients with dilated cardiomyopathy (DCM), where it is associated with higher rates of morbidity and mortality [1-3]. The traditional assumption is that LBBB is a consequence of the underlying disease process of DCM. However, DCM patients with LBBB compared to those with normal intraventricular conduction are more likely to exhibit increased left ventricular (LV) dilatation, depressed LV ejection fraction (LVEF), increased symptomatology and shorter survival [4]. Recently, three retrospective cohort studies demonstrate a poor prognosis of LBBB-CM patients despite receiving optimal guideline directed medical therapy (GDMT) [5-7]. The current GMDT recommend the use of CRT to patients with LBBB and left ventricular (LV) ejection fraction (LVEF) ≤ 35% after three months of HF medication may be less effective to this cohort [8-10]. However, CRT is emerging as a major therapeutic intervention to reverse LV remodelling and protection against HF events in patients presenting with a typical LBBB. LBBB is also one of the most powerful predictors of a super-response to CRT. The growing evidence suggests that in some patients, a longstanding LBBB may induce abnormal LV contraction pattern and ultimately a specific form of CM potentially reversible by CRT. The present review summarizes the current knowledge of the epidemiology, diagnostic, prognostic and therapeutic implications of LBBB-induced CM.
Historical context
The possible involvement of LBBB in the pathogenesis of LV systolic failure in patients with CM and HF is a recent discovery. Traditionally, LV systolic failure was associated with LV remodelling in the setting of intrinsic abnormalities in the function of cardiomyocytes and subsequent derangements in neurohormonal activation [11,12]. Only over the past decade did abnormalities in LV performance due to abnormal activation patterns gain widespread attention both in cardiology literature and in clinical practice. This recent attention was stimulated by the successful introduction of CRT as a novel device-based therapeutic option for patients diagnosed with end-stage HF and ventricular conduction delays in the mid-1990s [13,14]. Two decades later, several RCTs have demonstrated its beneficial potential in patients with chronic HF [15,16].
However, the initial reports on the pathologic consequences of abnormal electrical ventricular activation on mechanical cardiac performance appeared much earlier, immediately following the introduction of invasive and non-invasive imaging techniques into clinical cardiology [17]. These early studies described abnormal or delayed LV activation wave front lead to a reduction in LV efficiency and performance that is independent of cardiomyocyte contractile function [17-19]. McDonald [20] was the first to demonstrate the abnormal motion of the interventricular septum in LBBB on echocardiography. During early systole (at the time of aortic opening), the interventricular septum demonstrates a posterior motion, which may vary considerably, and followed by abnormal anterior motion later during the ejection phase. Subsequently, Fujii et al. [21] analysed 37 patients with LBBB and described three patterns of septal motion (Types A, B and C). Type A and B present early and abrupt posteriorly directed septal motion during the pre-ejection period after which septum motion is anterior nu type A and posterior in type B. Type C exhibits akinetic or diskinetic septal motion throughout systole. All patients with complete LBBB exhibit delayed posterior wall contraction [12].
The recent renewed interest in the significance of LBBB is attributable to the report from the Multicentre Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT) published in 2011. The report observed that only class I and II HF patients with LBBB derived benefits from CRT while patients with non-LBBB configuration did not derive any benefit [22]. The Medicare Implantable Cardioverter-Defibrillator Registry reported similar findings. Out of 14,946 patients who had received CRT and met standard QRS and LVEF criteria for CRT implantation, 69% had LBBB, 11% RBBB and 20% had non-specific intraventricular conduction disturbance. During a median follow-up of 40 months, non-LBBB patients had significantly higher early and late mortality than LBBB patients associated with a positive response to CRT [23]. A QRS duration ≥ 0.15 seconds predicted a more favourable outcome in LBBB patients but not in RBBB patients [12].
Definition of LBBB
Generally, LBBB is a type of interventricular conjunction disturbance. However, due to increased importance of distinguishing LBBB from diffuse intraventricular conduction disturbance, a closer look at the electrocardiographic (ECG) criteria is warranted. The dominant feature of intraventricular block in classical LBBB is a prolonged QRS complex (≥ 120 ms) caused by delayed activation of the LV accompanied by a characteristic morphology of the QRS complex [24-26]. In addition to conduction disturbances in specific parts of the intraventricular conduction system, a diffuse intraventricular conduction disturbance may exist in the LV due to more peripherally located regional conduction disturbances such as post-myocardial infarction. Such a diffuse or on-specific intraventricular conduction abnormality may also present with prolonged QRS interval (≥ 120 ms) but with a pattern of QRS that does not correspond to the classical patter of LBBB or RBBB [12].
The term “block” as used in LBBB does not necessarily imply a complete interruption of conduction but may be due to a major delay of conduction in some parts of the conduction system, which explains the variety of observable morphologies and frequently non-specific patterns of conduction delay [27]. Besides the typical prolonged QRS (>120 ms), there is a delay of the intrinsic deflection in leads V5 and V6 > 60 ms. Typical there is no septal (q) waves in leads I, V5, and V6 due to the abnormal septal activation from right to left. A narrow q wave may be present in lead aVL in the absence of myocardial pathology, and broad notched or slurred R waves can be observed in the left-sided leads I, aVL, V5, and V6.. The activation wave front from the RV proceeds slowly across the septum toward the late activated LV posterolateral segments and consequently the right-sided chest leads V1 and V2 present with an rS or QS pattern. Recently, Strauss et al. [28] emphasized on the importance of notched or slurred R waves in left-sided leads I, aVL, V5 or V6 as a classical feature of LBBB.
Sze, et al. [5] demonstrated that in LBBB patients, left ventricular dyssynchrony caused by abnormal conduction of electrical signal and subsequently mechanical dyssynchrony in the contraction of ventricular parts may lead to a specific kind of CM. Since the cause is a mission functionally left bundle, LBBB-CM requires a specific treatment – implantation of prosthesis of the left bindle, which is an LV lead. Delay in the application of this treatment prior to the failure of medical management over the course of 3 months delays effective treatment and may reduce treatment efficacy and result in the deterioration of the condition [4,5]. Thus, LBBB-CM may be defined as a specific type of CM characterized by LV dilatation and depressed LV systolic function without CAD or any other known aetiology in the setting of an abnormal conduction of electrical signal that is potentially reversible the institution of CRT.
Anatomy of LBBB
Anatomically, the left bundle arises from the His bundle on the left endocardial side of the muscular septum [29]. On rare occasions, His bundle may lie on the right side of the interventricular septum and the left bundle may actually traverse through the septum [30]. The proximal portion of the left bundle may vary in width (1 to 14 mm) influenced by the location of His bundle in relation to the septum [29]. The left bundle then broadens as it travels apically and divides into sub-divisions including the anterior and posterior sub-divisions. More commonly, the left bundle consists of multiple inter-connected sub-divisions that vary in number, size and morphology [30]. However, a simple division in to anterior and posterior fascicles has some clinical relevance. The anterior sub-divisions receive vascular supply from the left anterior descending artery, and the posterior sub-divisions receive vascular supply from the left anterior descending artery and posterior septal perforator branches of the posterior descending artery, which increases its resistance to ischemia and necrosis [27]. LBBB can be transient or rate-dependant. At faster heart rates, LBBB occurs because of impulse falling in the relative refractory period of the bundle branch cells (also known as phase 3 block). The exact mechanisms responsible for LBBB at a slower heart rate is controversial but the most explanation is that it is related to spontaneous depolarization in phase four renders the cells refractory to the next impulse [27,30].
Epidemiology/natural history
Since the introduction of invasive and non-invasive cardiac imaging, studies on conduction abnormalities in HF patients have increased. The prevalence of LBBB is significantly higher in HF patients (~33%) compared to the general population (~0.06% to 0.1%) [31]. LBBB is rare in patients younger than 50 years of age and is almost absent in patients younger than 35 years of age, which suggests it is an acquired disorder [29]. Population-based studies find the prevalence of LBBB increases steadily from less than 1% at the age of 50 years to approximately 6% by the age of 80 years [32,33]. Common factors associated with its development include LV hypertrophy (LVH) on the ECG, increased cardiac volume, hypertension, valvular heart disease, CMs, myocarditis and coronary artery disease [33-35]. However, some patient develop LBBB in the absence of any of these risk factors. What is of significant clinical interest is whether the newly diagnosed LBBB in the presence or absence of these risk factors can lead to increased mortality and morbidity? This is because it will affect the approach taken to treat asymptomatic LBBB patients.
Population-based longitudinal studies of LBBB patients reveal an increase or a trend towards increased cardiovascular mortality, sudden cardiac death, coronary artery disease and HF [36,37]. Few studies have revealed a significant effect of LBBB on overall mortality but the findings may be related to the limited number of patients and events in these studies. Patients who developed LBBB at a younger age (< 45 years) absent of any cardiovascular risk factors had favourable prognosis compared to LBBB patients with associated risk factors during or after the fifth decade of life [38,39]. These findings suggest that LBBB can be the consequence of intrinsic abnormalities in the conduction system or extrinsic insult from a variety of cardiovascular diseases. The clinical outcome of LBBB in these two distinct patient population is divergent. In the absence of consensus guidelines on evaluating these patients, a non-invasive evaluation for structural heart disease and ischemia is reasonable especially in LBBB patients with known cardiovascular risk factors.
LBBB and LV dysfunction
The pathologic effect of the LBBB on cardiac mechanical function ranges from minimal effect on some patients to a significant reduction in LV systolic function in other. This variability is attributable partly to the differences in the anatomical location of the bundle block [40]. Experimental studies have established a causal relationship between LBBB and systolic HF, where the induction of LBBB leads to immediate reduction in systolic function [41-43]. The recent history of CRT reflects the increasing understanding of the reversible harm induced by LBBB. In patients with systolic HF, the direct contribution of LBBB to LV dysfunction is substantial demonstrated by reverse remodelling and improvement in LVEF in most patients receiving CRT [44-48]. Two large multi-centre randomized trials – MADIT-CRT [49] and Resynchronization Defibrillation for Ambulatory Heart Failure Trial (RAFT) [50] demonstrated CRT to be a powerful intervention for the reduction of the combined end-point of HF hospitalization or death with beneficial effect limited to HF patients with LBBB. The MADIT-CRT also revealed that LBBB patients had the highest mortality and HF event rate compared with patients with other forms of wide QRS complex in the control arm (patients receiving implantable cardioverter defibrillator only). These patients had marked improvement by CRT, exhibiting the lowest primary endpoint event rate [51]. By 2012, the growing consensus associating CRT benefit with LBBB contributed to the Updated American Heart Association (AHA) and American College of Cardiology Foundation (ACCF) guidelines, in which class I indication was limited to symptomatic patients with LVEF ≤ 35% and LBBB [52]. In effect, the update recognized that in patients with depressed LVEF, conduction abnormality was a correctable pathological process.
Mechanisms of LBBB-induced LV dysfunction
In patients with isolated LBBB who appear to be healthy, LVEF is often normal or mildly reduced. In contrast, patients with HF and LBBB usually have a markedly depressed LVEF [53]. The mechanisms of LBBB-associated LV dysfunction is an altered pattern of ventricular activation and contraction [12,29,40]. Under normal conditions, impulse conduction spreads rapidly down His bundle branches, to the Purkinje system and subsequently synchronous activation of most of the LV endocardial surface. This synchronous pattern of activation results in efficient contraction at the expense of minimal energy consumption.
The presence of LBBB alters this synchronous activation and contraction [12,29]. The septal parts of the ventricle are activated much earlier than the lateral wall. The early-activated septum contracts when the LV lateral wall is fully relaxed. Instead of contributing to LV ejection, septal contraction displaces blood towards the lateral wall, which is stretched due to abnormally high preload. The activation of the lateral wall leads to its vigorous contractions according to the Starling mechanism, which displaces blood backwards to the septum that is stretched and displaced towards the RV. The stretched and displaced septum absorbs energy from the work performed by the LV lateral wall, which is wasted work in the septum [54]. The contraction of the LV free wall causes ejection of blood into the aorta. However, since the septum represents about a third of the LV mass, the loss of a large portion of the septal contribution to LV function adds substantial workload on the LV lateral wall (energy-inefficient contraction), which is a major stimulus to adverse remodelling in HF patients with LBBB [40]. Thus, during LBBB there is often hypertrophy of the LV lateral wall and thinning of the septum, which are reversible by CRT [55].
In addition, animal models reveal evidence of protein dysregulation concentrated on the LV lateral wall in animals after the induction of Dyssynchronous HF [56]. The discordant mechanical stretch alters cellular Ca++ transport resulting in a pro-arrhythmic state [57]. All these factors may contribute to the increased mortality in patients with LBBB and markedly depressed LV function [29,40].
Meta-analysis of diagnosis/management
The high prevalence of LBBB in patients with CM with depressed LVEF and its associated poor prognosis presents diagnostic and therapeutic challenges. The challenge is whether LBBB is a consequence or a cause of CM. However, increased benefits of HF patients with LBBB after treatment with CRT especially the reversal of LV dysfunction strongly suggests the pathophysiologic role of LBBB in the development of CM. However, there is no consensus on guidelines for the diagnosis of LBBB-induced CM. More importantly, patients with complete LBBB and markedly depressed LVEF (≤ 35%) do not respond to the current guideline recommended HF medical therapy [4,5]. These patients derive superior benefits from CRT compared to HF patients without LBBB. These findings adds to the growing evidence that, in many cases of non-ischemic CM with LBBB, this conduction abnormality is not a comorbid condition but rather the primary aetiology of CM. With a growing evidence suggesting LBBB is a reversible cause of CM with LV dilatation, may be it is time to recognize LBBB or dyssynchrony-induced CM as a separate clinical entity from DCM, which requires immediate specific treatment with CRT. Thus, the present meta-analysis aggregates current published evidence on LBBB-CM. The aim is to broaden the understanding of its epidemiology, diagnosis and treatment of LBBB-induced CM.
Electronic search for studies was performed on PubMed and Google Scholar. The key words used for the search were left bundle branch block (LBBB) cardiomyopathy, dyssynchrony cardiomyopathy, non-ischemic cardiomyopathy with LBBB, heart failure and LBBB, and cardiac resynchronization. Additional search was performed on reference lists of articles that met the inclusion criteria to identify relevant articles missed by the electronic search. There was no language restriction. The inclusion criteria were as follows. The study enrolled patients with non-ischemic cardiomyopathy with LBBB; received either CRT or optimal medical treatment; reported findings of the diagnosis or therapy. The clinical outcomes of interest were LV systolic function, LV mechanics and QRS duration. Only published data was used for analysis. Categorical data was expressed as frequency and percentage, continuous data as mean and standard deviation and dichotomous as mean differences or odds ratio and 95% confidence interval (CI). The inconsistency parameter (I2) was used to estimate the amount of heterogeneity across studies. Statistical significance was defined by P values < 0.05 and heterogeneity as P values < 0.10 or I2 > 25%). When heterogeneity was present, an explanation was sought in terms of methodological quality and different clinical characteristics. When no clinically significant differences between studies would be identified, the results of studies were pooled together using the random effect model (Table 1).
Table 1. Summary of characteristics of the included studies
Author [Ref#] |
Year |
Study Design |
Patient No. |
Male (%) |
Mean Age (SD) |
Inclusion Criteria |
Outcome Measures |
Key Findings |
Blanc [58] |
2005 |
Prospective Observational |
29 |
66 |
70 (7.7) |
LBBB; LVEF ≥ 45% |
Normalization of LVEF (> 50%) at 1 year |
Normalization of LV function at 1 year after CRT occurred in 17% of patients with LV pacing suggesting long-standing LBBB may be a reversible cause of CM |
Zareba [22] |
2011 |
Retrospective cohort |
1817 |
69 |
64 (11) |
LBBB>5 years; LVEF>50 NYHA II-IV |
∆ in QRS |
HF patients in NYHA I/II, LVEF ≤ 30% and LBBB derive substantial clinical benefits from CRT-D - reduced HF progression and reduced risk of VT. |
Vaillant [59] |
2013 |
Retrospective Cohort |
6 |
50 |
50.5 |
LBBB – QRS < 120 ms; LVEF ≤ 35% |
∆ in LVEF |
Support the existence of a specific LBBB-induced CM resolved by CRT. Its prevalence, time course and risk factors need to be prospectively studied |
Wang [6] |
2016 |
Retrospective cohort |
102 |
NR |
NR |
LBBB LVEF ≥ 45% without CAD |
∆ in LVEF |
GMDT does not confer significant improvement in LVEF in new onset LBBB-associated NICM at 3 months but a 35% are super-responders for CRT. Optimal timing for CRT requires further investigation |
Barot [60] |
2017 |
Retrospective Cohort |
264 |
NR |
73 (12) |
LBBB; LVEF ≤ 35% |
∆ in LVEF |
LBBB is associated with progressive LV dysfunction and DCM in the absence of CAD or other identifiable aetiology. The incidence of LBBB may be higher than reported - occurs even in patients with normal LVEF. |
Sze [5] |
2018 |
Retrospective cohort |
600 |
45 |
65 |
LBBB; LVEF ≤ 35% |
∆ in LVEF |
GMDT causes a smaller degree of LVEF improvement in LBBB patients compared with other QRS morphologies. Some LBBB patients may benefit from early CRT |
Wang [7] |
2018 |
Retrospective cohort |
123 |
NR |
NR |
IDCM with LBBB |
∆ in LVEF |
In LBBB-associated idiopathic NICM, earlier CRT is associated with more favourable cardiac remodelling. Delayed CRT may miss a critical period to halt and reverse progressive myocardial injury |
Emara [61] |
2019 |
Prospective Cohort |
101 |
19 |
42 (18) |
DCM with LBBB |
LV mechanics: LV strain/rate/twist |
LBBB is associated with more deterioration of LV mechanics and exaggerated LV dyssynchrony in IDCM patients |
Gentile [62] |
2019 |
Retrospective Cohort |
137 |
NR |
NR |
LBBB; LVEF ≥ 45% |
All-cause mortality, Htx and SCD |
LBBB does not affect mortality in IDCM patients intermediate after LVEF after OMT. Those with significant MR, LA and RV remodeling carry a higher risk of LVEF deterioration |
CAD: Coronary Artery Disease; CRT: Cardiac Resynchronization Therapy; HTx: Heart Transplantation; LBBB: Left Bundle Branch Block; IDCM: Idiopathic Dilated Cardiomyopathy; LA: Left Atrial; LVEF: Left Ventricular Ejection Fraction; NIYHA: New York Heart Heat association; Non-Ischemic Cardiomyopathy; NR: Not Reported
Study characteristics
Out of the 157 potential references yielded by the electronic search, only nine articles met the inclusion criteria and formed the final dataset for analysis [5-7,22,58-62]. Table 1 provides a summary of the characteristics of the included studies, which were recent, published between 2001 and 2019. A majority of the excluded studies were case reports of one to three patients or included patients with mixed HF conditions making data extraction of only LBBB patients unfeasible. In total, the nine studies enrolled 3,179 patients. The mean age of the patients was 60.75 years (range 42 to 73 years) with an equal gender representation (male = 49.8%; female 50.2%). Two studies [58,61] adopted a prospective cohort design while the remaining seven studies [5-7,22,59,60,62] adopted a retrospective cohort design. Seven studies (N = 2,909) compared the effect of optimal medical therapy or CRT between LBBB (n = 1,591) and non-LBBB (n = 1,318) patients. Only two studies [59,61] evaluated changes in LV mechanics (strain and strain rate) between LBBB and non-LBB patients with preserved or depressed LVEF.
Study outcomes
The prevalence of LBBB could not be determined because of a wide heterogeneity in the population studied, which ranged from HF patients, idiopathic DCM, non-ischemic CM and patients with a long-standing LBBB. Thus, there was no specific patient population to calculate the prevalence of LBBB. However, in the MADIT-CRT trial [22], 1,281 out of 1,817 patients with available sinus rhythm on ECG, 70% had LBBB. Sze et al. [5] studied a cohort of 659 patients with depressed LVEF (≤ 35%) selected from the Duke Echocardiography and found 17% had LBBB. Available data on LBBB-induced CM is too little to perform a meta-analysis that can provide valid findings. In addition, the included studies evaluated different functional and mechanical LV parameters further complicating a meta-analysis. However, the little data available provide useful insights into the role of LBBB in the development of LV dysfunction and subsequently CM.
Pooled results from three studies [5-7] show that three months of GMDT does not confer significant improvement in LVEF in patients diagnosed with CM and LBBB relative to those with CM and normal atrioventricular conduction (non-LBBB patients). Patients with CM and without LBBB had significantly higher standardized mean difference (SMD) in LVEF increase compared to CM patients with LBBB (SMD: -0.494%; 95% CI: -0.672 to -0.316; p = 0.000: Figure 1). In two studies [59,61], patients with CM and LBBB exhibited significantly impaired LV mechanics compared to non-LBB patients. The weighted mean difference (WMD) in global longitudinal strain (GLS) was significantly higher in non-LBB patients (WMD: 3.84%; 95% CI: 0.042 to 7.643; p = 0.047; Figure 2). Conversely, treatment with CRT showed beneficial outcomes to patients with CM and LBBB. Pooled analysis of two studies [58,59] showed significant improvement in QRS duration pre- and one-year post-CRT treatment in LBBB patients (WMD: 35.61 ms; 95% CI: 27.67 to 43.55; p=0.000; Figure 3) [58,59]. Early intervention with CRT also caused significant improvement in LVEF in patients with CM and LBBB compared to non-LBBB patients (WMD: 31.67%; 95% CI: 21.13 to 42.40; p=0.000: Figure 4) [58,59]. In addition, one study [9] reported 35% of patients with CM and a new-onset LBBB as super-responders for CRT. Finally, CRT treatment conveyed a protective effect for patients with CM and LBBB against cardiovascular death (odds ratio [OR]; 0.72; 95% CI: 0.48 to 1.08; p = 0.113; Figure 5) [7,22] and adverse HF events (OR: 0.57; 95% CI: 0.331 to 0.98; p = 0.042: Figure 6) [7,22].
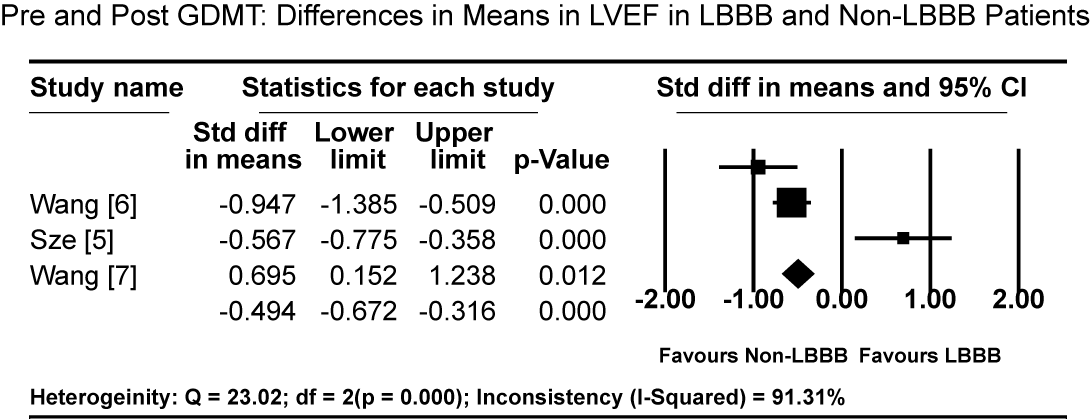
Figure 1. Pre and post GDMT: Differences in means in LVEF in LBBB and Non-LBBB patients
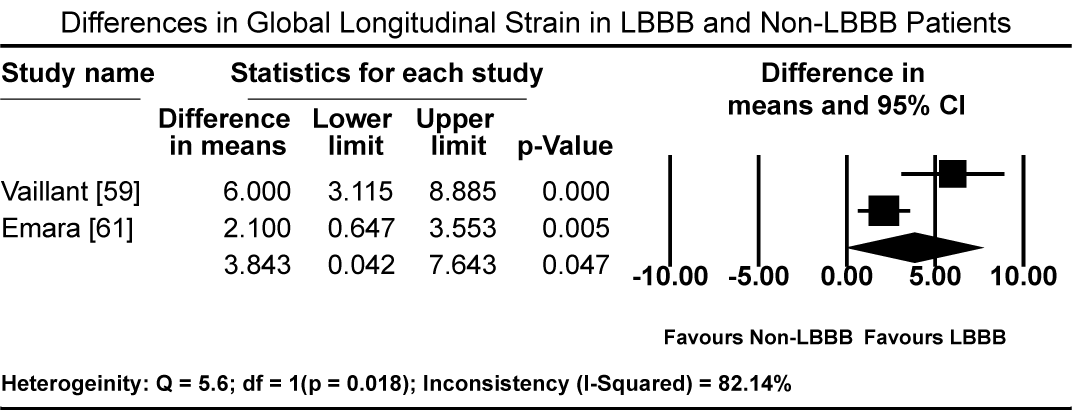
Figure 2. Differences in Mean GLS Pre- and Post-GDMT between LBBB and non-LBBB patients
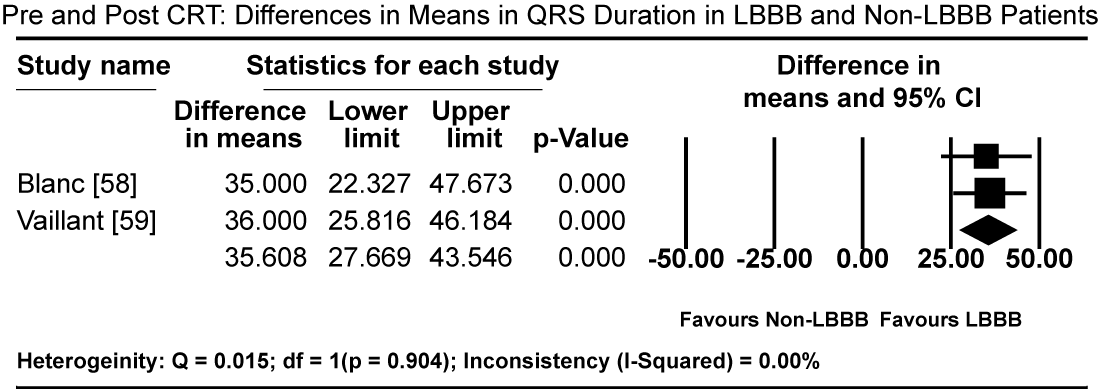
Figure 3. Differences in QRS Pre- and Post-CRT between LBBB and non-LBBB patients
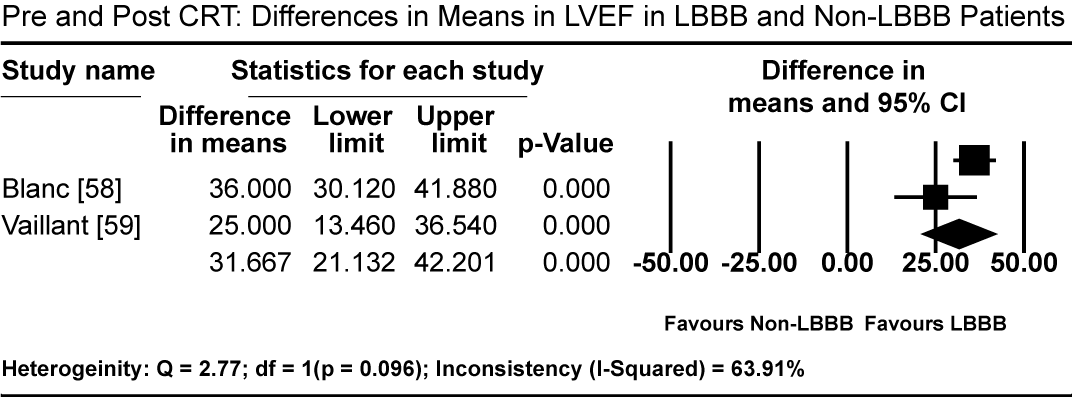
Figure 4. Differences in Mean LVEF Pre- and Post-CRT between LBBB and non-LBBB patients
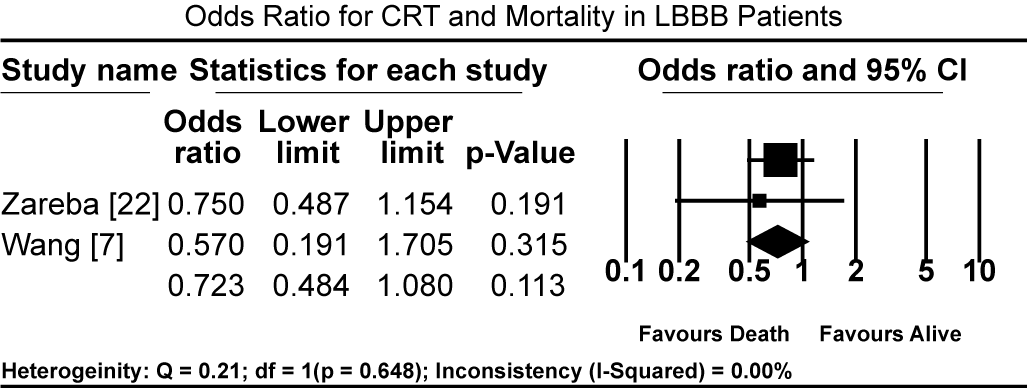
Figure 5. Odds Ratio for CRT and Mortality in LBBB Patients
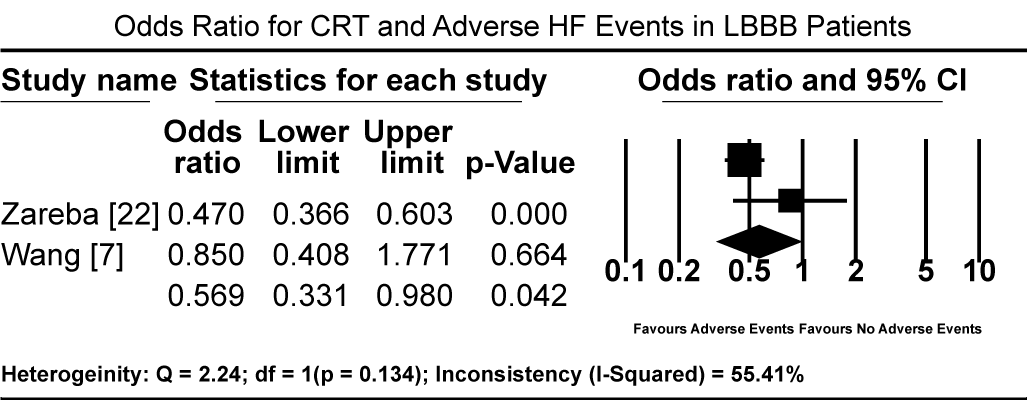
Figure 6. Odds Ratio for CRT and Adverse HF Events in LBBB Patients
The traditional assumption was LBBB occurred secondary to the underlying disease process of DCM. However, recent evidence suggest the possibility that, in some patients, long-standing LBBB may induce CM in the absence of any known cause [58]. This meta-analysis sought to examine evidence on LBBB-CM. The findings suggest that LBBB may be a reversible cause of CM in some patients. While GDMT for three months has been effective in DCM and HF patients, in a subset of these patients with LBBB, the benefits were not straightforward with some patients exhibiting worsening symptoms. However, early initiation of CRT is associated with significant improvement in LV function in CM patients with LBBB compared with non-LBBB patients. Furthermore, CRT in patients with CM and LBBB provided a protective effect against adverse cardiovascular events and cardiovascular mortality.
Diagnosis
Cardiomyopathy with LBBB has not been formally recognized as a distinct cardiac clinical entity. Instead, it is more often discussed under the DCM phenotype. Consequently, there are no consensus guidelines for the diagnostic and treatment of LBBB-induced CM. Similarly, in the present meta-analysis, there was no sufficient data to perform a meta-analysis on methods or features used to diagnose LBBB-CM patients. Instead, the review relied on the inclusion and exclusion criteria used by the individual studies to enroll patients. Inclusion criteria varied slightly among individual studies. DCM patients with LBBB, severe HF (NYHA III-IV) [58]; ischemic CM with NYHA I-II, LVEF ≤ 30% and QRS ≥ 130 ms [22]; new onset NICM, LVEF ≤ 35% and LBBB or narrow (< 120 ms) QRS [6,7]; LVEF ≥ 45% without CAD [60]; LVEF ≤ 35%, LBBB and wide QRS ≥ 120 ms (no LBBB) [5]; and DCM with LVEG > 35% [62]. Despite these variations, Vaillant et al. [59] provided a more comprehensive inclusion criterion that included almost all the diagnostic parameters in the individual studies. Thus, from the individual studies, the emerging shared criterion for the diagnosis of LBBB-CM includes the following six-point criteria.
- Normal sinus rhythm and > a 5-year history of typical LBBB;
- Preserved LVEF (>50%) at the time of diagnosis;
- A progressive decline in LVEF to ≤ 40%;
- The presence of major left heart mechanical dyssynchrony;
- No other known identifiable course of CM; and
- A super-response to CRT defined as LVEF ≥ 45% and a decrease in NYHA functional class within ≥ 12 months.
These diagnostic criteria formed the basis of patient selection in the individual included studies. Usually, the best clinical approach to assess and identify potentially reversible CMs is after the patient has received adequate treatment. Current potentially reversible forms of CMs include ischemic CM amenable to revascularization and tachycardia-induced CM amenable to restoration of sinus rhythm or control of ventricular rate [1]. Thus, in LBBB-CM, the most important or pathognomonic diagnostic parameter is super-response to CRT. Some studies defined LBBB-CM as DCM with LBBB yet the two diseases have diverging optimal treatment approaches. However, these LBBB-CM diagnostic criteria suggest a delayed diagnosis since it requires a prolonged history of LBBB, a progressive decline in LVEF over time and a marked positive response to CRT. Thus, there is need for additional prospective studies to recognize LBBB-CM as a separate cardiac clinical entity from DCM and to confirm and refine the current diagnostic criteria.
Treatment
Guidelines by both AHA and ESC on clinical interventions for patients with CM target to improve LV systolic function. The mainstay strategies include medical modulation of the renin-angiotensin-aldosterone axis or direct intervention on a reversible cardiac pathology such as CAD, valvular heart disease or tachycardia-induced CM or ischemic CM [1,63,64]. More recently, CRT has introduced the correction of electromechanical dyssynchrony as a powerful mechanism to induce LV remodelling to normalize LV systolic function [49,50,65]. The present meta-analysis also find divergent clinical management approach between CM patients with and without LBBB. Although current consensus recommend GDMT for at least three months before the consideration for CRT, current evidence suggest that it would delay the efficacy of treatment on LBBB-CM patients. Current findings associate GMDT administered for LBBB-CM patients for three months with non-benficial outcomes or even worsening of cardiac symptomatology while non-LBBB patients had significantly better LVEF improvement. Moreover, LBBB-CM patients had significantly impaired LV mechanisms evidenced with significantly lower GLS compared to non-LBBB patients.
Conversely, early initiation of CRT was associated with better clinical outcomes for LBBB-CM patients compared to non-LBBB patients. Treatment by CRT leads to normalization of QRS duration and significant improvement, even normalization of LV systolic function. CRT also led to a significant reduction in mortality and adverse HF events in LBBB patients. These beneficial outcomes were absent in CM patients without LBBB. The present findings support the current suggestions on the involvement of LBBB in the pathophysiology of CM. The current proposed role of LBBB in the pathogenesis of CM is mechanical dyssynchrony on ventricular contraction. The characteristic clinical feature of LBBB-CM is asynchronous septal wall motion, and most frequently, delayed lateral and/or posterior wall segments. This dyssynchrony in ventricular activation leads to inefficient contraction. In addition, the subsequent redistribution of the local ventricular workload provokes substantial changes in regional myocardial circulation and glucose metabolism along with structural remodelling [29,40,54,66]. Collectively, the current evidence consistent with cardiology literature, implicate LBBB as a cause of a specific type of CM reversible with correction of LBBB using CRT therapy.
Implications
The present findings have important clinical implications. They suggest that patients with CM, and LVEF ≤ 35% and LBBB demonstrate significantly less LV functional recovery compared to patients with normal atrioventricular conduction (or normal QRS duration). In some patients with LBBB-CM, the likelihood of a large improvement in LV systolic function (LVEF) is modest, even when the patients is under GDMT or revascularization. These findings suggests the need for more flexibility in the current treatment guidelines that recommend three months GDMT. The flexibility is important to address the unique presentation and prognostic of LBBB-CM patients. Most LBBB-CM patients are super-responders to CRT although myocardial pathology that is too extensive may not respond well to CRT. On the other hand, delaying CRT may miss a critical period to stop and reverse progressive myocardial damage. However, the optimal timing of CRT that lead to the greatest benefits for LBBB-CM patients remains unknown, requiring further prospective clinical trials to clarify the best time to initiated CRT. Indeed, LBBB-CM patient section for early CRT may require more precise estimations of the causal contributions of LBBB to the LV dysfunction in different clinical scenarios. Identifying potential CRT non-responders despite meeting current guideline criteria is also important to avoid violating the principle of primum non nocere (loosely translated to mean not acting is better than rash, uncertain action).
Left bundle branch block (LBBB) is a common ECG finding in patients with structural heart diseases and DCM. LBBB is often associated with significant heart disease resulting from myocardial strain, injury or hypertrophy although it can also occur in patients without any particular clinical disease. In isolation, LBBB does not pose any specific clinical concern and does not affect prognosis. LBBB is usually the consequence of DCM but in some cases it is the cause of CM. LBBB induces the development of CM through altering the pattern of ventricular activation (septum activates earlier than the lateral wall). The altered pattern leads to a dyssynchronous ventricular contraction, which increases pressure to the LV lateral wall leading to LV hypertrophy, dilatation and dysfunction. The criterion for diagnosis of LBBB-CM is a combination of a normal sinus rhythm, a history of LBBB, declining LVEF, LV mechanical dyssynchrony in the absence of any other known cause and a remarkable response to CRT. Although current guidelines for HF recommend GDMT for at least three months, CM patients with LBBB are non-responsive, and in some cases, may exhibit worsening cardiac symptoms. Treatment with CRT is the most efficacious associated with LV remodelling, reduced cardiac symptomatology, adverse cardiovascular events and cardiovascular mortality. The remarkable response to CRT is the key finding identifying LBBB as a reversible cause of CM. However, the optimal time to begin CRT or to identify non-responders who have met the clinical criteria for LBBB-CM remains unclear and warrants further studies for clarification.
- Bozkurt B, Colvin M, Cook J, Cooper LT, Deswal A, et al. (2016) Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation 134: e579-e646. [Crossref]
- Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, et al. (2006) Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation 113: 1807-1816. [Crossref]
- Arbustini E, Narula N, Tavazzi L, Serio A, Grasso M, et al. (2014) The MOGE (S) classification of cardiomyopathy for clinicians. J Am Coll Cardiol 64: 304-318. [Crossref]
- Wang NC, Adelstein EC, Singh M, Voigt AH, Saba S (2018) Left Bundle Branch Block–Associated Cardiomyopathies and Early Cardiac Resynchronization Therapy: Conceptualizing a Tailored Approach. J Am Coll Cardiol 71: 1943-1944. [Crossref]
- Sze E, Samad Z, Dunning A, Campbell KB, Loring Z, et al. (2018) Impaired recovery of left ventricular function in patients with cardiomyopathy and left bundle branch block. J Am Coll Cardiol 71: 306-317. [Crossref]
- Wang NC, Singh M, Adelstein EC, Jain SK, Mendenhall GS, et al. (2016) New-onset left bundle branch block–associated idiopathic nonischemic cardiomyopathy and left ventricular ejection fraction response to guideline-directed therapies: The NEOLITH study. Heart Rhythm 13: 933-942. [Crossref]
- Wang NC, Li JZ, Adelstein EC, Althouse AD, Sharbaugh MS, et al. (2018) New‐onset left bundle branch block‐associated idiopathic nonischemic cardiomyopathy and time from diagnosis to cardiac resynchronization therapy: The NEOLITH II study. Pacing Clin Electrophysiol 41: 143-154. [Crossref]
- Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, et al. (2013) 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 15: 1070-1118. [Crossref]
- Stevenson WG, Hernandez AF, Carson PE, Fang JC, Katz SD, et al. (2012) Indications for cardiac resynchronization therapy: 2011 update from the Heart Failure Society of America Guideline Committee. J Card Fail 18: 94-106. [Crossref]
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, et al. (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 18: 891-975. [Crossref]
- Haft JI, Herman MV, Gorlin R (1971) Left bundle branch block: etiologic, hemodynamic, and ventriculographic considerations. Circulation 43: 279-287. [Crossref]
- Breithardt G, Breithardt OA (2012) Left bundle branch block, an old–new entity. Journal of cardiovascular translational research 5: 107-116. [Crossref]
- Bakker PF (1994) Beneficial effects of biventricular pacing in congestive heart failure. Pacing Clin Electrophysiol 17: 820.
- Cazeau S, Ritter P, Bakdach S, Lazarus A, Limousin M, et al. (1994) Four chamber pacing in dilated cardiomyopathy. Pacing Clin Electrophysiol 17: 1974-1979. [Crossref]
- Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, et al. (2004) Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Eng J Med 350: 2140-2150. [Crossref]
- Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, et al. (2005) The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Eng J Med 352: 1539-1549. [Crossref]
- Abbasi AS, Eber LM, Macalpin RN, Kattus AA (1974) Paradoxical motion of interventricular septum in left bundle branch block. Circulation 49: 423-427. [Crossref]
- Curtius JM, Knueppel S, Meschig R, Balkenhoff K, Arnold G, et al. (1986) Course of left-ventricular contraction in left bundle-branch block and its hemodynamic effects. Z Kardiol 75: 138-146. [Crossref]
- Curtius JM, Nowitzki G, Kohler E, Kuhn H, Loogen F (1983) Left bundle-branch block: inferences from ventricular septal motion in the echocardiogram concerning left ventricular function. Z Kardiol 72: 635-641. [Crossref]
- McDonald IG (1973) Echocardiographic demonstration of abnormal motion of the interventricular septum in left bundle branch block. Circulation 48: 272-280. [Crossref]
- Fujii JU, Wantanabe H, Watanabe T, Takahashi NO, Ohta AK, et al. (1979) M-mode and cross-sectional echocardiographic study of the left ventricular wall motions in complete left bundle-branch block. Br Heart J 42: 255. [Crossref]
- Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt S, et al. (2011) Effectiveness of cardiac resynchronization therapy by qrs morphology in the Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 123: 1061-1072. [Crossref]
- Bilchick KC, Kamath S, DiMarco JP, Stukenborg GJ (2010) Bundle-branch block morphology and other predictors of outcome after cardiac resynchronization therapy in Medicare patients. Circulation 122: 2022-2030. [Crossref]
- Freedman HH (1971) Ventricular conduction defects. Diagnostic electrocardiography and vectorcardiography 163-92.
- Willems JL, de Medina EO, Bernard R, Coumel P, Fisch C, et al. (1985) Criteria for intraventricular conduction disturbances and pre-excitation. J Am Coll Cardiol 5: 1261-1275. [Crossref]
- Surawicz B, Childers R, Deal BJ, Gettes LS (2009) AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances. J Am Coll Cardiol 53: 976-981. [Crossref]
- Auricchio A, Fantoni C, Regoli F, Carbucicchio C, Goette A, et al. (2004) Characterization of left ventricular activation in patients with heart failure and left bundle-branch block. Circulation 109: 1133-1139. [Crossref]
- Strauss DG, Selvester RH, Wagner GS (2011) Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol 107: 927-934. [Crossref]
- Kumar V, Venkataraman R, Aljaroudi W, Osorio J, Heo J, et al. (2013) Implications of left bundle branch block in patient treatment. Am J Cardiol 111: 291-300. [Crossref]
- Massing GK, James TN (1976) Anatomical configuration of the His bundle and bundle branches in the human heart. Circulation 53: 609-621. [Crossref]
- Nikoo MH, Aslani A, Jorat MV (2013) LBBB: State-of-the-Art Criteria. Int Cardiovasc Res J 7: 39-40. [Crossref]
- Eriksson P, Hansson PO, Eriksson H, Dellborg M (1998) Bundle-branch block in a general male population: the study of men born 1913. Circulation 98: 2494-2500. [Crossref]
- Imanishi R, Seto S, Ichimaru S, Nakashima E, Yano K, Akahoshi M (2006) Prognostic significance of incident complete left bundle branch block observed over a 40-year period. Am J Cardiol 98: 644-648. [Crossref]
- Hiss RG, Lamb LE (1962) Electrocardiographic findings in 122,043 individuals. Circulation 25: 947-961. [Crossref]
- Ostrander LD Jr. (1964) Bundle-branch block: an epidemiologic study. Circulation 30: 872-881. [Crossref]
- Schneider JF, Thomas HE Jr, Kreger BE, McNamara PM, Kannel WB (1979) Newly acquired left bundle-branch block: the Framingham study. Ann Intern Med 90: 303-310. [Crossref]
- Fahy GJ, Pinski SL, Miller DP, McCabe N, Pye C, et al. (1996) Natural history of isolated bundle branch block. Am J Cardiol 77: 1185-1190. [Crossref]
- Rotman M, Triebwasser JH (1975) A clinical and follow-up study of right and left bundle branch block. Circulation 51: 477-484. [Crossref]
- Rabkin SW, Mathewson FA, Tate RB (1980) Natural history of left bundle branch block. Br Heart J 43: 164-169. [Crossref]
- Smiseth OA, Aalen JM (2018) Mechanism of harm from left bundle branch block. Trends Cardiovasc Med 29: 335-334. [Crossref]
- Vernooy K, Verbeek XA, Peschar M, Crijns HJ, Arts T, et al. (2005) Left bundle branch block induces ventricular remodelling and functional septal hypoperfusion. Eur Heart J 26: 91-98. [Crossref]
- Verbeek XA, Vernooy K, Peschar M, Van Der Nagel T, et al. (2002) Quantification of interventricular asynchrony during LBBB and ventricular pacing. Am J Physiol-Heart C 283: H1370-H1378. [Crossref]
- Liu L, Tockman B, Girouard S, Pastore J, Walcott G, et al. (2002) Left ventricular resynchronization therapy in a canine model of left bundle branch block. Am J Physiol-Heart C 282: H2238-H2H44. [Crossref]
- Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, et al. (2005) The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 352: 1539-1549. [Crossref]
- Vaillant C, Martins RP, Donal E, Leclercq C, Thebault C, et al. (2013) Resolution of left bundle branch block-induced cardiomyopathy by cardiac resynchronization therapy. J Am Coll Cardiol 61: 1089-1095. [Crossref]
- Sze E, Daubert JP (2016) Left Bundle Branch Block: Is it" Unsafe at Any Speed"? JACC Heart Fail 4: 904-906. [Crossref]
- Sze E, Daubert JP (2018) Left bundle branch block-induced left ventricular remodeling and its potential for reverse remodelling. J Interv Card Electrophysiol 52: 343-352. [Crossref]
- Gill EA, Poole JE (2015) Will the Real Left Bundle Branch Block Please Stand Up? J Am Coll Cardiol 66: 642-644. [Crossref]
- Moss AJ, Hall WJ, Cannom DS (2009) Cardiac resynchronization therapy for the prevention of heart-failure events. N Engl J Med 361: 1329-1338. [Crossref]
- Tang AS, Wells GA, Talajic M (2010) Cardiac resynchronization therapy for mild-to-moderate heart failure. N Engl J Med 363: 2385-2395. [Crossref]
- Zareba W, Klein H, Cygankiewicz I (2011) Effectiveness of cardiac resynchronization therapy by QRS morphology in the Multicentre Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 123: 1061-1072. [Crossref]
- Tracy CM, Epstein AE, Darbar D (2012) 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 60: 1297-1313. [Crossref]
- Linde C, Braunschweig F (2018) Cardiomyopathy and Left Bundle Branch Block: A Farewell to Drugs? J Am Coll Cardiol 71: 318. [Crossref]
- Vecera J, Penicka M, Eriksen M, Russell K, Bartunek J, et al. (2016) Wasted septal work in left ventricular dyssynchrony: a novel principle to predict response to cardiac resynchronization therapy. Eur Heart J Cardiovasc Imag 17: 624-632. [Crossref]
- Cvijic M, Duchenne J, Unlu S, Michalski B, Aarones M, et al. (2018) Timing of myocardial shortening determines left ventricular regional myocardial work and regional remodelling in hearts with conduction delays. Eur Heart J Cardiovasc Imag 19: 941-949. [Crossref]
- Spragg DD, Leclercq C, Loghmani M, Faris OP, Tunin RS, et al. (2003) Regional alterations in protein expression in the dyssynchronous failing heart. Circulation 108: 929-932. [Crossref]
- Ter Keurs HE, Zhang YM, Davidoff AW, Boyden PA, Wakayama Y, et al. (2001) Damage induced arrhythmias: mechanisms and implications. Can J Physiol Pharmacol 79: 73-81. [Crossref]
- Blanc JJ, Fatemi M, Bertault V, Baraket F, Etienne Y (2005) Evaluation of left bundle branch block as a reversible cause of non-ischaemic dilated cardiomyopathy with severe heart failure. A new concept of left ventricular dyssynchrony-induced cardiomyopathy. Europace 7: 604-610. [Crossref]
- Vaillant C, Martins RP, Donal E, Leclercq C, Thébault C, et al. (2013) Resolution of left bundle branch block–induced cardiomyopathy by cardiac resynchronization therapy. J Am Coll Cardiol 61: 1089-1095. [Crossref]
- Barot HV, Sharma S, Schwartzman A, Patten R (2017) Abstract 19033: Incidence of Left Bundle Branch Block: Associated Cardiomyopathy. J Cardiac Fail 23: S55
- Emara A, Badran HM, Abdou W, Fahim N, Fathi M, et al. (2019) Impact of Left Bundle Branch Block on Left Ventricular Mechanics in Patients with Idiopathic Dilated Cardiomyopathy. World J Cardiovasc Dis 9: 132-14
- Gentile P, Paldino A, Cannatà A, Artico J, Barbati G, et al. (2019) Left bundle branch block in dilated cardiomyopathy with intermediate left ventricular dysfunction: Clinical phenotyping and outcome correlates. Int J Cardiol 278: 180-185. [Crossref]
- Yancy CW, Jessup M, Bozkurt B (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62: e147–239. [Crossref]
- Nishimura RA, Otto CM, Bonow RO (2014) 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 148: e1-132. [Crossref]
- Cleland JG, Abraham WT, Linde C, Gold MR, Young JB, et al. (2013) An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J 34: 3547-3556. [Crossref]
- Akhtar MM, Elliott P (2019) Impact of left bundle branch block (LBBB) in dilated cardiomyopathy (DCM) with intermediate left ventricular systolic dysfunction (LVSD). Int J Cardiol 278: 199. [Crossref]






