Abstract
Background: Boundary layered lubrication of phospholipid bilayers character was explained based on a lamellar slippage model membrane. Our results reveal that the lamellar slippage model membrane shown less wear, and low friction creating an electrostatic repulsion between the bilayers.
Aim: To assess a decreased number of bilayers on the surface which eventually leads to abnormal cartilage wear and joint degeneration.
Methods: The authors examine surfaces topographical images and wettability of the bovine articular cartilage normal and depleted.
Results: The authors conclude that the lamellar slippage of phospholipid bilayers facilitates an almost frictionless lubrication in the joint.
Conclusion: This study showed that tissue surface changes its structure, which eventually leads to abnormal cartilage wear and joint degeneration. The amount of the structured synovial fluid is not enough to resist load and the friction rises rapidly. This behavior corresponds to the ruined joint system.
Key words
articular cartilage, lamellar slippage model, boundary layered lubrication, atomic force microscopy, wettability
Introduction
Cartilage has excellent biotribological properties with low friction and no wear/or very minimum wear in diarthrodial joints [1]. According to Linn and Sokoloff [2] ‘the secret of the low friction between the cartilage-bearing surfaces is that they never touch’. We can add ‘the secret of the supper low wear between the cartilage-bearing surfaces is lamellar mechanism of lubrication’. A morphological evidence demonstrates that phospholipid bilayers as the outermost lubricating lining of the articular surface [3]. Phospholipids in the biological material is highly self-organized biomolecules in aqueous media and their structure let them form spontaneously vesicles, lamellar phases, multibilayers, and membranes (Figure 1). The multilamellar structure of phospholipids, namely the surface amorphous layer (SAL), covers the natural surface of articular cartilage. It is concluded that a very high porosity (75 to 80 %) is a critical factor in providing excellent tribological properties of articular cartilage, by analogy to porous metal bearings (15 to 30%) [3].
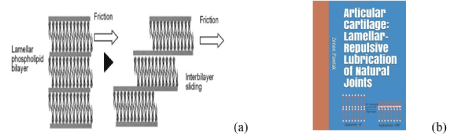
Figure 1. (a) Biological boundary layered lubrication of phospholipid bilayers and (b) Book cover “Articular cartilage: Lamellar-repulsive lubrication of natural joints” [1].
In contacting cartilage surfaces, the two multi-bilayers are against each other as opposing hydrophilic negatively charged surfaces with the electric double-layers resulting in repulsive electrostatic forces, which in the presence of pressurized water and macromolecules, e.g. glycoprotein named lubricin is capable of lubricating with low friction forces [4].
Methods
Articular cartilage specimens were prepared from the patella of 3-4-year-old bovine animals and stored at -20oC until testing. Using the nanoscale characterization and the SMENA-AFM imaging microscopy, the specimen was submerged in PBS solution ready for the scanning, for details go to [5].
Wettability
The contact angle was measured using a KSV CAM100 computerized tensiometer. A drop of the 0.155M saline solution was deposited on the air-dried cartilage surface. The contact angle test was repeated at least five times.
Results and Discussion
A boundary layered lubrication
Schematic illustration of biological boundary layered lubrication, and with the phospholipid bilayers friction mechanism in articular cartilage is presented in Figure 1. The surface amorphous layer (SAL) covering the articular cartilage as multi-bilayers has hydrophilic surface negatively charged and appears remarkably similar to that of graphite which is known as a ‘lamellated solid’ lubricant (uncharged). A typical interlamellar spacing is about 4.5 nm.
Layers of solid platelets in the tribological pair between surfaces, can align themselves parallel to the direction of relative motion and slide over one another with relative ease, and provide very low friction. There is strong interatomic bonding and packing in each layer and the layers themselves are relatively far apart and the forces that bond them are weak van der Waals ones. To perform effective lubrication some conditions are required for each platelet solid lubricant.
The lubrication mechanism proposed here is one, which liposomes, phospholipid lamellar phases and phospholipid sheets (Figure 2), slide over each other with minimal friction. In engineering lubrication graphite, h-BN and molybdenum disulfide have a similar layered structure performing boundary layered lubrication. The phospholipids have been ignored despite the “oily” nature of the articular surface and the presence of phospholipids in the concentration higher than lubricin [6]. The study upon on replacement the synovial fluid by saline (friction unaffected), and delipidization of cartilage surface (friction increased) [6,7], indicate a lipid lubricant is attached by adsorption to the articular cartilage. When PLs adsorption occurs on cartilage, surfaces are hydrophilic with wettability ~0°. The wettability of the resulting coating depends upon numbers of phospholipid bilayers. When the standard procedure [8-10] is used to measure contact angle (°) on the well rinsed and air-dry articular surfaces, the value of 100° to 104° is recorded. During the drying process, a change in surface energy leads to conformational changes in the surface phospholipid (flip-flop), the surface changes from bilayer (hydrophilic) to monolayer (hydrophobic) [4,11].
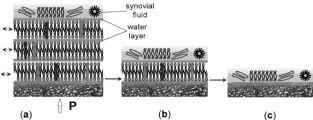
Figure 2. The three stages of the quality of a multibilayer structure over the articular surface of cartilage (AC) in human life: (a) normal healthy surface of AC (wettability ~ 103°); (b) unhealthy surface of AC (wettability 75°); (c) degenerated surface of AC (wettability < 60°). Note the typical interlamellar aqueous spacing of 4.5 nm. The lamellar spheres, liposomes, and macromolecules act like a roller-bearing mechanism between two cartilage surfaces.
Previous studies have demonstrated the presence of lamellated lubricant is imparted to the articular surface by surface-active phospholipids, and some basic aspects include (a) an electron micrograph of the articular cartilage surface of a human knee demonstrating an adsorbed lining similar structure in the lung [12-14], (b) The good correlation between the number of lamellae seen in the electron micrograph and the calculated from phospholipid recovered from solvent rinsing. (c) Thick bilayers can be deposited on various solid surfaces, (d) using phospholipid extracts from bovine joint deposited on flat quartz (3 bilayers), the friction coefficient was found 0.003 to 0.006 and wettability 96.2° [13], (e) the tendency for PLs to form multilaminar structures and bilayers as manifested in all natural membranes.
The atomic force microscope (AFM) was used to characterize the samples nanoscopically under the two surface conditions (normal, and depleted/ osteoarthritic) (Figure 3).
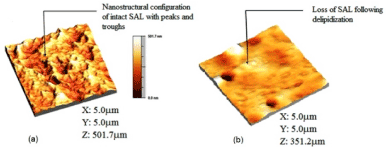
Figure 3. The atomic force microscopy 3D topographical image of (a) normal healthy bovine cartilage after image processing, showing the nanostructure arrangement of the surface amorphous layer with several peaks and troughs, (b) depleted/osteoarthritic cartilage after image processing, showing the loss of the membranous overlay (surface amorphous layer) of the articular surface [(length (X) and breadth (Y) of the scanned area, and average peak height of SAL.
The present results demonstrate that the biological lubrication mechanism, facilitated by a ‘lamellar-repulsive’ process is compatible with the presented data as well as those reported elsewhere [1,15-18]. Three or more bilayers are needed to achieve effective biolubrication at low shear stress resulting in low friction coefficient [1,19,20]. Anisotropy of mechanical properties, or in simple term, planes of weaknesses are characteristic of lamellar lubricants. If these lamellae are able to slide over one another at relatively low shear stress, then the multibilayer becomes a self-lubricating bearing [9,20]. The planes of low resistance allow relative movement between lamellae. The PLs molecule adheres strongly to the worn surface and the lamellar structure deforms at very low-stress levels. When the PLs multibilayer surfaces are brought into contact, there is an electrostatic attraction between the corresponding interfaces [13,20]. Thus, the cartilage surfaces of the phosphate groups are negatively charged, creating electrostatic hydration repulsion between the interfaces and making the slippage frictionless [21,22].
The cartilage interfaces share a hydration repulsive mechanism believed to be responsible for the low friction of the two negatively charged surfaces [1,22]. Electrostatic forces between AC surfaces in SF are consequences of (a) surface charging by (-PO4-) group, (b) and adhesive bio-macromolecules from SF on the cartilage surfaces. The effect of adhesion of the SF constituents onto the articular surface was to further reduce the surface charge density by 25% of its original value of 0.037 Cm-2 of young bovine cartilage [18]. The lubrication mechanism in joints according to the proposed ‘lamellar-repulsive’ scheme is a bimodal process which occurs simultaneously. Firstly, through lamellar activation of the bilayers which occurs when they slide over each other and secondly, through hydration repulsion and adhesion SF macromolecules on the PL membrane [20-22]. Charged lamellar aggregates, liposomes, and macromolecules act electrostatically on negatively charged cartilage surfaces [1,4].
The hydration repulsion of the negatively charged sliding surfaces can sustain a large load. The counter-ions trapped between the two negatively charged surfaces in the gap must be electroneutral [3,4]. The large hydration energy of the counter-ions effectively results in a strong hydration repulsion preventing contact of the cartilage surfaces [21,23,24]. The cartilage surface at pH ~ 7.4, composed of phospholipidic bilayers and compressed phosphate (-PO4-) groups, ensure an excellent hydration repulsion capacity [22]. In some circumstances, the pH of SF can be changed due to cartilage degeneration (osteoarthritis) but the friction coefficient remains unaltered. Serious biological changes in the structure of PL bilayers are noticed by increasing the friction coefficient [13,25].
As illustrated in Figure 2 when the multibilamellar PL lubricant present on sliding surfaces slide over one another with relative ease to provide very low friction, the interlayer share mechanism is believed to be responsible for the low friction of most lamellar solid lubricants. As far as the excellent solid-lubricating capacities of bilayers are concerned, a region of negative electrical charge is contained within the layers. Thus, the surfaces of the phosphate groups are negatively charged, and through creating an electrostatic repulsion between the layers and them make the interlayer slippage much easier. The relatively larger interlayer separation in PL bilayers is thought to result from electrostatic repulsion between the successive atomic layers of these lipid bilayers.
The low-friction behaviour of PLs in the aqueous electrolyte is an intrinsic property of the crystal structure, where hydrophobic weak forces are joined to form a bilayer. A PL multibilayer which displays low-friction behaviour will have most of the lamellar aggregates aligned parallel with the sliding direction. The friction coefficient of bilayers, like other lamellar solids, is largely determined by the ratio of shear strength to the specific load. There is a linear relationship with contact pressure, and thus the coefficient of friction decreases with increasing contact pressure.
The friction behavior of a multibilayer coating such as on cartilage surface usually follows a series of stages shown in Figure 2a. Typically, there is a long period of low, stable friction coefficient, with a little or no apparent wear. Phospholipidic lamellar spheres, lamellae, and macromolecules circulate between the contacting surfaces of AC, and can be very supportive for lubrication. Eventually, there is a breakthrough of the coating, degradation of the surface by biological causes. Typically, the PLs multibilayer undergoes deformation or fractures during the applications of a load. The lamellar aggregates become reoriented so that they are parallel to the sliding direction, and material transfers to the counter face. Enough liposomes and lamellar spheres can still be circulating in the system or stored in holes and troughs to ‘heal’ such ruptures followed by an irreversible low-friction behavior, Figure 2b. Finally, when the PL multibilayer structure is gone, Figure 2c, the protection and separation between the joint surfaces are not in place.
Conclusion
Solid lubricants in natural lubrication are characterized by phospholipid multibilayers in articular joints and phospholipid lamellar phases in synovial fluid. It was experimentally proven that a phospholipid (PLs) bilayer with a lamellar structure can act as an effective solid lubricant in friction and wear under biological test conditions. We present evidence of the outstanding performance of phospholipids and argue that this is due to their chemical inertness and hydrophilic-hydrophobic structure which imparts amphotericity and the ability to form lamellar structures that can facilitate functional sliding.
References
- Pawlak Z (2018) Articular Cartilage: Lamellar-Repulsive Lubrication of Natural Joints, Kindle Direct Publishing, 171pp. Print-book: https://www.amazon.com/dp/B07B42P1JY, e- book: https://www.amazon.com/dp/1976760283.
- Linn FC, Sokoloff L (1965) Movement and composition of interstitial fluid of cartilage. Arthritis and Rheumatism 8: 481-494.
- Hills BA (1988) The Biology of Surfactant, London; Cambridge University Press.
- Israelachvili JN, Wennerstrom H (1996) Role of hydration and water structure in biological and colloidal interactions. Nature 379: 219-225. [Crossref]
- Yusuf KQ, Motta N, Pawlak Z, Oloyade A (2012) A microanalytical study of the surfaces of normal, delipidized and artificially “resurfaced” articular cartilage. Connect Tissue Res 53: 236-245. [Crossref]
- Charnley J (1959) The lubrication of animal joints, London: Proceedings of the Symposium on Biomechanics. Institution of Mechanical Engineers 12–22.
- Little T, Freeman MAR, Swanson SAV (1969) Experiments on friction in the human hip joint, in Wright V (ed.) Lubrication and Wear in Joints: Proceedings of a Symposium organized by the Biological Engineering Society and held at the General Infirmary, Leeds, London, 110-116.
- Chappuis J, Sherman IA, Neumann AW (1983) Surface tension of animal cartilages it relates to friction in joints. Ann Biomed Eng 11: 435-449. [Crossref]
- Pawlak Z, Urbaniak 2021 Copyright OAT. All rights reserv(2013) Relationship between wettability and lubrication characteristics of the surfaces of contacting phospholipids-based membranes. Cell Biochem Biophys 65: 335-345. [Crossref]
- Pawlak Z, Urbaniak W, Hagner-Derengowska M, Hagner W (2015) The probable explanation for the low friction of natural joints. Cell Biochemistry and Biophysics 71: 1615-1621.
- Pawlak Z, Gadomski A, Sojka M, Urbaniak W, Beldowski P (2016) The amphoteric effect on friction between the bovine cartilage/cartilage surfaces under slightly sheared hydration lubrication mode. Colloids Surf B Biointerfaces 146: 452-458. [Crossref]
- Hills BA (2002) Surface-active phospholipid: a Pandora’s box of clinical applications. Part II. Barrier and lubricating properties. Intern Med J 32: 242-251. [Crossref]
- Hills BA (1989) Oligolamellar lubrication of joints by surface active phospholipid. J Rheumatol 16: 82–91.
- Ueda S, Kawamur K, Ishii N, Matsumoto S, Hayashi K, et al. (1985) Ultrastructural studies on surfaces lining layer of the lungs, Part IV. Resected human lung. J Jpn Med Soc Biol Interface 16: 34-60.
- Oloyede A, Gudimetla P, Crawford R, Hills BA (2004a) Consolidation responses of delipidized articular cartilage. Clin Biomech (Bristol, Avon) 19: 534-542. [Crossref]
- Oloyede A, Gudimetla P, Crawford R, Hills BA (2004b) Biomechanical responses of normal and delipidized articular cartilage subjected to varying rates of loading. Connect Tissue Res 45: 86-93. [Crossref]
- Trunfio-Sfarghiu AM, Berthier Y, Meurisse MH and Rieu JP (2008) Role of nanomechanical properties in the tribological performance of phospholipid biomimetic surfaces. Langmuir 24: 8765-8771. [Crossref]
- Trunfio-Sfarghiu AM, Berthier Y, Meurisse M-H, Rieu JP (2007) Multiscale analysis of the tribological role of the molecular assemblies of synovial fluid. Case of a healthy joint and implants. Tribology Int 40: 1500-1515.
- Higaki H, Murakami T, Nakanishi Y, Miura H, Mawatari T, et al. (1998) The lubricating ability of biomembrane models with dipalmitoylphosphatidylcholine and γ globulin. Proc Inst Mech Eng H 212: 337-346. [Crossref]
- Richter RP, Bérat R and Brisson AR (2006) Formation of solid-supported lipid bilayers: An Integrated view. Langmuir 22: 3497-3505. [Crossref]
- Klein J (2013) Hydration lubrication. Friction 1: 1-23.
- Cowley AC, Fuller NL, Rand RP, Parsegian VA (1978) Measurement of repulsive forces between charged phospholipid bilayers. Biochemistry 17: 3163-3168. [Crossref]
- Minassian A, O'Hare D, Parker KH, Urban JP, Warensjo K, et al. (1998) Measurement of the charge properties of articular cartilage by an electrokinetic method. J Orthop Res 16: 720-725. [Crossref]
- Schneck E, Sedlmeier F, Netz RR (2012) Hydration repulsion between biomembranes results from interplay of dehydration and depolarization. Proc Natl Acad Sci U S A 109: 14405-14409. [Crossref]
- Ballantine GC and Stachowiak GW (2002) The effects of lipid depletion on osteoarthritic wear. Wear 253: 385-393.



