Aim: This study was carried out to determine the effects of postnatal administration of nicotine on the pancreas of young Wistar rats.
Methods: A total of thirty female rats were used in the experiment; they were grouped into three-Group A served as the control group and received 0.1 ml normal saline in the first 14 days of postnatal life, Groups B and C served as the treated groups and received 0.05 ml of nicotine on the first 7 and 14 days of postnatal life respectively. The rats were weighed before and during gestation; the pups were also weighed while lactating on days 1, 4, 7, 10 and 14 before sacrifice on day 15. Tissue processing was done using Haematoxylin & Eosin and Periodic-Acid Schiff staining methods.
Results: There was significant decrease in the weight of the pups in treated groups when compared with those of the control group. Histological observation of the pancreas revealed distortion and degenerative changes in the morphology of the treated groups when compared with those of the control, while enzyme analysis of serum lactate dehydrogenase (LDH) and glucose-6-phosphate dehydrogenase (G-6-PDH) levels were elevated in the treated groups.
Conclusion: Exposure to lactational nicotine adversely affects the structure and functions of the pancreas in young Wistar rats.
lactational nicotine, pancreas, morphology, G-6-PDH, LDH
The critical period in development is a phase in the early stages of an organism's life during which it displays a heightened sensitivity to certain environmental stimuli and develops in particular ways due to experiences at this time [1]. Environmental chemicals play important roles in the development of metabolic disorders, especially when exposure occurs early in life [2]. Breastfeeding is recognized as the most appropriate way of providing ideal meal to meet the nutritional needs of the newborn and promoting optimal growth and development [3] Maternal smoking exposes the developing fetus and breastfeeding infant to the untoward effects of nicotine, as they may be exposed both to cigarette smoke as second-hand smokers and to nicotine transferred via breast milk [3]. Nicotine exposure in mammals is an independent risk factor for development of pathological conditions of the pancreas [4]. Studies indicate that the amount of nicotine found in breast milk is 2.9 times greater than that found in maternal blood plasma [5]. The amount of cotinine, the major metabolite of nicotine present in the urine of infants breastfed by smoking mothers, was on average ten times higher than that found in bottle-fed children whose mothers smoke [3]. Urinary cotinine levels in infants breastfed by smoking mothers is similar to those found in adult smokers [6].
With the extent to which mammals are exposed to nicotine, they are at risk of the various effects it poses to the pancreas, blood glucose, glucose metabolism, insulin, lactate dehydrogenase and glucose-6-phosphate dehydrogenase enzymes [7]; since this exposure occurs in utero or after birth during the lactation period. Nicotine plays a significant role in the induction of pancreatic pathophysiology. Hence, the aim of this study was to narrow down these roles and assess the effects of postnatal nicotine on the pancreas of young Wistar rats.
Experimental animals
Thirty female Wistar rats were used for this study. They were housed in wire-gauzed cages in the Animal House of the College of Health Sciences, University of Ilorin, at room temperature. Good ventilation, feeds and water were provided for the rats throughout the period of the experiment. Vaginal smearing was carried out as earlier described [8] and the oestrous phase of each female Wistar rat was determined. The rats were exposed to male rats overnight in their proestrous phase. The presence of mucous plug and sperm cells in the vaginal smears carried out in the early hours of the following morning confirmed mating and possibly pregnancy.
Treatment of experimental animals
The pregnant rats were categorized into three groups - A, B and C. 95% nicotine was obtained from British Drug House (BDH) Chemical Ltd, Poole, England. Following a titration procedure, a maximum dose of 13.76 mg/kg was tolerated by the rats. After littering, the mother rats were treated while lactating with intraperitoneal nicotine (13.76 mg/kg in 0.1 ml vehicle). Group A (control) was treated with 0.1 ml normal saline from postnatal day (PD) 1 to 14, while Group B and C received nicotine from PD1 to PD7 and PD1 to PD14 respectively.
Termination of administration
Treatment of animals was terminated on postnatal day 15, when pups for histological studies were euthanized using 20 mg/kg of ketamine intramuscularly. The pancreas was excised and fixed in 10% formalin. Histological demonstration of pancreatic cytoarchitecture was carried out in paraffin-embedded sections which were stained in Haematoxylin & Eosin and Periodic Acid Schiff (PAS) reaction. The remaining rats for enzymatic studies were sacrificed by cervical dislocation (to eliminate the interference of ketamine-induced changes in biochemical redox). The pancreas was harvested and homogenized in 0.25 M sucrose solution and then centrifuged. The supernatant of the homogenates was then subjected to enzyme-linked immunosorbent assay (ELISA) to quantify the levels of Lactate Dehydrogenase (LDH) and Glucose -6-Phosphate Dehydrogenase (G-6-PDH) in the homogenized pancreas.
Colorimetric assay for enzymatic studies
Enzymatic assay for G-6-PDH and LDH activities were carried out in carefully dissected pancreas of the rats using spectrophotometric techniques. G-6-PDH and LDH kits were procured from Cell Signaling Technologies, Danvers, USA. Equal weighing pancreatic tissues (0.085 g) were homogenized in 0.25 M sucrose. The tissue homogenate was centrifuged for 15 min in a centrifuge at 5000 rpm to obtain the supernatant containing organelle fragments and synaptosomes. The supernatants were aspirated into plain labelled glass cuvette placed in ice. G-6-PDH and LDH activities were assayed according to manufacturer’s instruction in the assay kit pack.
Data analysis
All data were analyzed using the GraphPad Prism® software (Version 6). G-6-PDH and LDH outcomes were plotted in ANOVA with Tukey’s multiple comparisons test. Significance was set at p < 0.05. The outcomes were represented in bar charts with error bars to show the mean and standard error of mean respectively. The body weights of the animals were plotted into bar charts using the Microsoft Excel application.
Morphological observation
The birth weight was lower in the treatment groups compared to the control group, however, a progressive increase in body weights was observed in all the groups through days 1, 4, 7, 10 and 14 (Figure 1). On PD4, the treatment groups had higher weights than the control with the animals in group C that were treated with nicotine for 14 days weighing more than those in group B treated with nicotine for only 7 days; the same was also observed on PD7. By PD10, the control group had gained more weight than the treatment groups and this progressed on to PD14. The weight of the animals in group B was slightly higher than those in group C on PD14.
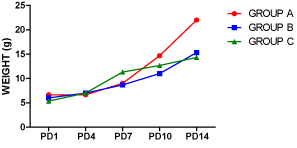
Figure 1. Average weight of pups on postnatal days (PD) 1, 4, 7, 10 and 14. ‘A’ was treated with normal saline, while B and C were exposed to lactational nicotine for the first 7 and 14 postnatal days respectively. The chart showed variations in body weights in relation to postnatal age.
Biochemical analysis
The activity of lactate dehydrogenase and glucose-6-phosphatate dehydrogenase were assayed using the homogenate of the pancreas of the pups on postnatal day 15. The result in figure 2 showed that there was a significant increase in the level of LDH in the pancreas of the treated groups when compared to the control group (p < 0.05), with the group that received nicotine for longer duration (14 days) having a higher level of activity than rats administered nicotine only for 7 days. When the two treated groups were compared, there was no significant difference between them.
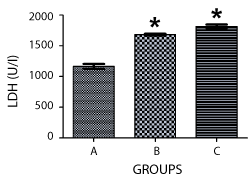
Figure 2. Graph showing the activities of lactate dehydrogenase (LDH) enzyme in the pancreas of the rats at PD 15. ‘A’ was treated with normal saline, while B and C were exposed to lactational nicotine for the first 7 and 14 postnatal days respectively, showing a significantly raised LDH activity in both treated groups compared to the control (p < 0.05).
The activity of G-6-PDH also increased in both groups exposed to nicotine when compared with the Control, with the group treated for 14 days having a higher level of activity (p>0.05). Similarly, the difference in activities of G-6-PDH in rats exposed to lactational nicotine for14 days and the Control group was statistically significant (p < 0.05) (Figure 3).
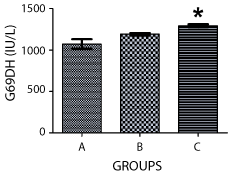
Figure 3. Graph showing the activities of glucose-6-phosphatase in the pancreas of the rats at PD15. ‘A’ was treated with normal saline, while B and C were exposed to lactational nicotine for the first 7 and 14 postnatal days respectively, showing a significant increase in activity in Group C compared with the control (p < 0.05).
Histological observations
The histological sections of the Control animals (Figure 4A) revealed alternating cellular and fibrous layers arranged in discrete units showing a well-stained structure, apparently normal architecture and numerous cells which were almost uniform in size, shape, and distribution within the laminae. These include clusters of endocrine epithelial cells (islets of Langerhans, (I); surrounded by many serous acini (A); the exocrine portion which is a compound acinar gland; and the larger interlobular ducts (D) lined by simple columnar epithelium. The initial portions of intercalated ducts (C) penetrated the lumens of the acini. Intercalated ducts merged to form larger interlobular ducts lined by columnar epithelium. The ducts and blood vessels (V) were located in connective tissue, which also provided a thin capsule to the entire gland and thin septa separating the lobules of secretory acini. The treatment groups (Figure 4B and 4C) had decreased intensity of staining and degeneration of the islet cells with figure 4C showing higher degree of degeneration. The ducts were also diminished due to hypertrophy of the glandular acini, while the blood vessels were difficult to view in Figure 4B and almost completely absent in figure 4C.
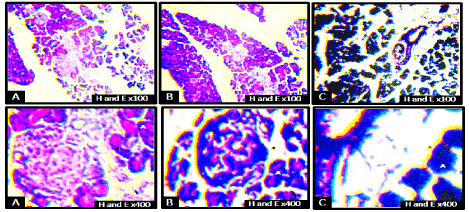
Figure 4. Photomicrographs of the general microarchitecture of the pancreas of rats on postnatal day 15 at low and high-power magnifications. Islets of Langerhans (I), glandular acini (A), interlobular duct (D), main duct (M), blood vessels (V) and intercalated duct (C). There was a decrease in the intensity of staining, degeneration of the islet cells, with plate C showing higher degeneration relative to B. The ducts were also diminished due to hypertrophy of the glandular acini:Blood vessels are difficult to view in group B and almost completely absent in group C when compared to the control group (H & E ×100 and ×400).
Figure 5 showed staining for PAS reaction demonstrating the general outline as more sparsely arranged in the control group, with the interlobar ducts diminished in treatment groups. Cellular structure was degenerated in the treatment groups when compared to the control group (Figure 5). There was reduction in PAS-positivity in the treated rats when compared to the control group.
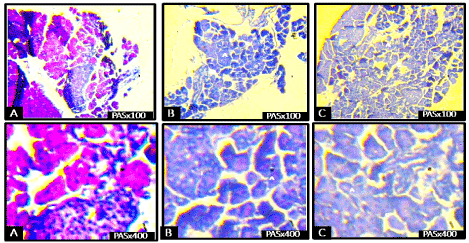
Figure 5. Photomicrographs of the pancreas of rats on postnatal day 15 showing reduction in the staining intensity (reduced PAS-positivity) in the treated rats when compared to the control group (PAS, ×100 and ×400).
Exposure to nicotine during the lactation period is usually associated with dramatic changes in body weights of the newborn. As observed in this study, lactational nicotine from the first day of birth resulted in reduced body weight. However, by the fourth day, exposed neonates have experienced an increase in body weights exceeding that of the un-exposed pups.
As noted by Santiago and Huffman [9], low birth weight usually accompanies prenatal exposure to nicotine-containing substances, which is followed by a possibility of accelerated gain in body weight. Nicotine exposure has been reported to cause glucose intolerance and impaired brain response to insulin in the offspring [10], and exposed children could experience a ‘catch-up growth’, with subsequent childhood obesity [11]. Alterations in the central endocrine control of body weight homeostasis could be responsible for the increased body weight associated with nicotine exposure, since the signals for body weight regulation and energy balance are ultimately integrated in the hypothalamus [12]. These changes result in increased appetite in offspring. Meanwhile, by PD 10, lactational nicotine did not further increase the body weights, rather the weight of the un-exposed pups picked up and rose above the treated animals, even till the termination of the study on PD 15. Very importantly, termination of nicotine exposure in a group of pups on PD 7 did not result in comparable weight gain (from PD 8 till PD 15) with the Control animals.
Nicotine at doses and durations tested in this study caused a significant rise in the level of lactate dehydrogenase (LDH) in the serum of animals compared to the Control group that received normal saline. LDH is an important enzyme of energy production in the cell, which serves as a marker enzyme for anaerobic carbohydrate metabolism, catalyzing the conversion of lactate to pyruvate [12]. High level of this enzyme is an indication of increased carbohydrate metabolism in treatment groups. Furthermore, LDH is a cytotoxic marker, which can be used to examine the extent of cellular damage [3]. Its increase is also suggestive of possible increased damage to pancreatic cells, underlying the pathological results obtained in this study.
Similarly, the level of glucose-6-phosphohate dehydrogenase (G-6-PDH) increased in animals administered with nicotine during lactation compared with the Control, with the likelihood of a more damaging effect in those exposed for a longer period, being very significant. G-6-PDH is an enzyme that catalyses carbohydrate metabolism through the glycolytic pathway [12], and a rise in the level of this enzyme would possibly increase carbohydrate metabolism (generation of energy) in postnatally treated rats.
The increase in the levels of LDH and G-6-PDH suggests an impairment in carbohydrate metabolism/energy production. However, more of carbohydrate metabolism occurred through the glycolytic pathway with comparatively higher enzyme activity. This can be explained in line with compromised cytostructures of the pancreas on exposure to nicotine.
Previous works have equally demonstrated postnatally a progressive mitochondrial damage and beta cell dysfunction of the pancreas when developing fetuses and neonates were exposed to nicotine [12,14], thus affecting both cellular structure and functions during the postnatal life of exposed offspring. Structural abnormalities were observed prior to the onset of glucose intolerance and progressively worsened with age even though nicotine exposure was discontinued at weaning [9,15].
In conclusion, lactation is a critical period in which nicotine may programme structural and physiological pathologies. Thus, caution should be taken, and advice should be given to mothers who actively ingest or are exposed to nicotine as it not only leads to pancreatic aberrations, but also has teratogenic effects which affect the offspring resulting from such pregnancies.
- Siegler RS (2006) How Children Develop, Exploring Child Develop Student Media Tool Kit & Scientific American Reader to Accompany How Children Develop. Worth Publishers, New York: 65.
- Behl M, Rao D, Aaqaard K, Davidson TL, Levin ED, et al. (2013) Evaluation of the association between maternal smoking, childhood obesity, and metabolic disorders. Environ Health Perspec 121: 170-180. [Crossref]
- Primo CC, Ruela PBF, Brotto LDA, Garcia TR, Lima EF (2013) Effects of maternal nicotine on breastfeeding infants. Rev Paul Pediatr 31: 392-397. [Crossref]
- Chowdhury P, Bose C, Udupa KB (2007) Nicotine-induced proliferation of isolated rat pancreatic acinar cells: effect on cell signalling and function. Cell Prolif 40: 125-141. [Crossref]
- Amir LH (2001) Maternal smoking and reduced duration of breastfeeding: a review of possible mechanisms. Early Hum Dev 64: 45-67. [Crossref]
- Mascola M, Van V, Tager I, Speizer F, Hanrahan J (1998) Exposure of young infants to environmental tobacco smoke: breast-feeding among smoking mothers. Am J Public Health 88: 893-896. [Crossref]
- Liu RH, Mizuta M, Matsukura S (2003) Long-term oral nicotine administration reduces insulin resistance in obese rats. Eur J Pharmacol 458: 227-234. [Crossref]
- Marcondes FK, Bianchi FJ, Tanno AP (2002) Determination of oestrous cycle phase of rats: some helpful considerations. Braz J Biol 62: 609-614. [Crossref]
- Santiago SE, Huffman KJ (2012) Postnatal effects of prenatal nicotine exposure on body weight, brain size and cortical connectivity in mice. Neuroscience Res 73: 282-291. [Crossref]
- Chen H, Iglesias MA, Caruso V, Morris MJ (2011) Maternal cigarette smoke exposure contributes to glucose intolerance and decreased brain insulin action in mice offspring independent of maternal diet. PLoS One 6: e27260. [Crossref]
- Chen H, Saad S, Sandow SL, Bertrand PP (2012) Cigarette smoking and brain regulation of energy homeostasis. Front Pharmacol 3: 147. [Crossref]
- Bruin JE, Petre MA, Raha S, Morrison KM, Gerstein HC, et al. (2008) Fetal and neonatal nicotine exposure in Wistar rats causes progressive pancreatic mitochondrial damage and beta cell dysfunction. PLoS One 3: e3371. [Crossref]
- Popoola N, Enaibe B, Adekomi D, Raheem S, Olajide O, et al. (2014) Effect of cigarette smoke on some enzymes of the descending aorta of adult Wistar rats (Rattus norvegicus). African J Cell Pathol 3: 25-29.
- Bruin J, Kellenberger L, Gerstein H, Mmorrison K, Holloway A (2007) Foetal and neonatal nicotine exposure and postnatal glucose: identifying critical windows of exposure. J Endocrinol 194: 171-178. [Crossref]
- Bruin E, Gerstein HC, Holloway AC (2010) Long-term consequences of foetal and neonatal nicotine exposure: a critical review. Toxicolo Sci 116: 364-374. [Crossref]





