Abstract
Long-chain polyunsaturated fatty acids (LCPUFAs) such as docosahexaenoic acid (DHA) and arachidonic acid (ARA) are essential for the maturation of the fetal brain, and retina in newborns. The concentration of LCPUFA in both organs increased dramatically from the third trimester until 24 months of life. DHA is one of the basic building blocks of nerve cells in the brain and retina. In the brain, it is found in the membrane of nerve cells (neurons) and synapses, and in the retina. It is also found in membrane of photoreceptor cells. It plays a structural role ensuring integrity, fluidity, permeability and greatly participates in the control of entry in these cells. It also plays a role in the production of molecules that protect these nerve cells from death, inflammation and oxidation, and stimulate the regeneration of nerve cells. Finally, it is involved in the various transmission systems between neurons at the synapse level (neurotransmission carried out by neurotransmitter molecules). We can therefore see through the action of these neurotransmitters the importance of DHA in memorization, attention, learning and self-control. Neurons lack the enzymes that would allow them to produce DHA from α-linolenic acid (ALA) or eicosapentaenoic acid (EPA). They must therefore be supplied with DHA through food, even if the liver and other brain cells produce it, but to a very small extent. The preferential transfer of LCPUFA to the fetus occurs across the placenta, compared with other fatty acids (FAs). The increase in LCPUFA DHA and ARA transferred from mother to fetus occurs late in pregnancy. Several studies have indicated that FA-activated nuclear transcription factors play important and complex roles in placental biology. Here, we review the recent roles of placental FA binding/transport proteins, and nuclear transcription factors in placental FA transfer to the fetus during fetal-placental growth and development.
Key words
Breast milk, fatty acid, long-chain polyunsaturated fatty acid, fatty acid binding/transport proteins, nuclear transcription factors, placenta
Introduction
Normal pregnancy is accompanied by transient changes in carbohydrate and lipid metabolism. During the in utero period, fetal adipose tissue is formed, with an accretion stage occurring from the start of the 3rd trimester of pregnancy. There are two varieties of adipocytes (or fat cells). White adipocytes and brown adipocytes, and consequently, two types of adipose tissue: white adipose tissue (WAT) which stores excess energy as triglycerides (TG). It regulates energy and nutritional metabolism via the action of adipocytokines. Brown adipose tissue (BAT) is ubiquitous in newborns. The uncoupling protein 1 (UCP1) of BAT allows dissipation of the electromagnetic proton gradient generated by the mitochondrial respiratory chain. The decoupling between energy consumption and ATP synthesis promotes the dissipation of energy into heat. BAT is the major site of thermogenesis in newborns, and is the main adipose tissue that is synthesized in the fetus.
The role and importance of LCPUFAs, such as Omega 3 (DHA) and Omega 6 (ARA), during pregnancy are indispensable for the development of the brain and retina of the fetus and infant. Breast milk (BM) is the only source of essential nutrients after birth.
Fatty acids, the main constituent of lipids, include in particular aliphatic carboxylic acids and their derivatives (methylated, hydroxylated FAs) and eicosanaoids [1,2]. The brain structures of the fetus are placed in place during pregnancy. During the last 3 months of pregnancy, the infant’s brain increases, which allows the fetus to build up its reserve adipose tissue and develop its retina. The accumulation of DHA in nerve cells begins as early as the perinatal period. This accumulation is an essential factor in the establishment of various cerebral and retinal functions. The main source of DHA for the fetus comes from the transplacental passage, but the FA composition of the amniotic fluid is very dependent on the mother’s diet. Some studies have shown that the concentration of DHA in the cord blood is higher in the blood of the fetus than in the mother, showing that the transfer of DHA to the fetus is promoted by the placenta. Maternal DHA can also come either from the diet or from the mobilization of its lipid reserves [3]. In the long term, the quality of maternal lipid intake could be at the origin of metabolic disorders perpetuating throughout life, favoring the appearance of diseases such as obesity and associated metabolic disorders in children.
There are two main families of essential LCPUFAs (EsLCPUFAs): omega-6 polyunsaturated FAs (or PUFA n-6), the precursor and major representative of which is essential linoleic acid (LA-18: 2n-6), and ARA (20: 4n-6), and omega-3 EsLCPUFAs (or n-3), the essential precursor of which is ALA (18: 3n), DHA (22: 6n-3), EPA (20: 5n-3). In premature children, but also in children born at term, the quantities of DHA synthesized appear insufficient with regard to their needs. The benefits of DHA supplementation in neurosensory development have been clearly demonstrated [4].
During pregnancy, DHA and ARA are transferred from the mother to the fetus across the placenta [5,6]. This transfer can be disrupted during a preterm birth, in which case, the newborn is nourished with FAs supplied in the diet and/or intravenously provided as a parenteral lipid emulsion. In children, omega-3 deprivation and therefore in DHA (for example by a deficiency in the mother’s milk, or in preterm infants without supplementation) causes a delay in the development of visual acuity, but also a poor development of behavior and cognitive capacities (ability to lear, to think) [7-9]. It has been proven that this deprivation induces a difficulty in carrying out certain movements. It is around the time of birth (neonatal period) that the nervous system stores DHA in the most active way. Here we find the importance of this ultimately short period when the brain must absolutely receive this supply of DHA for optimal psychomotor development of the child [10].
The FA composition of BM is influenced by maternal diet [11], maternal age [12], stage of lactation [13], and pregnancy duration [14]. Many factors influence fetal growth: gestational age, maternal weight before pregnancy, weight gain during pregnancy, parity, and maternal fetal diet. One of the main research findings is that levels of LCPUFAs (including DHA) are significantly higher in preterm BM than in BM from mothers delivering at term [15].
The aim of this review was to update the knowledge in the field of placental FA transport and metabolism, the relationship between pregnancy and LCPUFAs, and the regulatory roles of placental FA binding/transporter proteins and nuclear transcription factors in this association.
Literature search
We searched computerized databases and publications on EsLCPUFAs in pregnancies and preterm and term newborns. Initially, the MEDLINE, PubMed, PubMed Central®, ScienceDirect, and Edition Diffusion Presse (EDP) Sciences databases were searched for published studies. The publication year filters were: 1991, 1995, 1998, 2001, 2002, 2004-2009, 2011-2020 from PubMed/PMC; 2016 for ScienceDirect and 2005 for EDP Sciences. We also searched for relevant articles by using the following MeSH terms or keywords: LCPUFA, DHA, ARA, LA, ALA, EPA, omega-3 FAs, omega-6 FAs, prostanoids, eicosanoids, prostaglandins (PGs), leukotrienes (LTs), thromboxanes (TXs), protectins (PDs), maresins (MaRs), resolvins (Rvs), lipoxins (LXs), specialized pro-resolving lipid mediators (SPM), EsFAs, pregnancy, placenta, human BM, preterm, and term infants; fetal development; transport of FAs across the human placenta (HP); placenta fatty-acid-binding/transport proteins; nuclear transcription factors in HP tissue of pregnancies; sterol regulatory element-binding protein (SREBP); EsFA transfer and fetal development; and polyunsaturated fatty acid (PUFA) supply to fetus and brain. We included publications reported on analyses of FA and LCPUFA content pregnancies and in healthy term infants and preterm infants. Editorials, case reports, unpublished materials, submitted manuscripts, personal communications, and commentaries were excluded.
Classification of fatty acids
The lipid family is classified into six categories of components: (i) TG or triacylglycerols (TAG), (ii) glycerol phospholipids or phospholipids (PLs), (iii) sphingolipids, (iv) terpenoids, (v) sterols (cholesterol) or steroids, and (vi) FAs.
Fatty acids are saturated or unsaturated carboxylic acids. The FAs are grouped into distinct families depending on the number of bonds: (i) saturated FAs, which have no double bonds; (ii) monounsaturated FAs (MUFAs) that have only a single double bond; and (iii) PUFAs with double bonds ≥ 2. In unsaturated FAs, the chains structural configuration role is cis (more frequent) or trans (less frequent). Free fatty acids (FFAs) are obtained during digestion by lipases that separate FAs from TG.
Omega 6 is a family of PUFAs (or n-6 PUFAs), with as precursor LA, from which ARA and docosapentaenoic acid (DPA). Omega 3 families of PUFAs (or n-3 PUFAs), with as precursors (ALA), were synthesized from EPA and DHA. Among the non-essential fatty acid (N-EsFA), oleic acid and saturated FAs are present, including lauric, myristic, and palmitic acids.
PUFAs, LA, and ALA are EsFAs because they cannot be synthesized endogenously, and thus must be obtained from the diet [16]. The derivatives of ALA include the DHA required for brain growth and development [17], and EPA, the precursor of series-3 PGs. Linoleic acid is converted to ARA, the main precursor of PGs, TXs, and LTs. Diet may also provide DHA, EPA, and ARA.
Metabolism of fatty acids
Dietary lipids are composed of TGs (95%), PLs, cholesterols, and acylglycerols. The FAs are first transported into the bloodstream on lipoproteins (LPs), which supply adipose tissue, muscle, and liver. In the second step, FAs are metabolized by the liver and redistributed throughout the body. Through mitochondrial β-oxidation, all dietary FAs provide energy, and the majority of PLs contribute to cell membrane structures. Some FAs are esterified from the omega-6 and omega-3 series. The metabolism of essential PUFAs (EsPUFAs) leads to (i) the synthesis of long-chain constituents of cell membranes and (ii) oxygenated bioactive mediators such as ALA [18].
Transport of fatty acids through the mitochondrial membrane
The FFAs captured by the liver are activated as their corresponding acylcoAs by acylcoA synthases present in the microsomes and in the outer mitochondrial membrane, or the mitochondrial matrix. Long-chain acylcoA crosses the mitochondrial membrane through the carnitine acyltransferase system, where it is conjugated to a polar molecule, by carnitine transferase into the inner mitochondrial membrane, and into the mitochondrial matrix by acylcarnitine translocase.
In the mitochondrial matrix, acylcarnitine’s acyl group is transferred back to acylcoA (releasing carnitine) by carnitine acyltransferase II [19,20]. The mitochondrial metabolism of acetylcoA differs between extrahepatic and hepatic tissues. In extrahepatic tissues, acetylcoA is oxidized in the tricarboxylic acid cycle to produce CO2, H2O, guanidine triphosphate (GTP), and reduced forms that enter the electron transport chain to produce ATP [21]. In hepatic tissues, the acetylcoA produced by β-oxidation can undergo either complete oxidation in the tricarboxylic acid cycle or can be used to synthesize ketone bodies, which are then released into the circulation and transported to peripheral tissues (Figure1).
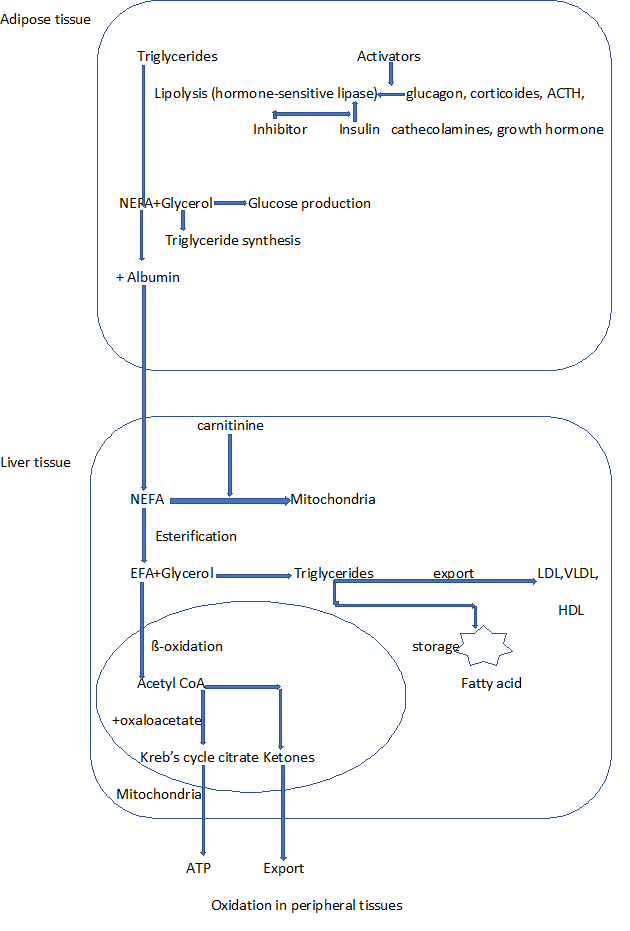
Figure 1: Production and metabolism of non-esterified fatty acid (NEFA) and esterified fatty acid (EFA)
Structural role of fatty acids
Lipoproteins, consisting of TGs, cholesterol, PLs, and apolipoproteins, are transported by proteins. There are five types of LPs: (i) chylomicrons are responsible for transporting TGs and dietary cholesterol taken up by the intestine to peripheral tissues; (ii) very low-density LPs (VLDL) are mainly synthesized by the liver and mainly by the intestine in order to export TGs; (iii) intermediate density LPs (IDL); and (iv) low-density LPs (LDL). They transport high-density LP (HDL) cholesterol to peripheral organs; (v) HDL transports cholesterol to the liver and serves as a store of apolipoproteins (Figure 1).
Lipids are transported between different organs from their place of absorption and synthesis to their place of storage or use. As lipids are insoluble in water, they cannot be transferred via simple diffusion in the plasma. Their transport is facilitated by LPs and albumin. It breaks down into four functions: (i) transport of lipids from food, (ii) transport of hepatic lipids, (iii) transport of cholesterol to the liver by LPs, and (iv) the transfer of FAs released by adipose tissue.
The lipid bilayer consists essentially of PLs (70-90%) and cholesterol, which increases membrane fluidity [20]. PLs act as constituent membrane interfaces, FA transporters, and emulsifiers. Linoleic acid, ARA and DHA are fundamental constituents of membrane PLs [22,23]. Thus, in photoreceptor cells of the retina, the reception of the photon and its translation into an electrical signal require conformational changes in retinal photopigment. These changes are modulated by the PL composition of the photoreceptor membrane in general and the level of DHA [24]. An indirect structural effect involves modulation of the biosynthesis of membrane PLs regulating protein kinase C and apoptosis.
Mechanisms of polyunsaturated fatty acids supply to the brain and retina
n-3 LCPUFAs taken during pregnancy, either in the form of supplements or in the form of food intake (e.g., fish and fish oil containing n-3 LCPUFAs, particularly DHA or EPA) could reduce the number of premature births (before 37 weeks of gestation) or very premature (before 34 weeks of gestation), the risk of pre-eclampsia (PE), hypertension induced by pregnancy, growth retardation intra-uterine, and reducing the risk of low birth weight. These LCPUFAs are essential for optimal neuronal development. Likewise, pregnant women taking the same LCPUFAs may have deleterious effects on pregnancy outcomes, such as an increased incidence of longer pregnancies (beyond 42 weeks of gestation), a larger intrauterine fetus, and an increase in newborn birth weight. In the fetus, LCPUFAs preferentially accumulate in the brain during the last trimester of pregnancy and in the first months of life. Maternal DHA is provided either from her diet or from the mobilization of her lipid reserves. The FA composition of maternal adipose tissue depends on the mother’s lifestyle and especially on her diet. Maternal circulating Omega 3, whether they are of food origin or that they come from its reserves, are primarily transferred to the fetus during pregnancy [25]. The incorporation of DHA into the PLs of the human brain occurs mainly during the period of rapid brain growth from the last trimester of pregnancy until the age of 2 years. The high concentrations of DHA and ARA in the brain and DHA in the retina suggest that these LCPUFAs play a key role in the functioning of the retina and brain. DHA and the retina could be involved in the regulation of neuroinflammation through their conversion into bioactive lipid derivatives. In the central inflammatory model, intake induced:(i) an increase in n-3 PUFA-derived lipid mediators, (ii) a decrease in n-6 PUFA-derived lipid mediators, and (iii) a decrease in inflammation in the hippocampus. Moreover, n-3 PUFA intake during the perinatal period did not affect the lipid composition of the brain immune microglial cells. PUFAs are mainly transported from the liver in the bloodstream to the brain by LPs such LDL receptors and VLDL or in the form of albumin complex. They can also be transported in esterified or non-esterified forms [26]. Albumin carries the non-esterified PUFAs (N-EPUFAs) form, derived from adipose TAG stores or esterified PUFAs (EPUFAs), such as lysophosphatidylcholine (LPC). Once in the brain, the FAs cross the blood-brain barrier either by passive diffusion (flip-flop mechanism) or by active transport proteins [27,28]. There are five types of transport proteins of FAs involved in the transport of PUFAs:(i) fatty acid translocase/cluster of differentiation 36 (FAT/CD36) is expressed in endothelial cells and microglia cells, and (ii) fatty acid binding protein (FABP) facilitates the separation between FAs and albumin [29]. FABPs are expressed in tissues that are rich in FAs and comprise several isoforms. To date, nine isoforms of FABP have been identified in the human genome. FABP-1, liver-(L-FABP), FABP-2, intestine-(I-FABP); FABP-3, heart-(H-FABP); FABP-4, adipocyte-(A-FABP); FABP-5, epidermal-(E-FABP); FABP-6, ileal-(IL-FABP); FABP-7, brain-(B-FABP); FABP-8, myelin-(M-FABP); and FABP-9, testis(T-FABP). Peripheral membrane fatty acid binding protein (FABPpm) and cytoplasmic fatty acid binding protein (cFABP) plasma leaflets assist PUFA transport, which may occur via integral membrane proteins. FABP binds a variety of ligands (LCPUFA, eicosanoids, and retinoids); (iii) fatty acid transport proteins (FATP) are a family of transmembrane transport proteins. This family contains six isoforms (1-6). FATP-1 is expressed in WAT and BAT and is regulated by various factors, such as peroxisome proliferator-activated receptor (PPAR). The other isoforms are broadly distributed in human tissue:(iv) Calveolae are small intracellular invaginations of plasma membranes that are formed from lipid rafts, making it possible to transport FAs [28]; (v) the major facilitator superfamily domain-containing protein D2A (Mfsd2a) is a major transporter of DHA LPC form [29] and FAT/CD36 [30,31]. FATP indirectly drives FA uptake by esterifying PUFAs to coA inside the cell, which effectively traps the nondiffusion acyl-coA-PUFA. DHA-containing LPC molecules are transported by Mfsd2a proteins. Mfsd2a is expressed in blood brain-barrier endothelial cells. It specifically transports DHA into the LPC. Once in the brain, E-PUFAs are released from membranes by phospholipase A2 (PLA2), which hydrolyzes esterified FAs in the sn position of PL. There are several forms of PLA2: secretory PLA2 calcium-dependent from group IIA or V (sPLA2), PLA2 calcium-independent from group VI (iPLA2), and plasmalogen-selective PLA2 (PIsETn-PLA2). FATP indirectly drives FA uptake by esterifying PUFAs to coA inside the cell, which effectively traps the nondiffusing acyl-coA-PUFA.
Prostaglandins: biosynthesis and biological effects of lipid mediators
PGs are molecules derived from PUFAs and belong to the eicosanoid superfamily. The precursor of protanoids, ARA, is produced by the hydrolysis of membrane glycerophospholipids by PLA2 and, to a lesser extent, by phospholipase C. The oxidation of ARA followed by reduction by COX1 and COX2 (also called PGHS1 and PGHS2), to be transformed into PGG2 (prostaglandin endoperoxydase G2) then into PGH2 (prostaglandin endoperoxidase H2). PGH2 is then converted by prostaglandin synthases (PGDs, PGEs, PGFs, PGIs, or TXAs) into PGs, PGD2, PGE2, PGF2α, or prostacylin (PGI2), or thromboxanes (TXA2, TXB2), respectively. These molecules are secreted and activate receptors with seven transmembrane domains coupled to G proteins, the G protein-coupled receptor (GPCR). There are nine members, DP1 and DP2 for PGD2; EP1-4 for PGE2, FP for PGF2α, IP for PGI2, and TP for TXA2 [30,31,32] (Figures 2 and 3).
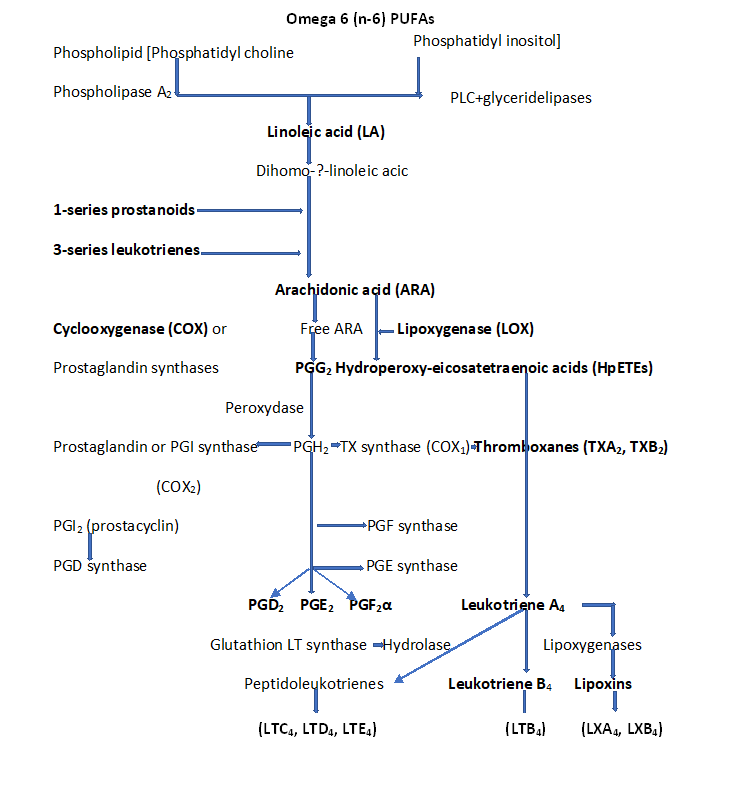
Figure 2: Biosynthesis of LCPUFAs via COX and LOX pathways. The conversion of ARA to eicosanoids by action of COX to prostaglandin endoperoxides, PGH2 (PGD2, PGE2, PGI2, PGF2α, TXA2, and TXB2), then by action of LOX include Lipooxin (LXA2), and 4 series leukotrienes (LTA4, LTB4, LTC4, LTD4, LTE4).
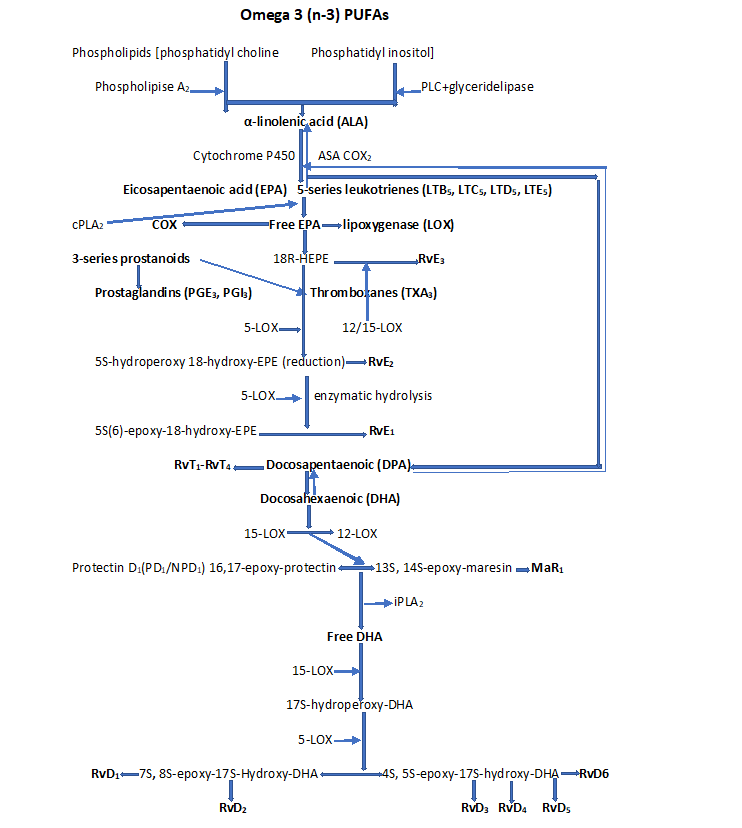
Figure 3: Pro-resolving lipid mediators. Pathway of D-series resolvins (RvD1, RvD2, RvD3, RvD4, RvD5, RvD6), maresins (MaRs), and protectins (PDs) from DHA, and E-series resolvins (RvE1, RvE2 and RvE3) from EPA biosynthesis derived from Omega-3 PUFA, and lipoxins [LXs (LXA4 and LXB4)] from ARA biosynthesis derived from Omega-6 PUFA; NPD1(neuroprotectins 1).
There are three main groups of inflammatory mediators:(i) pro-inflammatory cytokines including interleukin (IL-1, IL-6, IL-10, and IL-12 ; tumor necrosis factor-α (TNF-α), interferons (IFN); (ii) pro-inflammatory chemokines included CXCL,CCL; platelet activating factor (PAF); (iii) lipid mediators, including PGD2, PGE2, PGF2α, PGI2, and 15 d-PGJ2 ; TXA and TXB [30]; LTC4, LTD4, LTE4, LTF4, LTA4, LTB4 [30] (Figure 3), and SPMs such as MaRs, LXs, Rvs, and PDs [33,34]. The GPCRs, of which there are four main families (Gi/Go, Gq, Gs, and G12/13) are composed of α, ß, and ϒ subunits [32].
PGE2 has important effects on labor inductions, bleeding after delivery, and termination of pregnancy. PGE2 has a variety of functions in the central and peripheral nervous systems. It contributes to inflammation when bound to EP2 receptors (EP1, 2, -3, and -4 receptors of PGEs). It exerts diverse effects on cell proliferation, including apoptosis, angiogenesis, and inflammation. PGE2 is found naturally in the seminal fluid, menstrual flow, amniotic fluid, and placenta. It is produced by a variety of tissues, including bone marrow, skin, brain, spleen, and respiratory tract, and is abundantly produced by mast cells, platelets, and alveolar macrophages.
The synthesis of PGD2 is under the specific controlled by the enzyme prostaglandin D synthase (PGDS). There are two types of PGDS: lipocalin-PGDS (L-PGDS) and hematopoietic PGDS (H-PGDS). PGD2 undergoes dehydration to form a wide variety of metabolites: 15-deoxy-PGD2, PGJ2, or 15-deoxy-PGJ2. PGD2 binds to two GPCR receptors: the DP1 and the DP2. The metabolite 15-deoxy delta (12,14) -prostaglandin J2 has been identified as ligand PPAR [33-35]. PPAR has been found in adipose tissue, where it plays a key role in the regulation of adipogenesis [36]. After ligand binding, PPARs form a heterodimer with the retinoid X receptor (RXRα).
15-d-PGJ2 considered as product metabolite of PGD2 exerts its effects on cells by binding to PPAR. Stimulation of PPAR with 15-d-PGJ2 reduces the production of inflammatory proteins, such as gelatinize B [36-38]. It has been shown that the binding of 15-d-PGJ2 inhibits the production of cytokines (including TNF-α, IL-6, and IL-ß).
PGI2 inhibits platelet aggregation and induces vasodilation; hence, it counters the action of TXA2, a well-known vasoconstriction and inhibitor of platelet aggregation. The interaction between PGI2 and its receptor activates Gs proteins and induces a response that includes the generation of cAMP, followed by activation of protein kinase A, Gq, and Gi. Elevated levels of cAMP inhibit the AMP kinase pathway Gs plays a major role in PG receptor signaling because, PGI2/EP1 inhibits lipopolysaccharide (LPS)-induced p42/p44 [32,33].
Thromboxane A2 is an ARA-derived omega-6 PUFA. Aspirin inversely inhibits platelet COX-1, preventing the formation of PGH, and therefore TXA2 [39]. There are two types of TXA2 receptors (TPα and TPß), both of which are functionally coupled to heterotrimeric G-proteins. Activation of these components triggers a signaling cascade, and the central events in TXA2/TP signaling are the activation of phospholipase C, calcium release from intracellular stores, and activation of phosphokinase C (Figure2).
Specialized lipid pro-resolving lipid mediators are part of a large family of pro-resolving molecules enzymatically produced from the omega-6 and omega-3 FAs. These include LXA4 and LXB4 derived from ARA (Figure 2); RvE1-RvE3 derived from EPA; RvD1-RvD6, PD1 and PDX, MaR1 and MaR2 derived from DHA; RvT1-RvT4 derived from DPA, and their aspirin-triggered epimeric forms (AT-RvD1-AT-RvD6) generated via the action of LOXs and CYP450, and COX-2 (Figure 3). EPUFAs play a role in regulating the persistence and resolution of inflammation [39-41]. SPMs turn off the inflammatory response by acting on distinct GPCRs expressed in immune cells that activate dual anti-inflammatory and pro-resolution activities.
Maresins belong to the family of anti-inflammatory lipid mediators and DHA-derived SPMs. They are primarily produced in macrophages, where the phagocytosis of apoptotic neutrophils generates SPMs and 14-hydroxy-DHA (14-HDHA) [42,43]. Lipopolysaccharide, TNFα, IFN-ϒ, IL-4, -10, -13, immune complexes, and toll-like receptors (TLR) induce the differentiation of macrophages. MaR1 is generated by human macrophages via the conversion of 14-HDHA by 12-,15-LOX through a 13, 14-epoxide intermediate and by human platelet-neutrophil interaction via platelet 12-LOX-mediated conversion of DHA to 13S,14S-epoxy-maresin. The direct impact of MaR1 on smooth muscle and endothelial cells reduced the production of pro-inflammatory cytokines and decreased the activation of nuclear factor kappa-light chain-enhancer of activated B cells (NF-κB). MaR2 also has a powerful bioregulatory effect [44].
Lipoxins are metabolites of the ARA pathway. There are three major LOX genes involved in LX synthesis from ARA: 12-LOX, 5-LOX, and 15-LOX. LX can be synthesized by three major routes from ARA: (i) the platelet pathway, where LTA4 is acted upon by 12-LOX, and is converted to LX; (ii) the neutrophil pathway activated by 5-LOX, and the erythrocyte pathway activated by 15-LOX. ARA is converted to 15-hydroxyperoxyeicosatetraenoic acid (HEPE), which is subsequently converted to LXA and LXB; (iii) an aspirin-independent pathway that leads to the generation of 15 epi-LXA4 and 15 epi-LOXB4. Lipoxins and epi-lipoxins exert their anti-inflammatory effects by interacting with GPCR LXA4 (ALX) and GPCR 32 [45,46]. The LXs control the expression of the early growth response 1 gene (EGR1) and the transcription of pro-inflammatory cytokines IL-2 and TNFα in T-cells. LXs have been shown to increase the levels of PPAR and the neutrophil gelatinize-associated lipocalin gene.
Resolvins, biosynthesized from essential omege-3 PUFAs EPA and DHA as precursors, are thought to have anti-inflammatory and pro-resolving actions [42,45]. The biosynthesis of RvD1 and RvD2 requires the catalysis of 15-LOX and 5-LOX [42,47-49]. RvE1 and RvE2 are biosynthesized from EPA through aspirin-COX-2 and 5-LOX from the interaction of endothelial cells and leukocytes [50], and also via an aspirin-independent pathway through the CYP450. RvE3 is generated from 18R-HEPE through the 12/15-LOX pathway and can be synthesized in eosinophils [51,52]. RvE1 inhibits the synthesis of cytokines and cell adhesion molecules, NF-κB expression, and chemotaxis of polymorphonuclear leukocytes and dendritic cells to the inflammatory focus. RvE1 and RvE2 also regulate neutrophil chemotaxis and activate phagocytosis. RvD1 action is mediated by GPCR32 and ALX receptors [42].
Protectins are members of a class of SPMs. They are synthesized from DHA by 15-LOX-1 in a number of cells, including brain cells (microglia), monocytes, and CD4+ T-lymphocytes [42,53]. PD1 proceeds through the activity of 15-LOX-1. The binding of 15-LOX-1 to DHA leads to the formation of the (17S) -hydro (peroxy)-DHA intermediate to form a (16) -epoxy intermediate, which is hydrolyzed to PD1. The inhibition of NF-κB results in the downregulation of the pro-inflammatory gene COX-2, which is responsible for the release of PGs. In addition, PD1 protects retinal pigment epithelial cells against oxidative stress-induced apoptosis and blocks cytokine production in glial cells.
Role of the placenta on fatty acid requirements during pregnancy
The placenta is a unique ephemeral organ that physically and biologically connects the developing embryo to the uterine wall. Through pregnancy, the placenta provides the embryo and then the fetus, with water and nutrients it needs. Placental transfers also concern the elimination of fetal metabolic wastes that are released into the maternal blood and then eliminated (urea, uric acid, creatinine). The respiratory function of the placenta allows the delivery of O2 to the fetus and the evacuation of fetal CO2. The exchanges take place between maternal blood (rich in O2) and umbilical arterial blood (mixture of arterial and venous blood), poor in O2. Placenta is implicated in physiological functions during gestation, such as immunological tolerance between mother and fetus, hormonal production necessary for the maintenance of pregnancy, and fetal defense against environmental attacks [54,55]. The HP plays a crucial role in concentrating LCPUFAs and mobilizing maternal adipose tissue fat stores. Lastly, one of the essential roles of the placenta is to physically connect the fetus to the uterine wall and allow the formation of an exchange surface between the mother and fetus.
The corticosteroid-releasing hormone (CRH) produced in the paraventricular nucleus of the hypothalamus, controls the stress response via the anterior pituitary. During stress, two reactions are triggered at the level of the central nervous system, one leads to the release of adrenaline, norepinephrine and acetyl choline by the adrenal medulla, the other leads to the release of glucocorticoids such as cortisol. In prolonged stress, the production of CRH by the hypothalamus controls the production of adreno-corticotropin hormone (ACTH) by the anterior pituitary, which itself stimulates the production of glucocorticoids (cortisol) by the fetal adrenal cortex glands. Once released into the bloodstream, glucocorticoids exert negative feedback on the hypothalamus and pituitary gland causing CRH and ACTH levels to drop.
CRH increases exponentially throughout pregnancy until the near term of pregnancy. It acts on the placenta and the smooth muscles of the myometrium, but also on the pituitary-adrenal axis of the fetus. It also plays a role in the initiation and progress of labor, by stimulating the production of steroid hormones (estrogen and progesterone) produced by the placenta during pregnancy. The concentration of estrogen causes the formation of oxytocin receptors on the uterine myometrium, increases myometral contractility and maturation of the cervix. Estradiol increases the synthesis of glycosaminoglycans, and acts on collagenase, consequently the mature cervix. There is an increase in its ability to relax. Progesterone is essential for the maintenance of pregnancy, decreases the contractility of the myometrium and the formation of intercellular junctions, and an inhibition of cervical maturation. It reduces the effects of estrogen.
CRH stimulates the production of PGs and oxytocin by potentiating the action of these on the uterine muscle. The regulation of CRH is complex, with a decrease in placental production by progesterone and nitric acid (NO), and on the contrary an increase in its placental production secondary to glucocorticoids, on the one hand, and IL1, noradrenaline and acetylcholine on the other hand. Another action of placental CRH is to directly stimulate the production of steroids by the fetal adrenals (dehydroepiandrosterone sulfate) and indirectly, by stimulating the production of ACTH by the fetal pituitary gland. This could be in favor of the intervention of the fetal hypothalamic-pituitary-adrenal axis in the process of induction of childbirth.
During the last third of gestation, progressive protection in pregnancy increases the production of PGE2 by the fetal trophoblast, activating the fetal hypothalamic-pituitary adrenal axis, leading to an increase in fetal cortisol concentration. PGE2 are synthesized within the human fetal membranes, amnion, chorion, decidua and myometrium during pregnancy. They stimulate the synthesis of glycosaminoglycans and decrease the collagen concentration in the cervix. They increase the hydration of the cervix and the concentration of hyaluronic acid. Secretion of PGs during pregnancy from the placenta is increased with uterine distension. The placenta plays a particularly biochemical role with an increase in estrogen and a decrease in progesterone. At the same time, the adrenal and pituitary hormones of the fetus cause an increase in the number of gap junctions between cells. They allow the simultaneous contraction of the fibers of the myometrium and the passage of ions almost instantly from one cytoplasm to another, which causes uterine contractions and spontaneous onset of labor [55]. Placenta also exerts an endocrine function that is essential for the maintenance of pregnancy, fetal maturation, and parturition. It secretes several gestational hormones, such as progesterone and estrogen (estriol, estradiol, and estrone), and peptidic hormone (human chorionic gonadotrophin (hCG), lactogenic placental hormone (also called somatotrophin chorionic hormone)) into the maternal circulation.
The placenta is able to synthesize and store glycogen at the trophoblast level to ensure local glucose requirements by glycogenolysis. Pregnancy induces complex endocrino-metabolic changes. It has an overall "diabetogenic" effect on the metabolism, but this effect is variable depending on the period of pregnancy. The first part of gestation is characterized by an anabolic maternal phase during which lipid stores increase to subsequently meet maternal and fetal needs in late pregnancy and during breastfeeding. The second phase is called the maternal catabolic phase or fetal anabolic phase, in connection with a decrease in tissue sensitivity to insulin at level by up to 80%. The objective of which is to promote fetal growth. The placenta, which has an endocrin role during pregnancy, plays an important role in these mechanisms induced by the secretion of placental lactogenic hormone, placental growth hormone or even leptin and adiponectin.
Any pregnant woman therefore develops insulin resistance which requires increased secretion of insulin by the pancreas. Thus, those who already have, before pregnancy, a higher level of insulin resistance and/or a reduced capacity of the pancreas to secrete insulin, as in the case of obesity or a history of gestational diabetes, have higher blood sugar levels.
The fetal liver can synthesize FAs de novo, but much of fetal lipid is provided from the placenta, which indicates that all the Omega n-3 and Omega n-6 FAs acquired by the fetus during growth might cross the placental barrier, indicating a much higher concentration of ARA and DHA in the fetal blood. During the period from 22 weeks of amenorrhea to childbirth, the fetus remains totally dependent on the placenta for its supply of nutrients. The HP is a hemochorial, villus type where the maternal blood enters directly around the terminal villi without any intervening maternal vessel wall [56]. The placenta is made up of the maternal and fetal sides. It constitutes a protective barrier for the fetus. It is also made up of two contiguous membranes: the decidua and the embryonic trophoblast with many folds called chorionic villi. It contains several blood vessels allowing mother/fetus exchanges via the umbilical cord (cord connecting the fetus to the placenta). There are three umbilical vessels: two arteries that drain blood to the placenta, and a vein that drains blood into the fetus. Transport across the placental barrier takes place by different means: (i) passive diffusion (i.e., O2, CO2, H2O, and steroids); (ii) facilitated diffusion transporters of ions and organic molecules (glucose and amino acids); (iii) active ion transport using energy (ATP); (iv) direct endocytosis with degradation of molecules, and indirect, without degradation, thanks to receptors. The exchanges were dependent on maternal glucose levels. Glucose is transported by diffusion, facilitated by symport/Na+. Proteins do not pass through the placental membrane, because they are too large. Amino acids and peptides necessary for protein synthesis pass through facilitated transport (symport/Na+).
Considering all these functions described, the placenta would normally be able to ensure good growth and development of the fetus, but if we only consider reproductive function, this vital process is unfortunately inefficient, such as 50-60% of embryos that die before birth. Placental abnormalities can induce obstetric pathologies, such as (i) ectopic sites (placenta previa and placenta accreta), (ii) lack of placentation (PE), (iii) trophoblast disease (hydatiform mole and choriocarcinoma), and (iv) others (spontaneous abortion). To gain a better understanding of how the placenta could be involved in many obstetric pathologies, it has been speculated that the mutation phenomena implicating the inactivation gene could result in embryonic and fetal mortality in utero [55].
Fatty acid carriers in placental membranes
Once FAs are released by the lipases as N-EsFAs, they enter the cell through passive diffusion or by means of membrane carrier proteins. There are several membranes and cytoplasmic FA transports/binding in the HP [57]. These include FABP (40-kDa) which is an integral transmembrane protein located in both trophoblast membranes. It consists of an FA-binding protein plasma membrane (FABPpm) and placental plasma membrane FABP (p-FABPpm). FABPpm has been shown to promote the unidirectional flow of LCPUFA from the mother to the fetus. FAT/CD36 (88-kDa) and FATP (63-70 kDa) are present in both the microvillus and basal membranes in the HP. This location may favor the transport of FFA from the mother to the fetus, and vice versa. Its role consists of FA internalization. FABPpm, which specifically binds to LCPUFAs, was isolated from HP membranes [57]. Unlike FABPpm, FAT/CD36 is a multifunctional protein and has a number of putative ligands, including FFAs, collagen, thrombospondin, and oxidized LDL (ox LDL) (Figure 4).
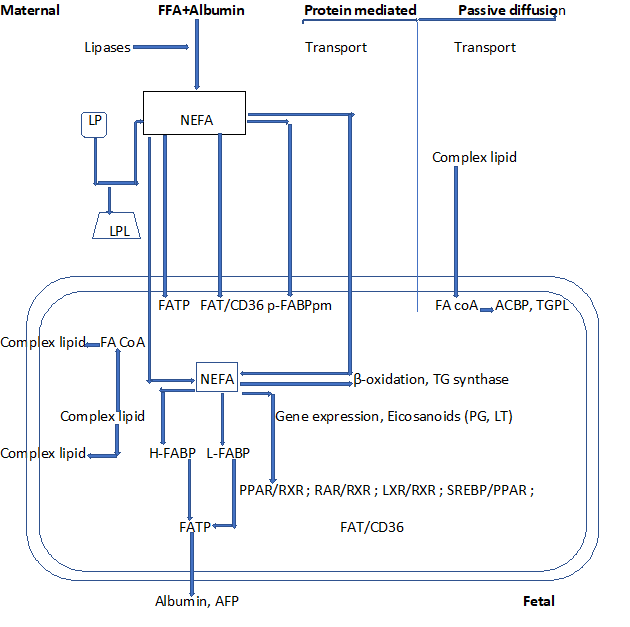
Figure 4: Fatty acids movement across placental tissue
LP: Lipoprotein; LPL: Lipoprotein Lipase; NEFA: Non-Esterified Fatty Acid; FATP: Fatty Acid Transport Protein; FAT: Fatty Acid Translocase; p-FABPpm: Placental Plasma Membrane Fatty Acid Binding Protein; H-FABP: Heart-Fatty Acid Binding Protein; L-FABP: Liver-Fatty Acid Binding Protein; TG: Triglyceride; PG: Prostaglandin; LT: Leukotriene; ACBP: Acyl-coA-Binding Protein; TGPL: Triglyceride Phospholipid; PPAR: Peroxisome Proliferator Activated Receptor; RXR: Retinoid X Receptor; LXR: Liver X Receptor; SREBP: Sterol Regulatory Element-Binding Protein; RAR: Retinoid Acid Receptors
The enrichment of LCPUFA in the fetal circulation could be related to their interaction with some FA carriers, such as p-FABPpm and FATP4. p-FABPpm may be directly involved in the uptake of DHA and ARA. The association between DHA dietary supplementation and placental transport protein gene expression in pregnant women showed that placental FATP-1 and FATP-4 gene expression was directly correlated with the percentage of DHA in the placental plasma PL. These data suggest that FATP-4 may be involved in the selective placental transport of DHA. FATP is a family of proteins that presents acyl-coA-synthase activity and may directly translocate FFA across the membrane as well as direct acyl-coA conversion, facilitating their metabolism within the cell.
The presence of other proteins involved in the transport of FAs has been demonstrated in the placenta, such as caveolae. Caveolae are flask-shaped invaginations that represent a subdomain of lipid rafts and are enriched in cholesterol, and a family of 21-24 kDa integral membrane proteins called caveolins. There are three different isoforms: caveolin-1 (Cav-1), Cav-2, and Cav-3. All three isoforms are found in human placental tissue. In HP, Cav-1 is strongly expressed in endothelial cells, with lower levels in cytotrophoblasts, mesenchymal cells, and syncytiotrophoblasts. Cav-2 and Cav-3 may play roles other than the formation of caveolae, such as regulation of Cav-1 expression and metabolism. Caveolae may control FAT/CD36 mediated FA uptake by increasing its surface availability [58]. Cav-1 and Cav-2 are upregulated by PPARϒ in human carcinomas [58].
The CRH carrier protein is produced mainly in the liver but also in the placenta and fetal membranes. The presence of this protein prevents the increase in CRH during the 3rd trimester of pregnancy. It has been observed a rapid decrease in this carrier protein 4 to 6 weeks before the onset of labor at term, which suggests that this phenomenon is likely to intervene in the process of induction of childbirth.
The expression of FAT/CD36 and FATP is under the control of PPARα in the liver and in adipose tissue. Several ligands for PPARϒ such as FAs, oxLDL derivatives, and 15d-PGJ2 have been identified. The ligands involved in activating PPAR/RXR heterodimers in the placenta may also activate the expression of FA binding/transport proteins in HP. Expression of p-FABPpm enables the placenta to recruit ARA and DHA, which may also act as ligands for PPAR and RXR. The presence of FAT and FATP in placental trophoblasts may help to deliver ligands (FA and eicosanoids) to PPARϒ for gene expression [57,59-61]. It has been suggested that the biomagnification of LCPUFA is related to the action of placental FABPpm and FATP-4. FATP-1 and FATP-4 exhibit both FA transport and acyl CoA synthase activities and facilitate FA trafficking and metabolism within the cell.
FABP isoforms present in trophoblasts regulate FAT within the cytosol. The FAs oxidized or re-esterified are directed towards fetal circulation via the placental basal membrane. It has been demonstrated to FABP-3 regulates the transport of n-3 and n-6 PUFAs in trophoblast cells. PPARα is critical for placental development and placental FA uptake. High concentrations of PGD2 have been reported in the HP, extra-placental membranes, and amniotic fluid [59].
Esterified fatty acids or LCPUFA from maternal circulation
Human placental transport of EsFA or LCPUFA from the maternal circulation into the fetal circulation is essential for proper fetal growth and development. During this period, recruitment of maternal LCPUFA, mainly DHA and ARA, is critical for rapid brain and other tissue growth. PPAR may be responsible for regulating the expression of FA transport or binding proteins in tissues such as adipose, liver, and skeletal muscle. On the other hand, FAs and their oxygenated derivatives directly or indirectly regulate several cellular processes such as differentiation and development. Therefore, these nuclear receptors may be involved in placental FA uptake by modulating the expression of FATP [59].
The placenta may capture N-EsFAs and FAs released from the membrane of maternal LPs by local endothelial lipases (ELs). It has been shown that the N-EsFA concentrations in maternal and fetal circulation increase during gestation and at the time of delivery. These concentrations are approximately three times higher in the maternal circulation than in the fetus. Maternal hyperlipidemia that occurs during gestation constitutes a source of FAs to the fetus, following their release from the maternal LPs by the placental LPL and EL, which is involved in FA uptake in placental tissues. This enzyme is also found in the placental microvillus membrane in contact with the maternal blood and trophoblasts. EL continues to be expressed at the end of pregnancy, while LPL is virtually absent in the trophoblast [59] (Figure 5).
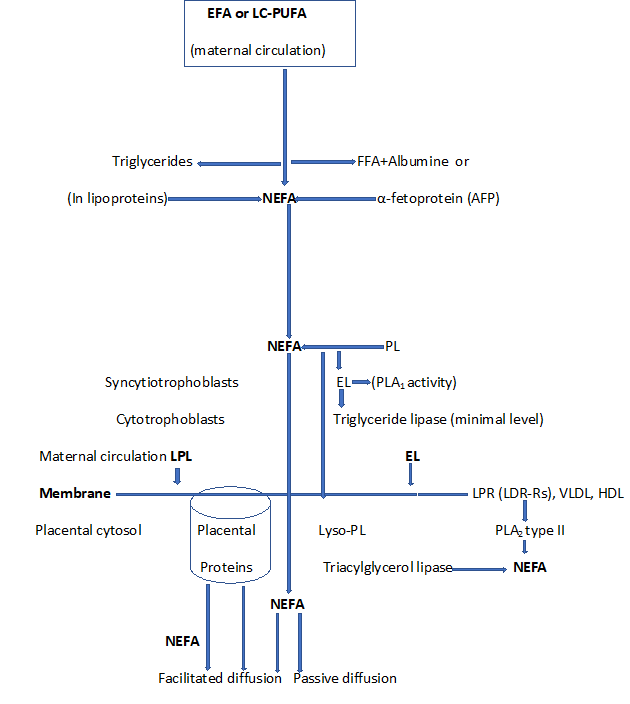
Figure 5: Placental fatty acid uptake processes
NEFA: Non-Esterified Fatty Acids; EFA: Esterified Fatty Acids; LCPUFA: Long Chain Polyunsaturated Fatty Acid; PL: Phospholipids; Lyso-PL: Lysophospholipids; R: Receptors; LPL: Lipoprotein Lipase; EL: Endothelial Lipase; PLA2: Phospholipase A2; PLA1: Phospholipase A1; FFA: Free Fatty Acid; LDL: Low-Density Lipoprotein; VLDL: Very Low-Density Lipoprotein; HDL: High-Density Lipoprotein
Maternal erythrocytes, which take lyso-phospholipids (Lyso-PL) into their membranes, constitute a potential store of LCPUFA. They are also vehicles for Lyso-PL transport to the placenta. Other lipases involved in the release of FAs from maternal plasma LP are PLA2 type II, which are mainly expressed in the placenta and choriodecidua. TAG hydrolase is expressed in the syncytiotrophoblast microvillus membrane. The placental tissue also showed LP receptors for HDL and LDL. After binding to these receptors, some LPs can undergo endocytosis to provide cholesterol to the placenta. This last mechanism could also provide FAs to placental tissue once they are released by intracellular lipases [55] (Figure 5).
Binding and transport proteins of fatty acids within the placenta
Once the FAs are in the cytosol, they bind to cytosolic FABPs that act in the cytoplasmic compartment through specific interactions with the endoplasmic reticulum, mitochondria, lipid droplets (LDs), and peroxisomes. The following FABPs have been described in the placenta: FABP-1, FABP-3, FABP4, FABP5, and FABP7. FABP-1 and FABP-3 were detected in the cytosol of human placental primary trophoblasts, as well as in human placental choriocarcinoma cells. FABP-4 and FABP-7 are isolated from trophoblasts [59].
Cyclopentenones (PGA1, PGA2, PGJ2 and δ12-PGJ2) bind strongly to FABP-1, which transports these ligands to PPAR through protein-protein interactions. Furthermore, differential effects of proteins have different roles in the intracellular trafficking of FAs in the placenta. Elevated expression of FABP-1 in placental trophoblasts during differentiation has been reported, once in the placenta, a proportion of the FAs is oxidized in the mitochondria to produce energy, and the other is incorporated into PLs.
During pregnancy, the placenta is the major source of PGs in intrauterine tissues. The fetal-placental unit synthesizes a range of eicosanoids, which might serve as ligands for PPARs and LXEs in the placenta. These PGs are synthesized in HP from ARA via the action of COX-1/2 and specific PG isomerase/synthases. PGD2 produced in HP and convert into PGJ2 can be converted to PPARϒ ligand [60]. In the PG synthesis pathways, the conversion of ARA and linoleate (hydroxyeicosatetraenoic acid (HETEs), and hydroxyoctadecadienoic acid (HODEs)), respectively, by LOX, has been shown to activate PPARs. Other oxidized FAs (such as 9-HODE, and 13-HODE), can activate PPARϒ suggesting that these endogenous PPARϒ ligands play a role as regulators of gene expression. It has been shown that the course of pregnancy can affect PPAR ligands produced in the fetal-placental unit [60].
Nuclear receptors and placentation
Nuclear receptors are required for the regulation of different functions, such as metabolism, placentation, and parturition. It can be divided into two groups: class I steroid hormone receptors, (i.e., thyroid, progesterone, estrogen, androgen, mineralocorticoid, and glucocorticoid receptors), and class II “orphans’’ receptors (so-called lipid sensors) (i.e., PPAR, liver X receptor (LXR) α and ß) [56], farmesoid X receptor (FXR), pregnane X receptor (PXR), retinoic acid receptor (RARs) α, ß and ϒ, and RXRs α, ß and ϒ). Each receptor’s modular structure features six conserved domains: A/B, C, D, E, and F [55], each of which has a specific function. PPARs are essential for placental development. There are three PPAR isotypes: PPARα, PPARβ/δ, and PPARϒ [61,62]. These receptors are involved in reproductive biology, tissue regeneration, cell differentiation, lipid metabolism, immune response, and disease processes (such as type II diabetes and cancers). PPARs have also been implicated in early pregnancy development (implantation, placentation, and trophoblast differentiation) (Figure 4).
PPARϒ is expressed in adipose tissue, intestine and immune cells, and at much lower levels in muscle, liver, kidney, pancreas and retina. It is strongly expressed in HPs, particularly in trophoblasts. It is also essential for placental development, trophoblast invasion, and differentiation of cytotrophoblasts into syncytiotrophoblasts, which form a barrier between maternal and fetal circulation [59]. The PPARϒ expression has been found in the granulosa, theca, and luteal cells, and may regulate the differentiation and proliferation of the ovarian cells, steroidogenesis, angiogenesis, and PG production. There are three isoforms of PPARϒ: PPARϒ1, 2, and 3. PPARϒ1 is expressed only in adipose tissue, colon, and liver. Like PPARϒ2, PPARϒ3 is restricted to adipose tissue and the colon. In HP, PPARϒ is specifically expressed in villus syncytiotrophoblasts and cytotrophoblasts [55,63]. Other endogenous PPARϒ ligands such as 9-HODE, 13-HODE, 15-PGJ2, 15-HETE, ARA, DHA, and LA, stimulated hCGß production in primary human trophoblasts. PPARs participate in uterine functions such as steroidogenesis, cytokine production, and angiogenesis during the estrous cycle and/or pregnancy [55,63]. Their activation may stimulate the production and secretion of hormones, such as gonadotrophin, which is required during pregnancy and fetal development.
PPARα has been detected in amnion, choriodecidua, placental syncytial, and cytotrophoblast cells at term, as well as in placental choriocarcinoma cell lines. The PPARα data suggest that it is involved in the development of the fetal epidermal barrier, in the inhibition of cell growth, and a decrease in hCGß secretion. It is activated directly by endogenous ligands such as ARA, DHA, LA, and EPA PUFAs and LTB4. It also transcribes genes involved in FA catabolism, thereby promoting FA clearance. The PPARα/RXR heterodimer binds to the DNA-binding peroxisome proliferator response elements (PPRE)/Direct Repeat 1. Oxygenated PUFA derivatives (prostanoids and LTs) induce binding to the RXR and thus to the target gene’s PPRE [64-66]. PPARα may not be essential for fetal-placental biology, unlike PPARϒ and PPARδ/ß.
PPARδ has been detected in human trophoblast cells. It is essential for fetal-placental growth and development and is involved in embryo implantation and decidualization. It stimulates cell growth and increases the differentiation and FA uptake. The endogenous ligands such as PGI2 analog carbaprostacyclin (cPGI2), FAs, ARA, and DHA of PPARδ, reduce the activity of 11ß-hydroxysteroid dehydrogenase type2 (11ß-HSD2), and expression at both protein and mRNA levels in cultured human placental trophoblasts. Placental 11ß-HSD2 plays a key role in controlling the level of fetal exposure to maternal glucocorticoids and levels of decidualization. cPGI2 enhances the heterodimerization of PPARδ and RXRα. Taken together, these data indicate that PPARδ is a regulating factor for fetal-placental growth and development [61].
There are two sub-types of LXR, LXRα and LXRß [56], both of which form heterodimers with RXRs. LXRß is ubiquitously expressed in all tissues. LXRα is closely related to tissues known to play important roles in lipid metabolism (such as liver, kidney, macrophages, small intestine, and adipose tissue), injury of vascular endothelial cells, and proliferation and invasion of trophoblasts. It acts through the transcriptional regulation of key target genes involved in lipid absorption, transport, synthesis, metabolism, and excretion. LXRα is expressed in the placenta of women with normal pregnancies. This expression is significantly upregulated in the placentas of PE patients, which is positively correlated with the extent of hypoxia. It also influences LP metabolism by modifying regulatory enzymes such as LPL, cholesterol ester transfer protein, and PL transfer protein [61,67]. Moreover, LXRß plays an important role in the inhibition of human trophoblast invasion. The transcriptional regulation gene is made by binding the heterodimer RXR/RAR or RAR/RXR. The two receptor families are stimulated by the active derivatives of vitamin A. The importance of RXRs in mammalian placentation and development and a harmonious pregnancy found to require a well-controlled supply and transformation of vitamin A. RARα, RXRα, and RXRß are ubiquitously expressed in embryonic and adult tissues, whereas RARß, RARϒ, and RXRϒ expression is more restricted [61,63,67]. The PPARϒ/RXRα heterodimer ligands have been shown to be important modulators of hormone synthesis in the human trophoblast. These ligands increase the transcription of hCGß. It has been suggested that the hCGß contains binding sites for PPARϒ/RXRα heterodimers.
The receptors (FAT/CD36) for oxLDL in trophoblasts may supply oxyterols through of oxLDL for activation of PPARϒ and LXRs and thus may increase FA synthesis. Maternal hyperlipidemia is a characteristic feature of pregnancy and corresponds to the accumulation of TGs in both VLDL and LDL. In PE, there is a further increase in plasma lipids compared to that in normal pregnancy. The maternal liver may play an important role in lipid homeostasis; however, a placental role in the development of hyperlipidemia has been increasingly evident. Similar to PPARα, activation of LXR reduced the secretion of hCGß in trophoblasts. The mechanisms of LXR-mediated inhibition of hCGß secretion may have a regulatory role in trophoblasts and countervailing. The effect of PPARϒ-induced increase of hCGß production thereby contributing to balancing the production of hCGß during pregnancy [55,63,64].
Expression of sterol regulatory element-binding proteins in placenta tissue of pregnancies
SREBPs are a family of transcription factors that regulate lipid biosynthesis and adipogenesis by controlling the expression of several enzymes required for cholesterol, FA, TAG, and phospholipid synthesis. There are three SREBP proteins encoded by two genes, SREBP1 and SREBP2. SRBP1 encodes two isoforms (SREBP-1a and SREBP-1c). SRBP-2 preferentially activates genes involved in cholesterol biosynthesis, whereas SREBP-1 activates genes required for FA synthesis [61,65]. To date, SREBPs have been shown to directly activate multiple genes involved in the synthesis and uptake of cholesterol, FAs, TG, PL (such as 3-hydroxy-3-methylglutaryl-coA synthase1, 3-hydroxy-3-methylglutaryl-coA reductase) [59,67]. Thus, SREBP-1c plays an irreplaceable role in the process of fat synthesis and is also a key adjustment factor to maintain the dynamic balance of FA in the placenta by investigating differential gene expression in placentas of PE pregnancies by means of gene chip technology [66]. In addition, SREBP-1c, acyl-coA synthase long-chain family member 3, FAs are all targets of LXRα and are all closely related to lipid metabolism [68]. SREBP controls intracellular lipid accumulation, which may be linked to insulin resistance and visceral obesity [69].
PUFAs stimulate the catabolic pathway by activating the transcription of FA transport proteins and mitochondrial peroxisomal β-oxidation enzymes. They reduce the lipogenic activity of liver tissue by repressing transcription factors and lipogenic enzymes, and regulating the expression of genes encoding LPL transcription, apolipoproteins for HDL and VLDL in the SREBP-1c pathway, and proteins controlling the development of adipose tissue, sugar metabolism, FA metabolism, and neurotransmission (neurotransmitter release, ion channels, and neural connection) [65].
In the liver, PUFAs (i) induce the transcription of enzymes regulating peroxisomal β-oxidation and the ketogenesis of FAs via the activation of PPARα, (ii) decrease the synthesis of malonyl-CoA, and (iii) inhibit the synthesis of enzymatic proteins that control the pathways for de novo lipogenesis of FAs (acylcoA synthase, acylcoA carboxylase, and FA synthase), bioconversion of PUFAs (delta 9, 6, 5-desaturases, and elongases), and the synthesis of TG (diacylglycerol acyltransferase) via the inactivation of SREBP-1c [70,71].
In adipose tissue, the regulation involves both PUFAs and their oxygenated derivatives including the isoform PPARδ, as well as the prostacyclin and PPARϒ. The SREBP-1c pathway is also involved in the regulation of HDL, VLDL, LPs, and apolipoprotein. The expression of genes involved in the brain metabolism of FAs and neurotransmission might also be regulated by PUFAs.
The roles of fatty acid metabolism in fetal-placental development
The fetus depends on the maternal supply of LCPUFA ARA and DHA. LCPUFA DHA is highly concentrated in the cell membranes of the retina and brain, where it acts as a modulator of membrane function, neurogenesis, photoreceptor differentiation, activation of the visual pigment rhodopsin, the function of ion channels, and the metabolism of neurotransmitters. The maternal transfer of DHA and ARA to the fetus during the 3rd trimester of pregnancy and into the postnatal period allows an appreciable amount of DHA to be stored in the maternal adipose tissue. This period of net mobilization corresponds to an exponential increase in fetal fat, during which 94% of all fat deposition in the fetus occurs. The maternal circulating concentrations of TAG, PLs, and N-EsFAs all increase during gestation. N-EsFAs can be oxidized within trophoblasts or re-esterified and stored as TAG in placental cells. Later, FAs can be released from cellular LDs by the action of TAG hydrolases during lipolysis [72,73].
Lipid metabolism takes place in two stages: (i) fat storage (1st and 2nd trimester of gestation) favored by overeating and increased lipogenesis;(ii) mobilization of reserves. From the 3rd trimester of gestation, lipolysis and the influx of FAs to the fetus predominate, linked to a state of insulin resistance and hyperestrogenism. This mobilization of reserves allows nutrients to be directed to the fetal placental unit. An increase in maternal hepatic production of VLDL was also demonstrated, associated with a decrease in their clearance by decreasing the activity of hepatic LPL and adipose tissue, as well as an increase in intestinal adsorption of dietary lipids. There is also an increase in the breakdown of TGs into FFAs and glycerol, which, once taken up by the maternal liver, will allow the synthesis of ketone bodies and maternal glucose, respectively. Ketones cross the placental barrier, and then the hematoencephalic barrier for the synthesis of brain lipids.
Maternal body fat accumulated during early pregnancy allows the accumulation of an important store of LCPUFAs derived from the maternal diet. During the last trimester of pregnancy, lipolytic activity in adipose tissue is increased. The FAs that are released, as well as FAs from dietary sources and hepatic overproduction of TAG, are responsible for increasing the amount of TAG in the maternal circulation. The increase in placental lipase activity during the last trimester of pregnancy results in a subsequent decrease in TAG storage. Placental LPL hydrolyzes TAGs from post-hepatic LDL and VLDL, but not the TAGs present in chylomicrons. Placenta FA metabolism may play a key role in guiding pregnancy and fetal outcomes.
Fatty acid supply to the fetus
Physiologically, the fetus may synthesize some saturated FAs and MUFAs de novo from glucose. However, the fetus depends on the placental supply of maternal EsFA and LCPUFA (especially ARA and DHA). They must be supplied to the maternal diet [74]. PUFAs exert many biological functions, especially during the perinatal period of rapid development of cell membranes, in the brain and retina. During pregnancy, the LCPUFAs ARA and DHA are transported actively and specifically across the placenta from the maternal circulation to the fetus. LCPUFA ARA and DHA are transported more actively and selectively than LA and ALA, explaining the higher concentrations of these FFAs in the fetal circulation. Thus, increasing maternal DHA intake during the last trimester of pregnancy effectively increased the DHA content of maternal plasma and red blood cells and, consequently, the status of DHA from the developing fetus (concentrations in cord blood). In contrast, maternal dietary intake of ALA appears to be less effective in increasing DHA status, due to the low rate of maternal conversion of ALA to DHA. During the lactation period, LA, ALA, ARA, EPA, and DHA found in the lipids of BM come from the mother’s diet (lipomobilization), but also from their mobilization from maternal adipose tissue partially constituted before and during pregnancy [75].
DHA is highly concentrated in the PLs of the brain and retinal membranes, making up about 25% of total FAs in the cerebral cortex and more than 30% in the retina. The incorporation of DHA into brain PLs occurs mainly during the period of rapid brain growth, from the last trimester of pregnancy. The main LCPUFAs found in BM are DHA and ARA. They can be synthesized in human tissues using EsFA precursors. It has thus been shown that the LA content of human BM is more closely related to that of adipose tissue than that of the mother’s diet; two-thirds of the LA found in BM would thus come from the process of lipomobilization of adipose tissue and only one-third directly from the mother’s diet. Consequently, PUFA concentrations in human BM strongly depend on maternal intake during the lactation period as well as during pregnancy.
The DHA and ARA contents in BM tend to vary as a function of the mother’s diet, nutritional status, and other factors. The DHA concentration in BM is lower and more variable than that in ARA. The level of ARA is relatively stable in BM and is biologically important because it consistently provides preformed ARA at a time when brain growth and development is critical. The majority of ARA in BM is derived from the maternal stores of ARA, rather than from the mother’s dietary LA [76-78].
Conclusions
The present review presents recent data on the role of regulatory transcription factors at different stages of placental development during pregnancy. Nuclear transcription factors, including LXRs, PPARs, and SREBPs, control fetal placental growth and development either individually or together. Early detection of LXRα and LXRß in HP suggested their involvement in choriovitelline and chorioallantoic placentation. The higher expression levels of LXRα in the human amniotic membrane play a crucial role in several mechanisms occurring in the placenta, such as hormone production, fetal-maternal tolerance, and lipid metabolism. PPARϒ expressed in the placenta is essential for placental development, trophoblast invasion, differentiation of cytotrophoblast into syncytiotrophoblast, and regulation of fat accumulation in trophoblasts. SREBP plays a role in the regulation of tissue differentiation, lipid homeostasis, inflammation, and apoptosis. It is an altered function of the placenta and may contribute to the development of disease (i.e., PE). These receptors are also FA sensors and may be involved in placental FA transport. This review also shows the existence of several membrane transport/binding proteins involved in the uptake of PUFAs. These include FAT/CD36, FATP, FABP, and CAV.
References
- Nilson AK, Lofquist C, Najim S, Helgreen G, Sävman K, et al. (2018) Long-chain polyunsaturated fatty acids decline rapidly in milk from mothers delivering extremely preterm indicating the need for supplementation. Acta Paediatrica 107: 1020-1027. [Crossref]
- Delplanque B, Gibson R, Koletzko B, Lapillonne A, Strandvik B (2015) Lipid Quality in Infant Nutrition: Current Knowledge and Future Opportunities. J Pediatr Gastroenterol Nutr 61: 8-17. [Crossref]
- Jacobson JL, Jacobson SW, Muckle G, Kaplan-Estrin M, Ayotte P, et al. (2008) Beneficial effects of a polyunsaturated fatty acid on infant development: evidence from the inuit of arctic Quebec. J Pediatr 152: 356-364. [Crossref]
- Smith SL, Rouse CA (2017) Docosahexaenoic acid and preterm infant. Matern Health Neonatal Perinatol 3: 22. [Crossref]
- Robinson DT, Martin CR (2017) Fatty acid requirements for the preterm infant. Sem Fetal Neonatal Med 22: 8-14. [Crossref]
- Qawasmi A, Landeros-Weisenberger A, Bloch MH (2013) Meta-analysis of LCPUFA supplementation of infant formula and visual acuity. Pediatrics 131: e262-272. [Crossref]
- Wang Q, Cui Q, Yan C (2016) The effect of supplementation of long-chain polyunsaturated fatty acids during lactation on neurodevelopmental outcomes of preterm infant from infancy to school age: a systematic review and meta-analysis. Pediatr Neurol 59: 54-61. [Crossref]
- Tam EW, Chau V, Barkovich AJ, Ferriero DM, Miller SP, et al. (2016) Early postnatal docosahexaenoic acid levels and improved preterm brain development. Pediatr Res 79: 728-730. [Crossref]
- Lapillonne A, Groh-Wargo S, Gonzalez CH, Uauy R (2013) Lipid needs of preterm infants: updated recommendations. J Pediatr 162: S37-S47. [Crossref]
- Innis SM (2014) Impact of maternal diet on human milk composition and neurological development of infants. Am J Clin Nutr 99: 734S-741S. [Crossref]
- Maas C, Franz AR, Shunova A, Mathes M, Bleeker C, et al. (2017) Choline and polyunsaturated fatty acids in preterm infants' maternal milk. Eur J Nutr 56: 1733-1742. [Crossref]
- Argov-Argaman N, Mandel D, Lubetzky R, Hausman Kedem M, Cohen BC, et al. (2017) Human milk fatty acids composition is affected by maternal age. J Matern Fetal Neonatal Med 30: 34-37. [Crossref]
- Bokor S, Koletzko B, Decsi T (2007) Systematic review of fatty acid composition of human milk from mothers of preterm compared to full-term infants. Ann Nutr Metab 51: 550-556. [Crossref]
- Granot E, Ishay-Gigi K, Malaach L, Flidel-Rimon O (2016) Is there a difference in breast milk fatty acid composition of mothers of preterm and term infants? J Matern Fetal Neonatal Med 29: 832-835. [Crossref]
- Cetin I, Alvino G, Cardellicchio M (2009) Long chain fatty acids and dietary fats in fetal nutrition. J Physiol 587: 3441-3451. [Crossref]
- Alessandri JM, Guesnet P, Vancassel S, Astorg P, Denis I, et al. (2004) Polyunsaturated fatty acids in the central nervous system: evolution of concepts and nutritional implications throughout life. Reprod Nutr Dev 44: 509-538. [Crossref]
- Alessandri JM, Guesnet P. (2005) Multiple facets of membrane lipids and the diversity of their action mode with special emphasis on the central nervous system. Reprod Nutr Dev 45: 529-533.
- Burdge G (2004) Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr Opin Clin Nutr Metab Care 7: 137-44. [Crossref]
- Niu SL, Mitchell DC, Lim SY, Kim HY, Salem N Jr, et al. (2004) Reduced G protein-coupled signaling efficiency in retinal rod outer segments in response to n-3 fatty acid deficiency. J Biol Chem 279: 31098-31104. [Crossref]
- Boyce JA. (2005) Eicosanoid mediators of mast cells: receptors, regulation of synthesis, and pathobiologic implications. Chem Immunol Allergy 87: 59-79. [Crossref]
- Sethi G, Shanmugam MK, Kumar AP (2017) SREBP-1c as a molecular bridge between lipogenesis and cell cycle progression of clear cell renal carcinoma. Biosc Reports 37: 20171270. [Crossref]
- McMullen PD, Bhattacharya S, Woods CG, Sun B, Yarborough K, et al. (2014) A map of the PPARα transcription regulatory network for primary human hepatocytes. Chem Biol Interact 209: 14-24. [Crossref]
- Clarke SD (2001) Polyunsaturated fatty acid regulation of gene transcription: a molecular mechanism to improve the metabolic syndrome. J Nutr 131: 1129-1132. [Crossref]
- Jump DB, Botolin D, Wang Y, Xu J, Christian B, et al. (2005) Fatty acid regulation of hepatic gene transcription. J Nutr 135: 2503-2506. [Crossref]
- Helland IB, Saugstad OD, Smith L, Saarem K, Solvoll K, et al. (2001) Similar effects on infants of n-3 and n-6 fatty acids supplementation to pregnant and lactating women. Pediatr 108: e82; 1-10. [Crossref]
- Liu JJ, Green P, Mann JJ, Rapoport SI, Sublette ME (2015) Pathways of polyunsaturated fatty acid utilization: implications for brain function in neuropsychiatric health and disease. Brain Res 1597: 220-246. [Crossref]
- Zhang W, Chen R, Yang T, Xu N, Chen J, et al. (2018) Fatty acid transporting proteins: Roles in brain development, aging, and stroke. Prost Leukot Essent Fatty Acids 136: 35-45. [Crossref]
- Kagawa Y, Yasumoto Y, Sharifi K, Ebrahimi M, Islam A, et al. (2015) Fatty acid-binding protein 7 regulates function of caveolae in astrocytes through expression of caveolin-1. Glia 63: 780-794. [Crossref]
- Yang Y-R, Xiong X-Y, Liu J, Wu L-R, Zhong QI, et al. (2017) Mfsd2a (Major facilitator superfamily domain containing 2a) attenuates intracerebral hemorrhage-induced blood-brain barrier disruption by inhibiting vesicular trancytosis. J Am Heart Assoc 6: e005811. [Crossref]
- Smith WL, Murphy RC. The eicosanoids: cyclooxygenase, lipoxygenase, and epoxygenase pathways. In D. E. Vance and J. E. Vance (eds): Chapter 13 : Biochemistry of lipids, lipoproteins and membranes. 2016, p1-31:(Sixth Edn).
- Narumiya S (2007) Physiology and pathophysiology of prostanoid receptors. Proc Japn Acad Ser Biophys Sci 83: 296-319. [Crossref]
- Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteed HH (2004) Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol 36: 1187-1205. [Crossref]
- Forman BM, Tontonoz P, Chen J, Brun RP, Spiegeman BM, et al. (1995) 15-deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 83: 803-812. [Crossref]
- Hata AN, Breyer RM (2004) Pharmacology and signaling of prostaglandin receptors; multiple roles in inflammation and immune modulation. Pharmacol Ther 103: 147-166. [Crossref]
- Sarah GH, Richard PP (2002) Prostaglandin D2, its metabolite 15-d-PGJ2, and peroxisome proliferator activated receptor-ϒ agonist induce apoptosis in transformed, but not normal human T lineage cells. Immunol 105: 23-34. [Crossref]
- Ricote M, Li AC, Wilson TM, Kelly CJ, Glass CK (1998) The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391: 79-82. [Crossref]
- Offermans S (2006) Activation of platelet function through G protein-couplet receptors. Circulation Res 99: 1293-1304. [Crossref]
- Harris SG, Phipps RP. (2002) Prostaglandins D2 its metabolite 15-d-PGJ2, and peroxisome proliferator activated receptorϒ agonists induce apoptosis in transformed, but not normal, human T lineage Cells. Immunol 105: 23-34. [Crossref]
- Serhan CN, Levy BD (2018) Resolvins in inflammation: emergence of the prosolving superfamily of mediators. J Clin Investig 128: 2567-2569. [Crossref]
- Elliot E, Hanson CK, Anderson-Berry AL, Norgren TM (2017) The role of specialized pro-resolving mediators in maternal-fetal health. Prost Leukot Essent Fatty Acids 126: 98-104. [Crossref]
- Ariel A, Serhan CN (2012) New Lives Given by Cell Death: Macrophage Differentiation Following Their Encounter with Apoptotic Leukocytes during the Resolution of Inflammation. Front Immunol 3: 4. [Crossref]
- Duvall MG, Levy BD (2016) DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur J Pharmacol 785:144-155. [Crossref]
- Hwang SM, Chung G, Kim YH, Park CK (2019) The Role of Maresins in Inflammatory Pain: Function of Macrophages in Wound Regeneration. Int J Mol Sci 20: 5849. [Crossref]
- Zhang MJ, Spite M (2012) Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu Rev Nutr 32: 203-227. [Crossref]
- Li C, Wu X, Liu S, Shen D, Zhu J, et al. (2020) Role of Resolvins in the Inflammatory Resolution of Neurological Diseases. Front Pharmacol 11: 612. [Crossref]
- Chandrasekharan JA, Sharma-Walia N (2015) Lipoxins: nature's way to resolve inflammation. J Inflamm Res 8: 181-192. [Crossref]
- Videla LA, Vargas R, Valenzuela R, Muñoz P, Corbari A, et al. (2019) Combined administration of docosahexaenoic acid and thyroid hormone synergistically enhances rat liver levels of resolvins RvD1 and RvD2. Prostaglandins Leukot Essent Fatty Acids 140: 42-46. [Crossref]
- Sato M, Aoki-Saito H, Fukuda H, Ikeda H, Koga Y, et al. (2019) Resolvin E3 attenuates allergic airway inflammation via the interleukin-23-interleukin-17A pathway. FASEB J 33: 12750-12759. [Crossref]
- Gilligan MM, Gartung A, Sulciner ML, Norris PC, Sukhatme VP, et al. (2019) Aspirin-triggered proresolving mediators stimulate resolution in cancer. Proc Natl Acad Sci U S A 116: 6292-6297. [Crossref]
- Choi G, Hwang SW (2016) Modulation of the Activities of Neuronal Ion Channels by Fatty Acid-Derived Pro-Resolvents. Front Physiol 7: 523. [Crossref]
- Aursnes M, Tungen JE, Vik A, Colas R, Cheng CY, et al. (2014) Total synthesis of the lipid mediator PD1n-3 DPA: configurational assignments and anti-inflammatory and pro-resolving actions. J Nat Prod 77: 910-916. [Crossref]
- Kytikova O, Novgorodtseva T, Denisenko Y, Antonyuk M, Gvozdenko T (2019) Pro-Resolving Lipid Mediators in the Pathophysiology of Asthma. Medicina (Kaunas) 55: 284. [Crossref]
- Serhan CN (2017) Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J 31: 1273-1288. [Crossref]
- Norwitz ER (2006) Defective implantation and placentation: laying the blueprint for pregnancy complications. Reprod Biomed Online 13: 591-599. [Crossref]
- Comptour A, Rouzaire M, Belville C, Bouvier D, Gallot D, et al. (2016) Nuclear retinoid receptors and pregnancy: placental transfer, functions, and pharmacological aspects. Cell Mol Life Sci 73: 3823-3837. [Crossref]
- Marceau G, Volle DH, Gallot D, Mangelsdorf DJ, Sapin V, et al. (2005) Placental expression of the nuclear receptors for oxysterols LXRalpha and LXRbeta during mouse and human development. Anat Rec A Discov Mol Cell Evol Biol 283: 175-181. [Crossref]
- Duttaroy AK (2009) Transport of fatty acids across the human placenta: a review. Prog Lipid Res 48: 52-61. [Crossref]
- Mattern HM, Raikar LS, Hardin CD (22009) The effect of caveolin-1 (Cav-1) on fatty acid uptake and CD36 localization and lipotoxicity in vascular smooth muscle (VSM) cells. Int J Physiol Pathophysiol Pharmacol 1: 1-14. [Crossref]
- Gil-Sánchez A, Demmelmair H, Parrilla JJ, Koletzko B, Larqué E (2011) Mechanisms involved in the selective transfer of long chain polyunsaturated Fatty acids to the fetus. Front Genet 2: 57. [Crossref]
- Tzeng J, Byun J, Park JY, Yamamoto T, Schesing K, et al. (2015) An Ideal PPAR Response Element Bound to and Activated by PPARα. PLoS One 10: e0134996. [Crossref]
- Jianhua L, Xueqin M, Jifen H (2016) Expression and clinical significance of LXRα and SREBP-1c in placentas of preeclampsia. Open Med (Wars) 11: 292-296. [Crossref]
- Matsuda S, Kobayashi M, Kitagishi Y (2013) Expression and Function of PPARs in Placenta. PPAR Res 2013: 256508. [Crossref]
- Duttaroy AK (2004) Fetal growth and development: roles of fatty acid transport proteins and nuclear transcription factors in human placenta. Indian J Exp Biol 42: 747-757. [Crossref]
- Díaz M, Bassols J, López-Bermejo A, Gómez-Roig MD, de Zegher F, et al. (2012) Placental expression of peroxisome proliferator-activated receptor γ (PPARγ): relation to placental and fetal growth. J Clin Endocrinol Metab 97: E1468-E1472. [Crossref]
- Pettinelli P, Del Pozo T, Araya J, Rodrigo R, Araya AV, et al. (2009) Enhancement in liver SREBP-1c/PPAR-alpha ratio and steatosis in obese patients: correlations with insulin resistance and n-3 long-chain polyunsaturated fatty acid depletion. Biochim Biophys Acta 1792: 1080-1086. [Crossref]
- Vasarhelyi B, Cseh A, Kocsis I, Treszl A, Györffy B, et al. (2006) Three mechanisms in the pathogenesis of pre-eclampsia suggested by over-represented transcription factor-binding sites detected with comparative promoter analysis. Mol Hum Rep 12: 31-34.
- Schaiff WT, Barak Y, Sadovsky Y (2006) The pleiotropic function of PPAR gamma in the placenta. Mol Cell Endocrinol 249: 10-15. [Crossref]
- Papacleovoulou G, Abu-Hayyeh S, Williamson C (2011) Nuclear receptor-driven alterations in bile acid and lipid metabolic pathways during gestation. Biochim Biophys Acta 1812: 879-887. [Crossref]
- Innis SM (2005) Essential fatty acid transfer and fetal development. Placenta 26: S70-75. [Crossref]
- Madrazo JA, Kelly DP (2008) The PPAR trio: regulators of myocardial energy metabolism in health and disease. J Mol Cell Cardiol 44: 968-975. [Crossref]
- Pawlak M, Lefebvre P, Staels B (2015) Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol 62: 720-733. [Crossref]
- Thorburn GD (1991) The placenta, prostaglandins and parturition: a review. Reprod Fertil Dev 3: 277-294. [Crossref]
- Haggarty P (2004) Effect of placental function on fatty acid requirements during pregnancy. Eur J Clin Nutr 58: 1559-1570. [Crossref]
- Lauritzen L, Hansen HS, Jørgensen MH, Michaelsen KF (2001) The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res 40: 1-94. [Crossref]
- Hadley KB, Ryan AS, Forsyth S, Gautier S, Salem N Jr. (2016) The Essentiality of Arachidonic Acid in Infant Development. Nutrients 8: 216. [Crossref]
- Guesnet P, Alessandri JM (2011) Docosahexaenoic acid (DHA) and the developing central nervous system (CNS) - Implications for dietary recommendations. Biochimie 93: 7-12. [Crossref]
- Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, et al. (2007) Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr 85: 1457-1464. [Crossref]
- Larqué E, Ruiz-Palacios M, Koletzko B (2013) Placental regulation of fetal nutrient supply. Curr Opin Clin Nutr Metab Care 16: 292-297. [Crossref]





