A serum ferritin concentration of <30 µg/L is the most sensitive and specific test for the identification of iron deficiency in patients with or without anemia. However, patients may be iron deficient at much higher concentrations of ferritin. Iron deficiency without anemia and with normal red blood count is a clinical challenge, and many patients have been diagnosed with a multitude of conditions ranging from hypothyroidism to depression to chronic fatigue syndrome over the years when they have sought help for their often debilitating symptoms. The key to a correct diagnosis are assessment of the serum ferritin concentration and a meticulous medical history focusing on the possibility of life-long blood losses and diseases such as celiac disease. Differential diagnostic causes for the symptoms must be sought for. The mainstay of therapy is oral iron in sufficient doses for at least 6 to 9 moths together with serum ferritin monitoring. Some patients who do not respond to oral iron treatment may need intravenous iron. The longer the iron deficiency has lasted, the more challenging the therapy may be. Some iron deficient patients without anemia may have had the condition for over a decade, and may not fully recover. The amount of human suffering, the loss of quality of life and the indirect costs to society caused by iron deficiency are huge.
Iron deficiency is the most common nutritional deficiency. Several studies in Western countries have shown that 3–9% of children have iron deficiency before puberty. Some 11–33% of young women have iron deficiency after menarche, and 3.5–13% of males are iron deficient after having passed the growth spurt in adolescence. The prevalence of iron deficiency is constantly high, at 9–22%, among menstruating women, but among adult males the prevalence settles to around 1–2%. After menopause, the prevalence of iron deficiency among females approaches the prevalence of males and is 1.4–4% [1-3]. It has been estimated that 25–40% of females have iron deficiency anemia at some stage in their life [3,4]. Still, iron deficiency without anemia is much more common than iron deficiency anemia.
Excluding major blood loss, iron deficiency ensues as the end result of a long period of negative iron balance, i.e., when iron losses exceed iron intake or there are increased demands [5,6]. First, iron stores are gradually and progressively depleted and only then anemia may develop. Clinical data is emerging and showing that many patients may remain in prelatent or latent stages of iron deficiency without progressing to anemia [2,3,7-9].
During my 30-year carrier as a consulting internist I have met hundreds of patients, mainly menstruating females, who have sought medical advice for prolonged (1–35 years) fatigue, brain fog, muscle and joint pains, weight gain, headache, dyspnea, palpitations (sometimes associated with sleep disturbances), arrhythmia, lump in the throat or difficulty in swallowing or restless legs. Over time, the patients have often received a spectrum of diagnoses and corresponding treatments: subclinical hypothyroidism, fibromyalgia, burnout, overtraining, asthma, somber mood extending from melancholy to severe therapy-resistant depression, chronic fatigue syndrome and chronic Lyme disease. It is important to include iron deficiency without anemia as a differential diagnostic possibility, because this type of iron deficiency is very often associated with symptoms that severely impair the patient’s performance and quality of life and may even hinder the patient from overcoming the ordinary challenges of everyday life and may cause permanent disability.
Evaluation of iron balance
The body contains about 3–4 grams of iron, most of which resides in the iron stores of the body and bound to hemoglobin, muscle tissue and enzyme proteins of metabolism.
Males lose, on average, 1 mg and women 2 mg of iron daily as the surface of the skin and mucous membranes of the gut are sloughed off; among women, menstrual bleedings account for the higher loss of iron. These losses are compensated for by increased iron absorption from the small intestine. Of a daily intake of 10–14 mg of iron, 5–15% (or about 0.5–2.0 mg) is absorbed. This takes place regardless of the daily liberation and reuse of 20–25 mg of iron by the body. A regular occidental diet does not always provide a sufficient amount of iron. This is the case especially at times of body growth, pregnancy, hemorrhage (e.g., heavy or prolonged menstrual bleeding) or high-level physical training [3,7]. One milliliter of blood contains about 0.5 mg of iron. At times of enhanced iron needs, the body may increase iron absorption, provided that iron is available in the diet. The absorption of iron from the gut into the body compartments is regulated by complicated physiological mechanisms [1,3,10].
Iron deficiency anemia
Anemia is defined as reduction of the blood hemoglobin concentration to less than 120 g/L for women and less than 130 g/L for men [11]. There are several forms of anemia and these cannot be differentiated from each other by the hemoglobin value alone. The most common form of anemia is, however, iron deficiency anemia and iron deficiency must always be considered in the diagnosis of anemia. Iron staining of a bone marrow aspiration sample is an invaluable method to assess iron stores, but the procedure is often not easily available (Table 1).
Table 1. Different stages of iron deficiency (*A significant part of these patients may never progress to overt anemia).
|
Prelatent |
Latent* |
Preanemia* |
Anemia |
Hemoglobin |
Normal |
Normal |
Often reduced but within the reference range |
Reduced |
Bone marrow iron |
Reduced |
Reduced |
Absent |
Absent |
Serum ferritin |
Reduced |
<50-70 μg/l |
<30 μg/l |
<15 μg/l |
Transferrin saturation |
Normal, >30% |
Normal, 20–30% |
15–20% |
<15% |
Serum transferrin receptor |
Normal |
Normal |
Often increased |
Increased |
Mean corpuscular volume/ hemoglobin concentration |
Normal |
Normal but close to lower limit of reference |
Slightly reduced |
Unequivocally reduced |
Symptoms |
May exist |
Common |
Common and may be debilitating |
Classical symptoms of anemia |
Iron constitutes an essential cofactor of tens of enzymes in our body, and the activity of these enzymes is reduced in iron deficiency. Iron is required for these enzymes to work properly. These enzymes, e.g., cytochrome c, cytochrome oxidase, α-glycorophosphate oxidase, succinic dehydrogenase, thyroid peroxidase and aconitase catalyze at least 180 biochemical reactions necessary for energy metabolism, brain function (e.g., dopamine metabolism, conduction of nerve impulses) and thyroid hormone synthesis [2,3,12,13]. This helps us to understand why the symptoms of iron deficiency are so extremely variable. Still, it is surprising that some more or less symptom-free patients with proven iron deficiency of long duration experience dramatic exacerbation of the symptoms of iron deficiency in connection with an acute condition or stress.
For the assessment of anemia, the blood count must always be reviewed, addition to the blood hemoglobin concentration [5,6]. A reduced erythrocyte size (E-MCV or median corpuscular volume) <80 fl and/or mean corpuscular hemoglobin (E-MCH) <27 pg indicate iron deficiency anemia. Iron deficiency anemia usually emerges gradually, and it is more important to follow the changes of the hemoglobin concentration and the other blood count values over time than just to evaluate a single blood count sample. Modern electronic patient charts make such an evaluation over time a rather simple endeavor. In a patient with symptoms, blood count changes in the direction of iron deficiency may imply the presence of iron deficiency even years before the stringent criteria of iron deficiency anemia are fulfilled. Because of the slow progression, especially young patients may have extremely low hemoglobin values – even as low as 40–60 g/L – at the time when iron deficiency anemia is diagnosed.
Ferritin
Ferritin is the name of a family of complex molecules which bind iron [3]. One ferritin molecule may bind up to 4,500 iron atoms. The correlation between serum ferritin and body storage iron levels is strong. Almost one third of the body iron is bound to ferritin, and thus changes in the body’s iron stores are reflected by the concentration of ferritin in the serum. The ferritin concentration rises also in inflammatory and infectious conditions. In this sense ferritin acts like the erythrocyte sedimentation rate (ESR) or the concentration of C-reactive protein (CRP), but the ferritin value does not necessarily parallel the changes of these inflammation markers. It is also possible that the ferritin value is falsely high despite normal ESR and CRP values. The ferritin increases also as a result of alcohol consumption, obesity and fatty degeneration of the liver. Apparently, an individual’s serum ferritin concentration is at least partly genetically determined (Figure 1).
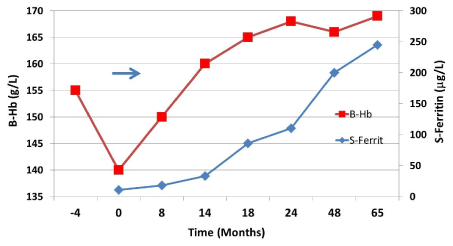
Figure 1. The hemoglobin value of a young man, age 35, had been about 165 g/l in numerous blood samples taken over several years before the consultation.
He had had rather profuse bleedings from hemorrhoids during a few years before attending (time point 0). His hemoglobin 4 months before had been slightly lower than his previous normal (154 g/l). At the time of the first appointment, the patient reported fatigue with a duration of about two months and now his hemoglobin was 141 g/l and his blood count mildly microcytic (E-MCV 81 fl) and hypochromic (E-MCH 27 pg). It was postulated that the condition was due to prolonged blood loss from the hemorrhoids and further examinations were deferred. The hemorrhoids were treated surgically, and the patient was prescribed 100–200 mg of iron daily by mouth, which he took only for less than 4 months (arrow). By that time, the patient was clinically healthy, and his hemoglobin concentration reached the pretreatment value within 2 years. He consumed a regular diet. Interestingly, the ferritin value continued to increase for 3 years, i.e., even long after the hemoglobin had reached the normal value for him. This phenomenon reflects how the iron stores were replenished over a period of time which exceeded the time to reach a normal hemoglobin value. This patient's individual ferritin concentration exceeds 250 µg/L. Iron deficiency may be corrected also if the patient follows a regular diet. Since iron deficiency moves the iron balance in a positive direction, this facilitates replenishment of iron stores, albeit very gradually.
Low ferritin (<30 µg/L) is unequivocally the best (most sensitive and most specific) indicator of iron deficiency [1,10] (Table 1). However, the patient may be iron deficient even at higher serum ferritin concentrations than 30 µg/L [14-17] (Figure 2). For pediatric patients the limits are probably only slightly lower than for adults [2,18].
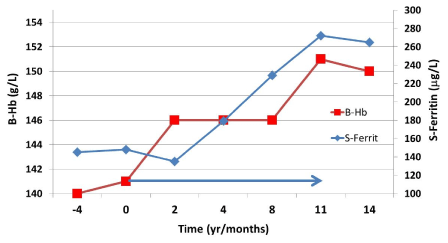
Figure 2. The female patient, age 51, had had fatigue, reduced mental awareness and restless legs for several years before her appointment.
The patient was slightly overweight and had type 2 diabetes which was treated with oral medication. Mild hepatosteatosis had been diagnosed by sonography. The patient had had her blood count and serum ferritin determined already four years previously (time point -4); the blood count, ESR and CRP were normal. She had always had profuse menstrual bleedings. At the appointment, her “normal” ferritin (~140 µg/L) was considered to be due to her liver condition, and following differential diagnostic examinations, she was started on oral iron treatment. After two months on iron treatment her condition began to improve and after about eight months, she was symptom-free. She was on oral iron treatment for 11 months (arrow, Fig. 2).
Patients with true iron deficiency anemia, as judged by a negative bone marrow iron staining finding, may have a serum ferritin concentration close to 50 µg/L [14]. Patients with the restless leg syndrome should be considered iron deficient when their ferritin concentration is <75 µg/L [19]. Furthermore, patients lacking iron stores in their bone marrow may present with serum ferritin levels of close to 100 µg/L [15]. Anker et al 2009 [16] found that iron deficiency in patients with heart failure impairs the quality of life, irrespective of the presence of anemia. They defined iron deficiency as a ferritin level <100 µg/L or, at 100–299 µg/L with transferrin saturation <20% [16].
Under ordinary clinical circumstances ferritin should not be measured when the patient has some acute febrile infection or other active inflammatory condition which is reflected as a high ESR or CRP value, because the values of ferritin and other markers of iron might be misleading. There is, on the other hand, no indication for measuring the ESR or CRP in connection with regular assessment of ferritin [18].
Soluble transferrin receptor (sTfR) and transferrin saturation (TS)
sTfR is released by erythropoietic precursors in proportion to their expansion and to the degree of iron deficiency. The sTfR value increases especially in situations when bone marrow iron stores are completely depleted [2,14,15], but not by inflammatory conditions. Thus, the sTfR is a valuable additional tool for diagnosing iron deficiency if the diagnosis is otherwise in doubt. However, if the patient has ingested even a small amount of iron the value of sTfR as a diagnostic tool may be lost (Figure 3).
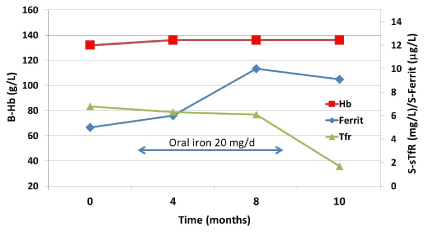
Figure 3. A small dose of iron in a young female iron deficient patient without anemia causes a very modest increase in ferritin concentration (from 5 to 9 µg/L) but decreases the soluble transferrin receptor (sTfR) concentration to the normal range.
The transferrin saturation (TS%) is calculated from fasting serum iron and transferrin concentrations. Although the serum iron concentration decreases in iron deficiency anemia, the serum iron concentration is highly volatile and it is, as such, unsuitable for the diagnosis of iron depletion [2]. The synthesis of transferrin is inversely related to iron stores, i.e., when iron stores are depleted, more transferrin is synthesized and vice versa. When the TS% falls below 15%, the supply of iron to the bone marrow is not sufficient to meet the needs for hemoglobin production [3].
Symptoms related to iron deficiency
Iron deficiency may cause a myriad of symptoms [3]. Judging only by the symptoms, the differential diagnosis ranges from hypothyroidism and depression to MS and hypochondria. Some symptoms are quite common: fatigue, headache, difficulty to concentrate, memory lapses, reduced mental awareness, absent-mindedness, dyspnea, muscle and joint pain, hair loss and weight gain. Other symptoms may be rare or very rare. For example, nail clubbing is mentioned as a symptom of iron deficiency, but in my practice, I have encountered this sign only once in a patient with iron deficiency during the last 10 years. Pica is also a rare symptom with an assumed prevalence of 1–3 per 1000 iron deficiency patients. One patient had an appetite for ice cubes and soiled potato peels, another patient disclosed having ordered clay which was then consumed. A third patient described an irresistible desire to throw herself on the floor of parking garages and lick the floor made of concrete; to her, the scent of concrete generated this exceptional urge. Various nutritional preferences do surface in conjunction with iron deficiency. A couple of patients reported a development of strong desire to rice cakes; the crunching sound when eating gave satisfaction. I still learn of new iron deficiency symptoms, which fade off as the iron deficiency is corrected by iron substitution therapy. It is a pity to hear patients recall how they have been informed that their symptoms cannot be to iron deficiency, but whose symptoms have disappeared iron therapy.
The non-anemia patients have often been extensive examined as in- and out-patients during many years and may be unable to work because of extreme fatigue and they spend most of the day in bed, and are unable to work out or have a normal life because they have no energy i.e. their quality of life is close to zero. The associated costs are also huge (Table 2).
Table 2. Symptoms described by anemic and non-anemic patients with iron deficiency.
Common |
Less common |
Exceptional fatigue* Reduced mental awareness* Absent-mindedness and poor concentration* Headache* Hair loss* Muscle and joint pain* Edema* Shortness of breath and dyspnea* High resting heart rate and rapid acceleration of heart rate during exertion; palpitations* Weight gain* Loss of initiative Memory lapses Difficulties finding words Vertigo Depression Anxiety Globus, pharyngeal irritation and phlegm, dysphagia, cough# Heartburn Cold hands and feet Diaphoresis or no sweating Restless legs# Reduce aerobic performance, myalgia associated with even slight muscle strain Sleeping disturbances Abnormal menstruation Mild pyrexia (<38oC) Dry skin and pruritus Easy bruising |
Rash Stinging tongue discomfort and pain Abdominal symptoms (which often respond to exclusion of milk and grain products from the diet) Loss of appetite Nausea Dysesthesia (pins and needles) of the extremities Weight loss Visual disturbances and blurred vision Inexplicable fluctuations in blood glucose values of diabetics (especially if treated with insulin) Irritability and anger tantrums Feeling sick Tinnitus and buzzing of the ears Muscle cramps Hot skin Poor heat tolerance Syncope / Episodes of memory loss Pale skin Low and variable blood pressure Rapid (within a few months) impairment of academic skills, e.g., in school or student life Falling asleep involuntarily Pica disorder (eating substances not suitable for human consumption)# Nail clubbing# Plummer-Vinson syndrome# |
Iron deficiency without anemia
Iron deficiency without anemia is a condition that has been known for decades [7,20,21], but it has moved into focus only in recent years [1,2,8,10,17,22-24]. Patients with iron deficiency are much more common than patients with iron deficiency anemia and the diagnosis of both of these conditions may be quite tricky. Some patients with iron deficiency without anemia may have had profound symptoms for many years while their blood count has been normal throughout. The symptoms seem to be more severe and more variable than those in patients with iron deficiency and anemia (Table 2), probably because their diagnosis of iron deficiency is more delayed than among patients with overt anemia.
Based on my personal clinical experience, about two out of three patients with iron deficiency do not have anemia nor do the MCV or MCH values even imply iron deficiency. A diagnosis of iron deficiency may be very difficult to reach under these circumstances [17]. The diagnosis of iron deficiency in these patients relies primarily on a careful and detailed patient history with special emphasis on symptoms (Table 1). This is crucial for the physician to consider the likely causes for any iron losses (Table 3). Further diagnostic hints are provided by knowing what illnesses (diagnoses) the patient has previously had or may have had, what illnesses she/he may currently have, what symptoms have become worse and what symptoms have not responded to previous treatments (Table 4). Often patients have undergone examinations over the years in specialty units at costs that may well exceed 100,000€.
Table 3. Causes of iron deficiency, Lifetime losses of blood/iron must be taken into account.
Common |
Less common |
Children: Maternal iron deficiency during pregnancy Puberty (growth burst and increased blood volume; females: menarche and menstruation) Heavy menstruation Pregnancies, blood loss at delivery Blood donations Celiac disease Ulcerative colitis and Crohn’s disease Chronic use of some analgesics Testosterone treatment |
Intestinal bleedings (polyps, tumors, occasionally hemorrhoids) Atrophic gastritis (achlorhydria) Helicobacter infection Chronic use of proton pump inhibitors Competitive athletics & runners Hemorrhage in connection with accidents and surgery Nutritional deficits Eating disorders and vegan diet Bariatric surgery Hemorrhage from tumor in urinary tract |
Table 4. Erroneous diagnoses and findings in patients with iron deficiency without anemia.
Common |
Less common |
Hypothyroidism Melancholy to severe, therapy-resistant depression Migraine Asthma Fibromyalgia Poor improvement of fitness on exercise Unremitting weight gain Heavy menstruation Esophageal reflux/heartburn Irritable bowel syndrome Heart condition without objective findings |
Overtraining Burnout Inexplicable childlessness Poor indoor air quality Osteopenia/osteoporosis Disturbed behavior (children) Ménière’s disease ADHD Incipient dementia Chronic fatigue syndrome Narcolepsy |
Iron deficiency may often be diagnosed on the basis of the serum ferritin concentration. However, for about 10% of the patients the result is equivocal. In this situation, further diagnostic support may be gained by determination of the plasma concentrations of transferrin receptor, iron and transferrin to calculate the transferrin saturation. Occasionally, knowing the concentration of serum hepcidin may be useful [3]. If these laboratory assessments are needed, the symptoms of the patients are often multifactorial in origin and not caused purely by iron deficiency. Then interpretation of these laboratory results may be a true challenge.
Differential diagnosis
The symptoms experienced by persons with iron deficiency are not pathognomonic, although the saying that “usual diseases are common” fits very well. It is important that the physician considers the etiology: What causes the iron deficiency in the patient (Table 2)? Among the common causes for iron deficiency are heavy menstrual bleedings and hemorrhages in connection with pregnancy and delivery among females and multiple blood donations, celiac disease and ulcerative colitis in both genders.
There are several conditions and illnesses with symptoms mimicking iron deficiency. Examples are hypothyroidism, profound vitamin D deficiency, vitamin B12 deficiency, celiac disease, testosterone deficiency, abnormal calcium metabolism, sleep apnea, heavy smoking and even occult malignancy [3,17]. Adequate differential diagnostic consideration is, of course, needed. There are innumerable patients with iron deficiency who have been put on thyroid hormone medication despite normal thyroid function. This is due to the fact that the symptoms of hypothyroidism and iron deficiency are very similar, and the serum thyrotropin activity tends to increase in iron deficiency [22,25]. In this setting, increased TSH-values are due to the enzyme thyroid peroxidase, a hormone that contributes to thyroid hormone synthesis. Thyroid peroxidase contains an iron moiety and in iron deficiency the function of the enzyme is disturbed. The symptoms may abate transiently when the patient takes thyroid hormone, but the iron deficiency must be corrected as soon as possible, during which time the need of thyroxin often decreases. Patients with iron deficiency tend to tolerate thyroid hormones poorly. Once the iron stores have been replenished, it is best to discontinue thyroid medication, if possible. Since both hypothyroidism and iron deficiency are common, it is by no means rare for a patient to have both conditions simultaneously, possibly even complemented with other comorbidities which further accentuate symptoms.
Treatment of iron deficiency
Although there are some recent meta-analyses on the use of iron supplementation by patients with iron deficiency but without iron deficiency anemia [8,24,26], the benefits of iron treatment remain equivocal. The study summaries find some reasons for this: the amount of daily iron supplementation was too small (less than 100 mg), the treatment duration/follow-up was too short and the ferritin goal was set too low. Despite a lack of robust scientific data, iron deficiency must be treated when diagnosed [2,17,24].
The impact of blood loss or poor iron absorption must be minimized, and blood donation is not allowed during iron deficiency. If menstrual bleedings are heavy, tranexamic acid may be considered to reduce blood loss and the patient should be advised to discuss other treatment options to reduce menstrual blood loss with her gynecologist. Often, heavy menstrual bleeding is reduced, as iron deficiency is corrected.
When the patient has iron deficiency anemia and is put on iron substitution therapy, the hemoglobin value will normalize first, and only after this will iron begin to accumulate for metabolic use and storage (Fig. 1). Interestingly, even if the iron deficient patient has not been diagnosed with anemia, a rise in the hemoglobin concentration of more than 10–20 g/L is not uncommon during iron supplementation treatment [10,26]. This is a sign of severe iron deficiency and a rise in the hemoglobin concentration on a trial of iron administration may often be used as a test to corroborate the diagnosis (diagnosis ex juvantibus). If the patient does not have iron deficiency anemia, the hemoglobin concentration will not increase during iron supplementation, nor does the hemoglobin increase above the genetically and individually determined hemoglobin value.
Iron deficiency must always be corrected initially with oral iron preparations available at pharmacies, if possible. Occasionally, this is problematic, because intolerance to oral products is individual and no single product is superior another. If needed, different products may need to be tested before a suitable one is found for the patient. The most common adverse effects of oral iron are diarrhea, constipation, abdominal pain, abdominal cramps, nausea and heartburn. Teeth discoloration and skin reactions may occur.
A common recommendation is (100–) 200 mg of oral iron daily [1,10], but also much less has been recommended [2,27]. The hemoglobin value may normalize also on a small daily dose of iron, but a low dose seldom suffices for metabolic needs or for replenishment of the iron stores of the body. Occasionally it may be more tolerable for the patient to take one iron pill every other morning, three mornings per week than to take iron pills every day [27]. The adult maximum oral dose of iron is 5 mg/kg or no more than 400 mg daily, divided into three doses. However, less than 5% of patients tolerate such a high dose. If a patient with severe iron deficiency tolerates a high daily dose of iron, it is certainly worth using although the benefit may not be clear cut.
Oral iron treatment is continuous and there is no need for keeping treatment breaks before blood sampling, except in certain specific circumstances [27]. Before blood sampling for serum iron and transferrin, a 10-hours fast (including no iron supplements) is necessary. Under certain problematic situations the iron treatment break must be at least two days.
Iron inhibits the absorption of thyroid hormones, and hence it is best to take the thyroxin tablet in the morning and the first iron pill around noon. Iron absorption is reduced by milk, calcium and magnesium products and by proton pump inhibitors. Also, coffee, tea and cereal products affect negatively the uptake of iron. At higher doses of iron intake, these diet restrictions have less importance. The impact of vitamin C as an uptake enhancer diminishes also when the iron dose increases. The role of the diet as a treatment modality of iron deficiency is generally modest, but once iron deficiency has been corrected, dietary iron is a significant iron homeostasis-maintaining factor.
The blood count and serum ferritin should be assessed 1–2 months after the start of oral iron supplementation – especially if the patient has had frank iron deficiency anemia or severe symptoms – to check that the anemia or the ferritin concentration are being corrected. One month of treatment with iron pills (100 mg twice daily) increases the hemoglobin level in iron deficiency anemia at least by 10–20 g/L [10].
The ferritin concentration might not change very much before the hemoglobin value normalizes. During oral iron treatment the serum ferritin concentration increases often by 0.5–4.0 µg/L per week of treatment, but the treatment response varies from patient to patient and depends on the amount of ingested iron and concomitant iron losses. Later, blood testing may be repeated at intervals of 2–4 months. After the anemia has been corrected, the ferritin goal is >100 µg/L, but it may be even much higher (Fig. 1). The blood count and ferritin concentration should be checked at least twice within the year following discontinuation of iron therapy [17].
The longer the iron deficiency has lasted, the more complex treatment usually becomes. This situation arises usually among patients who have never had anemia. If iron deficiency has persisted for at least 10–15 years, iron therapy, treatment follow-up and clinical response are often fraught with challenges, and the patient may need treatment and follow-up for up to two years. In my opinion, iron deficiency with a duration of more than 15–25 years may need years of treatment and follow-up, and complete recovery in terms of symptom recession is still not certain. While on therapy, these patients experience a weekly increase in serum ferritin of maybe more than 8–10 µg/L but thus may not be accompanied by clinical improvement. Paradoxically, the patient may experience symptom exacerbation after treatment start (Figure 4). Another difficult situation arises when the initial increase in ferritin is less than 0.2 µg/L per week on treatment despite an oral iron dose of 100 mg twice daily. This situation is difficult for the patient and treating physician; management requires faith and trust of both parts on each other and a reliable doctor-patient relationship [17].
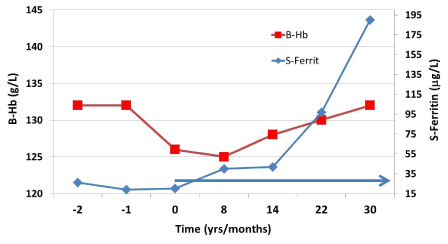
Figure 4. Judging from the patient’s history and symptoms, her iron deficiency had prevailed for more than 20 years, although both the ferritin and the hemoglobin values were available only at 2 years before the patient's first office visit (time point -2).
The hemoglobin value had been stable for at least 10 years before the patient’s visit. Appropriate differential diagnostic procedures were performed with normal results, after which iron therapy was started. After the first 14 weeks of treatment the ferritin value increased by about 0.15 µg/L per week and then by about 9 µg/l per week. The patient’s symptoms began to subside just before the follow-up visit at 7.5 months (30 weeks). The horizontal arrow indicates the time when the patient took iron pills at the same dose (200 mg/d).
Administration of iron by infusion is never the primary treatment option for symptomatic iron deficiency. If intravenous treatment is considered, this implies that treatment of the patient is a challenge [1,2,10,28]. Iron by infusion may, especially in the beginning of treatment, correct the iron deficiency and iron deficiency related symptoms slightly faster than iron by mouth, but after completion of intravenous iron therapy, follow-up continues for at least two years [17,29].
Intravenous iron is usually resorted to when the patient does not tolerate oral iron at all or at insufficient doses, and when iron deficiency is severe and a rapid treatment response is needed. Iron infusions are an exceptional treatment and there is a risk of serious allergic reactions: anaphylaxis (incidence approximately 1/10,000 infusions), serious respiratory failure (1/1000 infusions) and choking with a febrile reaction (1/100 infusions). When such reactions occur, the infusion must be stopped. Generally, iron infusion is well tolerated [30] and may be provided in any health care unit where allergic reactions can be treated and where the infusion is supervised by a physician with experience in iron infusion therapy.
However, the truly demanding phase of treatment starts after the infusion: adverse events requiring attention may occur, although they are seldom severe or longstanding (>30 days). Under optimal conditions, a treatment response may be expected in 3–30 days after the infusion, but only about 25% of the patients respond completely after the first infusion (500 mg) and about 5% of the patients need five infusions or more. The infusion should preferably not be repeated until after at least 30–60 days of the previous infusion. Problems after iron infusions tend to be the more marked the longer the iron deficiency (without anemia) has lasted.
The most dreaded complication is a situation I call “iron utilization disturbance”. It is a constellation where, after an initial increase in hemoglobin of 10-20 g/L and an above expected increase in ferritin concentration, the patient’s condition deteriorates significantly after the infusion and the ferritin level remains high. This condition may persist for weeks or months and patients must be made aware of this possibility before the infusion. I have the impression that this phenomenon occurs approximately once per 100 infusions in patients who have had iron deficiency (without anemia) for at least 10 years. The pathophysiological basis of this phenomenon is difficult to understand based on current knowledge [3].
Before starting infusion treatment it is impossible to evaluate how many infusions the patient will need for clinical response. The increase in serum ferritin induced by iron infusions is the larger the higher the ferritin has been at baseline (before infusion treatment), but the correlation is weak (Figure 5). This only shows that the serum ferritin level is not an unequivocal measure of the body’s iron stores.
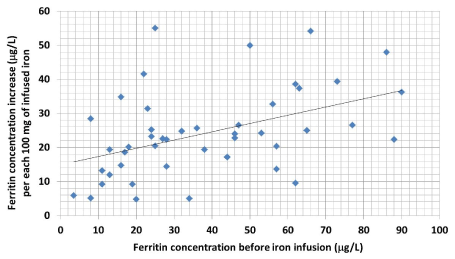
Figure 5. Rise in ferritin level per 100 mg of infused ferric carboxymaltose in relation to the ferritin level before the first infusion. The figure shows that it is not possible – using the preinfusion serum ferritin value – to estimate with certainty how well the amount of storage iron will be corrected by infusion treatment.
Iron deficiency is very common and may be ranked among the most common public health concerns today. Diagnosing iron deficiency, especially if there is no iron deficiency anemia, is a challenge for the clinician. Iron deficiency without anemia seems to be an autonomous clinical condition which needs special attention, as has been suggested earlier [9]. Clearly, we have yet much to learn about iron deficiency and iron metabolism and how they relate to the spectrum of symptoms experienced by the patient. Iron deficiency is a real and harsh disease which may lead to severe symptoms and work incapacity. The longer the duration of iron deficiency, the more difficult it is to treat. The treatment of iron deficiency is often carried out with too small doses of iron and for too short a time. The treatment response must be followed up with assessments of the blood count and the serum ferritin concentration. Follow-up must continue for at least one year after normalization of the hemoglobin and ferritin concentrations.
- Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L (2016) Iron deficiency anaemia. Lancet 387: 907-916. [Crossref]
- Clénin GE (2017) The treatment of iron deficiency without anaemia (in otherwise healthy persons). Swiss Med Wkly 147: w14434. [Crossref]
- http://docshare.tips/wintrobes-clinical-hematology-13th-edition_588de152b6d87fa7058b4aa4.html
- Looker A, Dallman P, Carrol M, Gunter E, Johnson C (1997) Prevalence of iron deficiency in the United States. JAMA 277: 973-976. [Crossref]
- Umbreit J (2005) Iron deficiency: A concise review. Am J Hematol 78: 225-231. [Crossref]
- Peyrin-Biroulet L, Williet N, Cacoub P (2015) Guidelines on the diagnosis and treatment of iron deficiency across indications: a systematic review. Am J Clin Nutr 102: 1585-1594. [Crossref]
- Sinclair LM, Hinton PS (2005) Prevalence of iron deficiency with and without anemia in recreationally active men and women. J Am Diet Assoc 105: 975-978. [Crossref]
- Yokoi K, Konomi A (2017) Iron deficiency without anaemia is a potential cause of fatigue: meta-analyses of randomised controlled trials and cross-sectional studies. Br J Nutr 117: 1422-1431. [Crossref]
- Al-Jafar HA (2017) HWA: Hypoferritinemia without anemia a hidden hematology disorder. J Family Med Prim Care 6: 69-72. [Crossref]
- Camaschella C (2015) Iron-deficiency anemia. N Engl J Med 372: 1832-1843. [Crossref]
- (http://www.who.int/vmnis/indicators/haemoglobin.
- Kim J, Wessling-Resnick M (2014) Iron and mechanism of emotional behavior. J Nutr Biochem 25: 1101-1107. [Crossref]
- Pino JMV, da Luz MHM, Antunes HKM (2017) Iron-restricted diet affects brain ferritin levels, dopamine metabolism and cellular prion protein in a region-specific manner. Front Mol Neurosci 10: 145-161. [Crossref]
- Punnonen K, Irjala K, Rajamäki A (1997) Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood 89: 1052-1057. [Crossref]
- Mast AE, Blinder MA, Gronowski AM, Chumley C, Scott MG (1998) Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem 44: 45-51. [Crossref]
- Anker SD, Comin Colet J, Filippatos G (2009) Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 361: 2436-2448. [Crossref]
- Soppi E (2018) Iron deficiency without anaemia - a clinical challenge. Clin Case Rep 6: 1082-1086. [Crossref]
- Oatley H, Borkhoff CM, Chen S (2018) TARGet Kids! Collaboration. Screening for iron deficiency in early childhood using serum ferritin in the primary care setting. Pediatrics 142: 6.
- Picchietti DL, Hensley JG, Bainbridge JL, Lee KA, Manconi M (2015) Consensus clinical practice guidelines for the diagnosis and treatment of restless legs syndrome/willis-ekbom disease during pregnancy and lactation. Sleep Med Rev 22: 64-77. [Crossref]
- Dallman PR, Beutler E, Finch CA (1978) Effects of iron deficiency exclusive of anaemia. Br J Haematol 40: 179-184. [Crossref]
- Sawada T, Konomi A, Yokoi K (2014) Iron deficiency without anemia is associated with anger and fatigue in young Japanese women. Biol Trace Elem Res 159: 22-31. [Crossref]
- Soppi E (2015) Iron deficiency is the main cause of symptom persistence in patients treated for hypothyroidism. Thyroid 25: A-74.
- Johnson S, Lang A, Sturm M, O'Brien SH (2016) Iron deficiency without anemia: A common yet under-recognized diagnosis in young women with heavy menstrual bleeding. J Pediatr Adolesc Gynecol 29: 628-631. [Crossref]
- Houston BL, Hurrie D, Graham J (2018) Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron deficient adults: a systematic review of randomized controlled trials. BMJ Open 8: e019240. [Crossref]
- Ravanbod M, Asadipooya K, Kalantarhormozi M, Nabipour I, Omrani GR (2013) Treatment of iron deficiency anemia in patients with subclinical hypothyroidism. Am J Med 126: 420-424. [Crossref]
- Pasricha SR, Low M, Thompson J (2014) Iron supplementation benefits physical performance in women of reproductive age: a systematic review and meta-analysis. J Nutr 144: 906-914. [Crossref]
- Moretti D, Goede JS, Zeder C (2015) Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 126: 1981-1989. [Crossref]
- Krayenbuehl PA, Battegay E, Breymann C (2011) Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood 118: 3222-3227. [Crossref]
- Sharma R, Koch TL, O'Brien SH (2014) Effect of intravenous iron therapy on quality of life in non-anemic iron-deficient young women with fatigue. Blood 124: 4858.
- Aksan A, Isik H, Radeke HH, Dignass A, Stein J (2017) Systematic review with network meta-analysis: comparative efficacy and tolerability of different intravenous iron formulations for the treatment of iron deficiency anaemia in patients with inflammatory bowel disease. Aliment Pharmacol Ther 45: 1303-1318. [Crossref]





