In this abstract we review the pancreatic cancer epigenetic mark up and the impact of related therapies on preclinical and clinical outcome. We explore the potentials of combination therapies with targeted drugs in a protocol called multitargeted epigenetic therapies (MTET) with a specific focus on intratumoral hypoxia and angiogenesis. We share our invitro data as well as a review of case studies of advanced pancreatic cancer treated with such protocol and we conclude that such combination therapy appears to be superior in the cases studied to the standard of care alone. Further clinical trials are needed to support such hypothesis and validate the companion diagnostics associated with the therapy.
Background: Pathogenesis of pancreatic cancer has been attributed solely to genetic mutations; however, recent advancements in the epigenetic science have demonstrated that aberrant activation of epigenetic pathways contributes significantly to the pathogenesis of the disease. The identification of these aberrant activated epigenetic pathways has revealed enticing targets for the use of epigenetic inhibitors to mitigate the phenotypic changes driven by these cascades. These targets include EZH2, the histone methyltransferase G9a/GLP. The role of intratumoral hypoxia and creation of 3 dimensional spheroids is critical for pancreatic cancer growth and dissemination. These pathways have been found to be responsible for overactivation of growth signaling pathways and silencing of tumor suppressors and other cell cycle checkpoints.
Epigenetics is defined as the type of inheritance not based on a particular DNA sequence but rather traits that are passed to the next generation via DNA and histone modifications as well as microRNA-dependent mechanisms. Key tumor suppressors that are well established to play a role in PDAC may be altered through hypermethylation, and oncogenes can be upregulated secondary to permissive histone modifications. The potential to “reprogram” cancer cells to revert them to a healthy state presents great promise and merits further investigation. Current studies have attributed the rapid progression of the disease to epigenetic changes such as DNA methylation alterations and histone tail modifications. HDAC inhibitors have been used successfully both to mitigate therapeutic resistance in cancer cells as well as to manipulate the microenvironment of the tumor cells increasing their sensitivity to standard chemotherapeutics.
pancreatic cancer, precision medicine, epigenetic therapies, liquid biopsy
A combination of histone deacetylase inhibitors and DNA demethylators were applied under MTET protocol to the pancreatic cell lines in 3-dimensional cell culture model. A viability assay demonstrated significant reduction of cell migration and viability. Formulations for compound X defined as polyphenols (QC). Please see below images. Further patients were treated with similar combination and ratio of drugs and their response measured through serial circulating DNA assays by Guardant 360 Laboratory. 8 cases of advanced pancreatic cancer with or without intra/ extrahepatic metastasis were randomly selected and their outcome was evaluated using a biomarker dependent assay with liquid biopsy. These cases will be discussed in full detail in this article. All 8 cases passed their expected survival with this regimen of therapy. One had complete remission and cured.
Circulating tumor DNA (ctDNA) analysis was performed via Guardant360 as previously described. Briefly, two 10 mL tubes of blood were collected in Streck Cell-Free DNA BCT (Streck, Inc.). Plasma was isolated via centrifuge, and cfDNA was extracted from the plasma and labeled wth nonrandom oligonucleotide barcodes (IDT, Inc.). This cfDNA was then used to prepare sequence libraries which were enriched by hybrid capture (Agilent Technologies, Inc.) and sequence by paired0end synthesis (NextSeq 500 and/or HiSeq 2500, Illumina, Inc.). Variant analyses were performed using the locked Guardant360 bioinformatics pipeline and reported unaltered by post hoc analyses. All decision thresholds were determined. Base call files generated by Illumina's RTA software (v2.12) were demultiplexed using bcl2fastq (v2.19) and processed with a custom pipeline for molecule barcode detection, sequencing adapter trimming, and base quality trimming.
Both invitro and in vivo results were consistent with the proposed hypothesis. There was significant tumor inhibition reported in the 3-dimensional cell culture model (Figure 1).
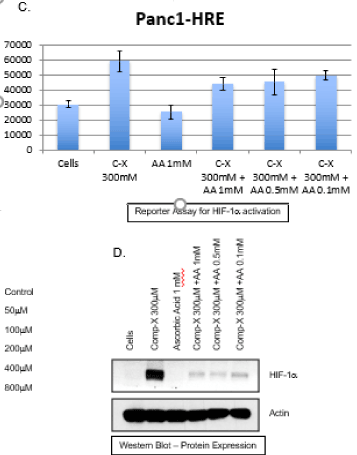
Figure 1. 3-dimensional cell culture model for pancreatic cell Pnc1- HRE and response to the MTET therapy.
There was also significant improvement in tumor burden and markers for tumor dissemination manifested by both imaging studies and labs, post therapy. The decline in markers were reported in 2 weeks post therapy. Liquid biopsy by measuring circulating DNA, (through Guardant 360 lab) was performed to confirm tumor burden response.
Case 1: 70 years old female with Stage II locally advanced pancreatic CA with obstructive pattern, increased bilirubin, diagnosed and consulted for Chemotherapy in August 2017, refused such option, at Mayo clinic.
Upon her arrival, she had pain and fatigues, unable to perform her ADLs, and her initial labs showed that she had high liver enzymes, Lyme titers, and tumor markers.
Immediately she was started on daily IV epigenetic therapies. She had a great response to the therapies provided here manifested by her improved function and quality of life.
Her bilirubin dropped down to 2.2 from 2.9, her CEA dropped from 7.6 to 6.2, her liver enzymes normalized. Her albumin increased from 3.2 to 3.4. Her ALK-P dropped from 140 to 86, Her serum Her2 level dropped from 34 to 27. Her western blot Lyme titers reduced (IgG P 58 and 23 non detectable post therapy). Her QOL drastically improved. Her neurogenic bladder recovered with the treatment, as a result she was able to empty her bladder again. This is extremely rare to see any recovery from spinal neuroborreliosis. Her lumbosacral MRI confirmed there was no sign of cord compression or spinal stenosis, dated 9/2/17.
Her labs were repeated again on 9/21/17 and it showed complete resolution and response of CTC. Her circulating DNA was undetectable post therapy.
Further she started metronomic dose of capecitabine at 500 mg twice a day in combination with IV epigenetic therapies. Her scan was repeated on 10/3/17 which showed resolution of the pancreatic mass around the stent as well as biliary tract. The inflammation in other areas of pancreas as well had resolved. There were no new lesions seen. The pancreatic mass also was stable.
This magnitude of response continued following therapies, as a result patient exceeded her life expectancy by 2 years.
Case 2: 60 years old male with history of stage four pancreatic cancer who was diagnosed in 9/2017, status post abdominal pain, with adenocarcinoma of pancreas metastasized to his liver, exploring any potential therapies, as his oncologist had given him less than 3 months to live and referred him to hospice. He was seen at our clinic, and initial labs drawn which showed increased tumor markers CEA, CA 19.9 as well as angiogenesis markers, VEGF and IL-8. He was immediately started on daily IV epigenetic therapies which he received for two weeks when his labs were repeated.
Post therapy his VEGF decreased from 248 to 70. His IL-8 also dropped from 114 to 106 and 99 to 48. (plasma and serum)
His c DNA showed significant alteration in following genes (FGF2, C Myc, EGFR, PI3K, CDK4, CDK6, NF-1, KIT, TP53, PDGF, KRAS, BRCA1. These alterations did not change with taking Lynparza alone.
He did not receive any other modalities of care neither changed his diet or supplements. His quality of life improved drastically, and his pain medication requirement significantly decreased. He was able to walk again independently.
He started to do clinically well. His pain completely stopped. His lower extremities edema was improved. His appetite was significantly better. His labs show significant reduction in his VEGF from 280 down to 70 (normal). Measured on 12/8/17 and 12/29/17, respectively. His interleukin 8 also dropped from 280 down to 48.
His labs were repeated on 2/8/18 and it showed that his TGF had dropped form 9317 down to 3365. (measured on 12/8/17.
On 2/6/18 the c DNA was repeated and it showed significant reduction in his KRAS MAF (from over 50 percent down to 5.6), TP53 down from over 50 percent down to 5.1 percent, , All alteration, BRCA1, MTOR, ARID1, KIT, PDGF, FGFR2, PI3K, Myc, CDK4, CDK6 were resolved (Figure 2).
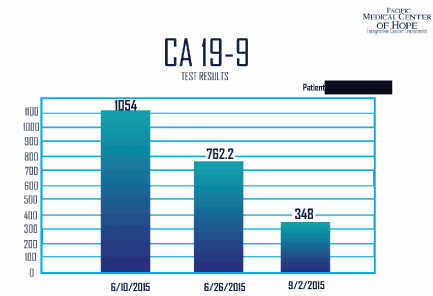
Figure 2. Pre and post therapy tumor markers.
Further he stopped the epigenetic therapies on 12/25/17, and disease progressed despite continuing his conventional therapy, Lynparza. (Lynparza alone was not sufficient to retard tumor growth). His tumor markers were checked upon his return to the clinic, and it showed CA 19.9 of 45988, measured on 2/8/18 from 8565 on 12/8/17. (This was doubled expected tumor growth when the epigenetic therapies stopped).
In summary, the tumor growth was significantly reduced when epigenetic therapies were applied in conjunction with PARP inhibition in this case. The tumor showed significant reduction in its heterogeneity as well as BRCA fingerprint in liquid biopsy confirming such conclusion.
Case 3: 64 years old male with history of pancreatic cancer with numerous liver mets, diagnosed after a prolonged course of abdominal pain and weight loss, for months. Abdominal CT is suggestive of the tumor 2.5 cm X 2.5 cm in pancreatic head along with liver mets. The mass in pancreas was entraping the vasculture and adjacent areas. He was jaundiced and had lost weight by pounds every day and lost his appetite. He was seeing Stanford oncology and had been given 5 percent chance of response to the recommended therapy which consisted of neoadjuvant chemotherapy and whipple was out of the table and the intention of therapy was palliative at best. He then decided to refer and be treated at our center. Initial evaluation showed significant pain, jaundice and elevated tumor markers. Immediately he was started on IV daily epigenetic therapies and started to show improvements in his function and ability to eat immediately after the first week. He also had no pain this time and his ECOG score improved.
We repeated the blood test looking at the tumor markers after 7 treatments. All his tumor markers dropped substantially. His CA 19.9 decreased from 110 to 80-, CEA dropped from 6.4 to 6.1, LASA (Lipid associated sialic acid dropped from 21 to 15 (normalized). His serum Her 2 also dropped from 17.3 to 11.7 (measured on 10/15/14) and further down to 9.2 (measured on 11/21/14). His PSA which also incidentally was reported to be at 7.7 dropped down to 3.7 (normalized). His CTC was repeated post therapy (purified DNA technique) which showed resolution of all CTC in serum, measured by CK20 and telomerase activity.
Then he was forced by his oncologist to start Gemzar and Abraxane per protocol, and he accepted. After in combination with epigenetic therapies. His clinical response continued with decreased tumor marker CA 19.9 measured on 11/17/14 at 64 from 80. He then decided to withhold the chemotherapies and just do the epigenetic therapies. His markers continue to drop. His CEA dropped to 2.2 (normal) from 6.4 and his CA 19.9 dropped to 30 (normal) from 107. (measured on 12/15/14).
Such an initial response in all tumor markers in a patient who has received only Epigenetic therapies, is very promising in pancreatic cancer and deserves further evaluation in clinical trials.
Case 4: 65 years old female with history of stage four pancreatic cancer status biopsy of a large 14 cm mass at the head of the pancreas, along with metastatic deposits in the liver extensive disease in pelvis as well as abdominal peritoneal carcinomatosis presented to our clinic for evaluation and treatment. The patient was complaining of loss of appetite, diarrhea and weight loss. The initial evaluation on 6/10/15, showed negative circulatory tumor cells, but positive circulatory DNA for ERBB2. The tumor markers were elevated both CA 19.9 (1054) and CEA (11.9). We immediately stared her on combination therapy with MTET (Multitargeted epigenetic therapy) that she received on daily basis. Other treatments included Firmagon and Artemisinin and cellebrex.
After five days, her symptoms relieved and she started to eat. ECOG score improved and she was able to gain weight and function normally. After two weeks, her labs were repeated and showed stable to a reduction of 20 percent in her tumor marker. His CA 19.9 dropped to 762.2 (on 6/26/15) from 2000 and 1054. On 9/2/15 her CA 19.9 had dropped down to 348 from 1054, and CEA down to 8.9 (from 11.9), (Figure 2).
Her circulatory DNA was repeated, and it showed complete resolution of the ERBB2 alteration. (Not detectable c DNA).
This response in a quick fashion can be a preliminary finding that could potentially correlate with a better outcome. We yet are awaiting to correlate the findings with her overall survival.
Case 5: 62: Patient is a 61 years old female with history of pancreatic neuroendocrine cancer diagnosed in 2006 status post FOLFOX and Avastin until 2008, with excessive toxicity ( neuropathy and weight loss), further treated with Afinitor with initially good response, status post progression of disease confirmed in her PET scan on 12/2012 which showed diffuse metastatic involvement of liver and pancreas with several abdominal and pelvic as well as mesenteric LNs, all progressed.
She was referred to us by her oncologist to be evaluated and treated with IV epigenetic protocol. Her initial labs confirmed increased pancreatic poly peptide, CA 19.9 and CA 125 as well as VEGF and Neuron specific enolase and Chromogranin A on 2/28/13.
She immediately was started on the treatment program on daily basis. Her labs were repeated on 3/12/13 after receiving 7 treatments which showed decreased VEGF from 374 to 334 and her CA 19.9 dropped slightly from 71 to 69. Her Chromogranin A also dropped from 164 to 150 from 2/28/13 to 3/12/13. Her Neuron specific enolase dropped from 525 to 229. (measured on 3/29/2013.
Her VEGF was measured on 3/29/13 and it dropped from 374 to 315. Her liver enzymes also dropped (AST from 73 to 66, and ALT from 41 to 27). Her IGF-1 dropped from 125 to 109 and her LDH from 385 to 325, all measured on 3/29/13.
Case 6: 75 years old male with history of pancreatic cancer diagnosed in 1/15 after experiencing back pain for 6 months. He had seen many alternative doctors received IV vitamin C and peroxide (3 each), had seen a surgeon who has indicated that the tumor was too advanced to be removed, (inoperable)
Referred to us for evaluation and treatments, his last CA 19.9 was reported at 7800, complaining of the back pain was taking advil daily, along with cannabois oil to relieve the pain, he refused chemotherapies. After prolonged discussions he accepted to be seen and referred to an oncologist. He is reporting a resolution of the pain in his back completely after only two sessions of IV treatments. His labs indicated his CA 19.9 dropped from 7800 down to 5300, status post 10 treatments, which he received on daily basis 5 times a week.
Other markers for cancer also dropped. His LASA (Lipid Associated Sialic Acid) dropped from 24 to normal (16). His Interleukin 8 (serum) also normalized from 68 to 34. (measured respectedly on 3/2/15 and 3/12/15).
His quality of life improved and he was able to function better and with no pain.
His Circulatory tumor analysis confirmed the response to the treatments he received in laboratory. Second analysis showed complete resolution of the CTC confirmed post therapy, measured respectedly on 3/4/15 and 3/26/15 (Figures 3&4).

Figure 3. Circulating DNA pre and post therapy.
Figure 4. Circulating tumor cells pre and post therapy
Such response in matter of just 10 treatments is valuable and suggest further needs to evaluate such method of treatment in a larger scale. The fact that the circulatory tumor cells resolved completely only after 10 treatments is extremely important pointing into improved patient survival as a surrogate marker.
Case 7: 68 years old male with history of metastatic pancreatic cancer diagnosed in March 2017, status post an episode of DVT , treated with Lovenox. He further has seen his oncologist Dr Eric Cheung who has recommended FOLFIRI, per verbal reports, further decided to switch to Gemzar and Xeloda.
He was hesitant to do cytotoxic chemotherapy, so refused and self-referred to us.
Upon his arrival, he was complaining of some mild to moderate pain in his abdomen mainly at night.
His labs were drawn, and it showed significantly elevated FGF-2 at 18 (normal less than 6.5), her liver enzymes were all elevated. His ALK-P was at 477, His AST was at 212, his ALT was at 231.
Hi abdominal CT showed a mass with the size of 5.0 cm X 4 X 3.5 cm in the tail of the pancreas as well as too many to count liver lesions in all segments.
He was immediately started on Iv epigenetic therapies, which he received on daily basis for ten treatments for two weeks. He did not receive any chemotherapy neither changed his diet, nor received any supplements.
He felt better after 3 days of treatments; his pain was alleviated. His QOL improved. His labs showed reduction in his liver enzymes. Hiss ALK-P dropped from 477 to 346, his AST to 130, his ALT to 115. His LDH also dropped from 562 to 523. His CA 27.29 dropped from 304 down to 257. His CA 15.3 dropped from 126 to 92.8. His CEA dropped from 2160 to 2000, and his TGF dropped from 8899 to 5590 (normalized) (measured on 5/30/17 and 6/9/17 respecetedly). His Transforming growth factor also dropped from 8899 down to 5590 on 6/7/17.
His Caris report confirmed presence of KRAS mutation, as well as ATM, MLH1, MSH2, PMS2, confirming the MSI.
His abdominal Ct was repeated on 6/30/17, and it showed stable tumor size at 5X4 cm compared with his prior CT of 4/23/17.
His labs were repeated again on 7/14/17 and it showed further reduction of bilirubin this time, down to 4.6, albumin increased from 2.2 to 2.7. CA 15.3 dropped from 188 to 180, CEA dropped from 3408 to 2556, LDH decreased from 509 to 433. His ECOG score was improved from 3 to 1 (dated 7/14/17). These results were obtained after total of 23 treatments.
The pedal edema was improved, and he is now able to walk independently again. His labs show improved markers, CEA has come down to 2100 (400 units dropped in 6 days). His Hemoglobin i up to 8.4 from 7.9, His Bilirubin is down to 3.7 from 4.6 in 6 days, all liver enzymes improved to almost normal range.
On 8/4/17, his labs were drawn again, and it showed continuous reduction. His Bilirubin was down to 1.9, ALK- P was down to 423, CEA down to 1266, and LDH at 344. His c DNA was repeated after 60 days, and 30 treatments, and it showed drastic results. His c DNA MAF of KRAS dropped from 35.8 percent down to 2.3 percent, TP53 dropped from 49 percent to 1 percent. Four alterations at ARID1, FGFR1, and RAF1 all became undetectable. (Figure 5).

Figure 5. circulating tumor cells pre and post therapy

Figure 6. circulating tumor cells pre and post therapy
Further his labs measured on 8/15/17 showed reduction of bilirubin to 0.8, CEA down to 305 (Figure 7).
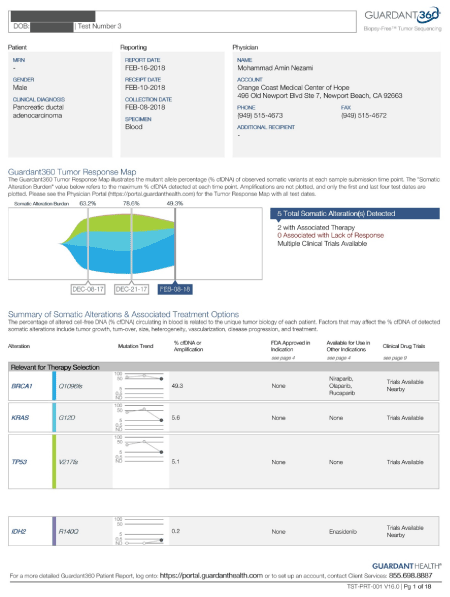
Figure 7. Circulating tumor cells pre and post therapy
His CT scan in August 2017, confirmed response to therapy with reduction of all metastatic disease in liver, as well as original tumor with 50 percent volumetric size reduction ( 5.4 cm to 3.2 cm).
This magnitude of response in a patient with positive MSI, is very exciting and can be translated into larger clinical spectrum, by randomized controlled trials.
Case 8: Pancreatic cancer status post whipple and two cycles of abraxane and gemzar, seeking complimentary treatments, asymptomatic at this time. He was diagnosed after an episode of severe jaundice in April, which was followed by nausea and vomiting subsequently he was seen at cedars sinai and treated with chemotherapies, in June 2013.
His pathology was reported moderately differentiated adenocarcinoma, with negative nodes, however his surgical report showed suspicious areas especially at the uncinate process of pancreas, which was not biopsied and has very close margin to surgical incision. The pathology was also positive for neural invasion. The molecular profiling by Caris report was also showing lost PTEN which made his cancer more resistant in the long run with higher risk of recurrence. I did order labs to identify the markers, as VEGF, FGF-2, IGF-1 and interleukin 8. We followed him very closely. Our invitro studies suggested improved response with our therapy along with the Gemzar as his regimen with chemosensitizing efficacy. He received total of 15 IV epigenetic therapies per MTET protocol in 45 days. His tumor markers were monitored, and he was restaged after three months with a whole body PET/ Ct scan, which showed no residual activity of his disease. At the time this article is being published he is still in complete remission/ cured after 5 years (Figures 8-12).
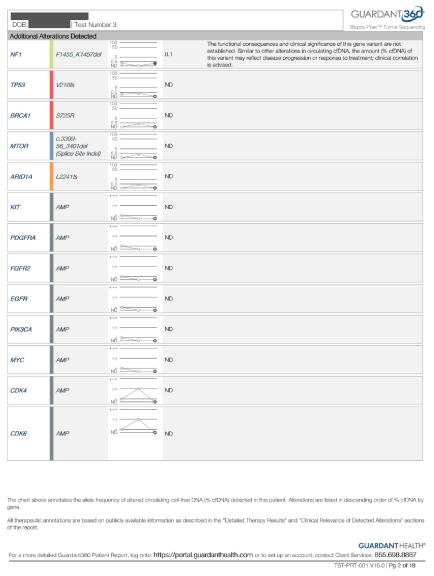
Figure 8. Circulating tumor cells pre and post therapy
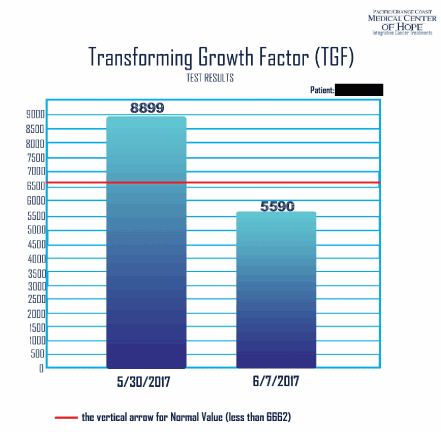
Figure 9. Tumor markers pre and post therapy
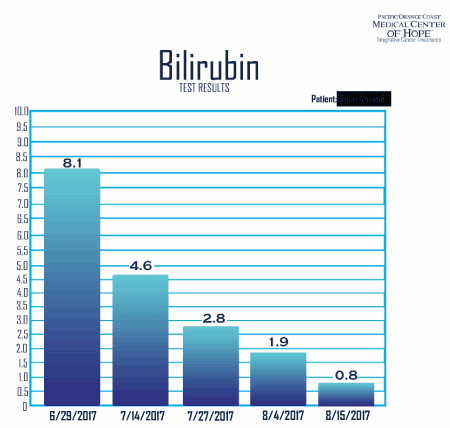
Figure 10. Tumor markers pre and post therapy
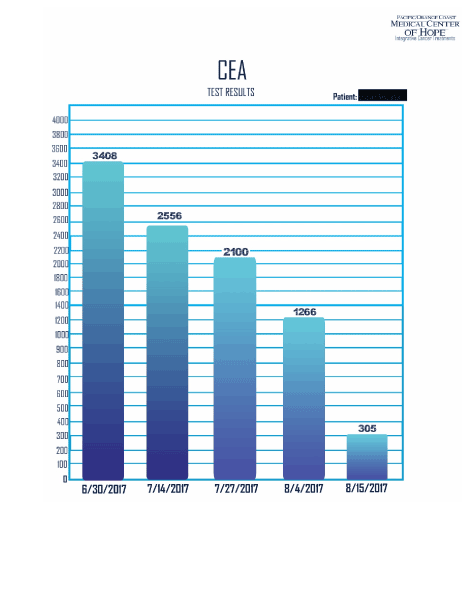
Figure 11. Tumor markers pre and post therapy

Figure 12. Circulating DNA pre and post therapy

Figure 13. Circulating DNA pre and post therapy
We conclude that addition of epigenetic targeted therapies could potentially enhance the tumor response in advanced pancreatic cancer. Also, the multitargeted epigenetic therapy was found to be independently effective in the cases treated here by direct reduction of tumor biomarkers in circulating tumor cells and/ or circulating DNA. Further clinical trials are needed to expand our knowledge in this area of oncology.
- Brooke D Paradise, Whitney Barham, Martín E. Fernandez-Zapico (2018) Targeting Epigenetic Aberrations in Pancreatic Cancer, a New Path to Improve Patient Outcomes? Cancers (Basel) 10: 128. [Crossref]
- Pappano WN, Guo J, He Y, Ferguson D, Jagadeeswaran S, et al. (2015) The histone methyltransferase inhibitor A-366 uncovers a role for G9a/GLP in the epigenetics of leukemia. PLoS ONE 10: e0131716. [Crossref]
- Pan MR, Hsu MC, Luo CW, Chen LT, Shan YS, et al. (2016) The histone methyltransferase G9 as a therapeutic target to override gemcitabine resistance in pancreatic cancer. Oncotarget 7: 61136-61151. [Crossref]
- Van Vlerken LE, Kiefer CM, Morehouse C, Li Y, Groves C, et al. (2013) EZH2 is required for breast and pancreatic cancer stem cell maintenance and can be used as a functional cancer stem cell reporter. Stem Cells Transl. Med. 2: 43-52. [Crossref]
- Miele E, Valente S, Alfano V, Silvano M, Mellini P, et al. (2017) The histone methyltransferase EZH2 as a druggable target in shh medulloblastoma cancer stem cells. Oncotarget 8: 68557-68570. [Crossref]
- Vaswani RG, Gehling VS, Dakin LA, Cook AS, Nasveschuk CG, et al. (2016) Identification of (R)-N-((4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-methyl-1-(1-(1-(2,2,2-trifluoroethyl)piperidin-4-yl)ethyl)-1h-indole-3-carboxamide (CPI-1205), a potent and selective inhibitor of histone methyltransferase EZH2, suitable for phase I clinical trials for B-cell lymphomas. J. Med. Chem 59: 9928-9941. [Crossref]
- Huang L, Holtzinger A, Jagan I, BeGora M, Lohse I, et al. (2015) Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat. Med 21: 1364-1371. [Crossref]
- Mc Cabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, et al. (2012) EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492: 108-112. [Crossref]
- Chen YT, Zhu F, Lin WR, Ying RB, Yang YP, et al. (2016) The novel EZH2 inhibitor, GSK126, suppresses cell migration and angiogenesis via down-regulating VEGF-A. Cancer Chemother. Pharmacol 77: 757–765. [Crossref]
- Mc Cleary-Wheeler AL, Lomberk GA, Weiss FU, Schneider G, Fabbri M, et al. (2013) Insights into the epigenetic mechanisms controlling pancreatic carcinogenesis. Cancer Lett 328: 212-221. [Crossref]













