Background: Oral squamous cell carcinoma isone of the leading cancers in India. Itis frequently preceded by a potentiallymalignant disorder called oral leukoplakia (OL), which is linked to widespread high-risk oral habits. In the state of Chhattisgarh, India, tobacco-chewing is one of the most common habitsin both males and females. However, the impact of lifestyle factors in etiology of OL in this region has not been investigated, and their evaluation could help develop translational strategies to reduce cancer incidence and improve overall public health.
Materials and methods: Sixty patients with oral patches and clinical diagnosis of OL were enrolled. History and clinical presentation were recorded, brush cytology carried out and the patients followed-up after one year.
Results: Mean age at presentation of OL was 43.4±12.6 years. OL was predominantly associated with chewing tobacco in both genders. The habit was reported in 82.6% (OR=7.07, p<0.0001) male and 62.5% (OR=5.1, p<0.02) female OL patients. Among tobacco chewers, increased risk of developing OL in males was 5.04-fold (p = 0.0006) higher relative to females. The most common site of involvement of OL was buccal mucosa (75%). Patients who chewed tobacco and consumed alcohol for > 10 years had increased incidence of non homogeneous OL. At one-year follow-up, patients with or without any oral habit, who did not show any improvement, were 21% and 60%, respectively. Literacy rates in patients who complied vs. those who did not comply with follow-up surveillance were 100% and 67%, respectively.
Conclusion: Awareness program against habit of chewing tobacco, with special focus on males at 20-year age group, may reduce the occurrence of OL in this region. OL patients with no history of oral habit should be followed-up more frequently. Increase in literacy rate may help in improving patient compliance to follow-up surveillance.
lifestyle, oral leukoplakia, high risk oral habits. tobacco chewing, chhattisgarh, translational, compliance
Oral squamous cell carcinoma (OSCC) is sixth commonest cancer worldwide [1]. It is also the most common form of oral cancer comprising almost 90% of all oral malignancies [1]. It is a one of the leading cancers in India and constitutes 30% of all new cancer cases and most of the cases (60%) present with advanced disease, leaving very limited treatment option [2]. It is often preceded by persistentoral potentially malignant disorders (OPMDs), a group of oral lesions commonly presented as white patches called oral leukoplakia (OL) [3]. India has the highest prevalence of OL primarily due to several forms of high-risk oral habits. Its prevalence ranges from 0.2 to 8.2% depending on geographic regions [4-7]. Though most of the cases regress spontaneously, some may progress to OSCC with a variable transformation rate from 0.1 to 34% [5,8-10].
The common risk factors for OLarehigh risk oral habits which include chewing tobacco, betel nut, smoking tobacco and alcohol consumption with variable contribution in different populations [11]. There is a significant difference in the pattern of high-risk oral habits in western countries and India. While in western countries, smoking tobacco and alcohol are dominant high-risk oral habits, in India, smokeless form of tobacco chewing is a wide spread oral habit. This includeskhaini, gutkha, and snuff, either used separately or as an ingredient of pan masala or betel quid [12].Unlike diffuse effect of tobacco smoking, soluble form of tobacco causes localized exposure with high concentration of tobacco leading to more penetrating and injurious impact on oral mucosa. The differences in potentially risky oral habits in different geographical regions within India provide a natural opportunity to study theirimpact on etiology of OL in light of specific environmental and lifestyle factors and/or gender of consumers. This will allow formulation ofan overall preventive health policy as well as policies suited for specific regions.
Though there are several studies on premalignant lesions from various regions of India [13,14], most of them lack in appropriate number of hospital-based control population and thus may not represent the background risk factor exposure within general population. Moreover, most of these studiesfail to provide any assessment which translational implication may have to undertake community based preventive strategy.
It has been proposed that oral cancer and premalignant lesions mostly affect malessuggesting that the high risk associated with these habits is gender-specific [15]. However, contradictory reports from different parts of the world regarding gender specific vulnerability to oral canceralso exist. In fact, several recent reports suggest that compared to men, there is increased risk among women with the habit of smoking and alcohol intake for developing oral cancer [15-17]. There are other studies which claim that estrogen, a gender specific hormone may have protective role contributing to low occurrence of oral cancer in young females compared to males [18,19]. Therefore, it remains to be determined if and how the high-risk oral habits differentially impact males and females with relevance to development of precancerous oral conditions.
A thorough investigation of oral habits and their consequence in light of differences in gender, geographical location, environment and lifestyle factors will not only identify specific risk factors but also the populations which could be at risk. This in turn will allow development of strategies for prevention, identification and regular follow-up of vulnerable groups as well as early diagnosis of oral cancer, improving the clinical outcome of disease.
In state of Chhattisgarh, located in central India, the use of soluble forms of tobacco and alcohol consumption is very high in the general population. This region is also unique from rest of the country in having a high percentage of tribal population. Many residents are below poverty line. As per National Family Health Survey (NFHS-4) 2015, unlike other parts of India, the high-risk oral habits in this region are prevalent in both male and female population [20]. So, this provides a natural opportunity to study the impact of oral habitsand gender on various characteristics of OL relative to control population. Moreover, there is no report from this region, evaluating the impact of the prevalent risk factors in occurrence of OL and oral cancer. It is, therefore, extremely important to formulate an evidence-based preventive strategy against OL and oral cancer in this region which is unique with respect to prevalence of oral habits as well as socioeconomic features.
In this hospital-based pilot study, weevaluated the impact of major contributing risk factors in occurrence of OLin this region to identify thehigh risk individuals/groups for implementing effective awareness and surveillance programs for them. This is the first pilot study in this region designed with clear translational goals which include: (1) Identifying target population on whom awareness program should be focused for effective prevention of OL; (2) Impact of educational backgroundon patients’ compliance on follow-up surveillance; (3) Impact of gender on vulnerability; and (4) Comparison of the impact of high risk oral habits in causation of OLwith other parts of the world.
Study was carried out at Multidisciplinary Research Unit (MRU), Pt. Jawaharlal Nehru Memorial Medical College, Raipur, Chhattisgarh, India, following approval by institutional ethical committee. Patients who attended ENT and dental clinic with oral lesion and clinically diagnosed as leukoplakia and willing to participate in the study were referred to MRU for enrollment. After taking informed consent from the patients, the history was taken, and brush biopsy conducted for cytological assessment. In suspected cases, punch biopsy was carried out in ENT OPD. Patients having history of chronic illness and use of steroid treatment for were excluded from the study.
Background control population: As the exposure happened much earlier than the start date of the study, we used the data for the general background population to compare the high-risk lifestyle factor from the published fact sheet of Annual Health Survey Chhattisgarh 2010-2011, conducted on 1220077 population of Chhattisgarh [21].
Statistical analyses: The data were analyzed using following software:
https://www.calculator.net/standard-deviation-calculator.htmlhttps://www.medcalc.org/calc/odds_ratio.php
Association between gender predisposition, tobacco use, oral lesions and clinical characteristics were expressed as odds ratio with 95% confidence intervals.
The present study was conducted over a period from June 2016 to September 2018. Total 60 patients of OL were examined during this period. Out of 60 patients, 56 were from different parts within Chhattisgarh state, whereas 4 patients were from adjacent areas outside the state. For comparingthe exposure with the background control population, we used Annual Health Survey 2010-11 fact sheet Chhattisgarh, which was conducted on 1220077 population over the 16 districts of Chhattisgarh [21].
Patient profile
Out of 60 OL patients, majority (31.66%) belonged to the age group of 31 to 40 years. Mean age of the overall OL patients were 43.4±12.6 years. Although the mean age of male OL patients (43.25±12.6) was slightly more than that of female OL patients (44.37±13.29), this difference was not statistically significant (p=0.8) (Table 1). In OL patients,the proportion of males was significantly higher than the females(86.66% vs.13.33%) as compared to this ratio in general population (50.81% males vs.49.19%females) (p<0.0001). Literacy rate in the OL patients (83.34%) was not statistically different as compared to that in general population (74.75%; p=0.12). Complete educational profile of OL patients is shown in table 2.
Table 1. Age distribution of oral leukoplakia patients
Age (Y) |
% of OL patients (n) |
Mean age (Mean±SD) |
21-30 |
16.66(10) |
43.4±12.6 |
31-40 |
31.66(19) |
Male |
Female |
41-50 |
23.33(14) |
43.25±12.6 |
44.37±13.29 |
>50 |
28.33(17) |
Table 2. Educational profile of OL patients in CG
Education |
% of OL patients ,(n) |
p-value
(with general population) |
Literate |
83.33 (50) |
Up-to 10th |
51.66(31) |
0.12
|
>10th |
31.66(19) |
Illiterate |
16.66(10) |
total |
100(60) |
|
Distribution of high-risk oral habits in OL
Out of 60 patients, frequency of no oral habit, smoking, tobacco chewing, alcohol, pan and areca nut were 13.3, 30%, 80%, 41.66%, 10% and 6.66% respectively (Table 3). Out of 52 males in OL patients, 49 (94.3%) had at leastone of the high-risk oral habits, whereas in 8 females, 5 (62.5%) had similar oral habits (Table 4).
Table 3. Prevalence of high-risk soral habits in OL patients
Type of high-risk habit |
% of OL patients (n) |
No habit |
13.3 (8) |
Smoking |
30 (18) |
Chewing tobacco |
80 (48) |
Alcohol |
41.66 (25) |
Pan |
10 (6) |
Areca nut |
6.66(4) |
Total |
100(60) |
Table 4. Gender distribution and risk association of major high-risk habits in OL patients (*statistically significant)
Major life style factors |
Over all (n) |
Male %,(n/N) |
Female %, (n/N) |
P value |
Total OL patients |
60 |
86.66 (52/60) |
13.33 (8/60) |
<0.0001* |
Oral habit
|
Total
(at least one habit) |
54 |
94.3(49/52) |
62.5(5/8) |
|
%Male |
OR
(95%CI) |
P |
%Female |
OR
(95%CI) |
P |
Smoking |
18 |
34.61
(18/52) |
3.77
(2.13-6.68) |
<0.0001*
|
0 |
11.7
(0.67-202.87) |
0.09
|
|
Chewing tobacco
|
48 |
82.6 (43/52) |
7.07
(3.45-14.52) |
<0.0001*
|
62.5
(5/8) |
5.16
(1.23-21.60) |
<0.02*
|
|
Alcohol |
25 |
48.07
(25/52) |
2
(1.16-3.45) |
<0.0123*
|
0 |
0.73
(0.04-12.75) |
0.83
|
|
NO habit |
6 |
5.7(3/52) |
37.5(3/8) |
|
Table 5. Gender-specific distribution of OL patients addicted to smokeless tobacco in comparison with corresponding general population of Chattisgarh. (*statistically significant)
Sex |
% of ChewingTobacco in OL |
% of total Chewing Tobacco in general population |
OR(95%CI) |
p-value |
Male |
89.6 |
63 |
5.04(1.99-12.72) |
0.0006* |
Female |
10.4 |
37 |
Frequency of oral habits in OL patients were compared with the corresponding habit in general local population of Chhattisgarhfrom the annual fact sheet data [21]. Among the male OL patients, frequency of smoking was 34.61% compared to 12.29% in general male population, indicating that smoking is associated with a significant 3.77-fold increase in the risk for development of OL (OR=3.77; 95%CI: 2.13-6.68, p<0.0001). Frequency of chewing tobacco among the male OL patients was 82.6% compared to 40.29% in general male populationthus suggesting that chewing tobacco is associated with statistically significant 7.07-fold increase in the risk of OL (OR=7.07; 95%CI: 3.45-14.52, p<0.0001). Habit of drinking alcohol among male OL population was 48.07% compared to 31.59% in general male population with statistically significant risk association (OR=2, 95%CI: 1.16-3.45, p=0.0123;Table 4). In our study female OL patients did not smoke or consumed alcohol. However, the frequency of chewing tobacco among the female OL patients was 62.5% compared to 24.4% in general female population indicating a statistically significant risk association (OR=5.16 95%CI: 1.23-21.60, p<0.02; Table 4).
Among the OL group having habit of chewing tobacco, 89.6% were males and 10.4% females, whereas individualswith this habit in general population were 63% males and 37% females. This suggests that among tobacco chewers,risk of developing OL was 5.04-fold higher (OR=5.04, 95% CI: 1.99-12.72, p = 0.0006) in males relative to females (Table 4).
Duration of oral habit
Mean duration of exposure (in years) to smoking, chewing tobacco and alcohol was 21.27±13.54, 11.05±9.27 and 12.62±8.09, respectively (Table 5).
Clinical Profile of OL lesion
Anatomical Site distribution: The most common site of involvement of OLwas buccal mucosa (75%), followed by dorsum of tongue (13.33%) and lips (11.66%) (Table 6 and 7). Tongue which is one of the common sites for malignant transformation [22,23], was the predominant site of OLin patients who did not have any high risk oral habit, whereas it was buccal mucosa in the group which had these habits [24-37]; the association of presence or absence of high risk habits with the specific location was statistically significant (p£0.0007, Table 8). Interestingly malignant transformation rate of OL in patients having no high-risk oral habit is also reported to be high [38-40].
Table 6. Duration of exposure of high-risk oral habits in OL in Chattisgarh
Habit |
Duration of Exposure (n=54) |
1-5 (Y)
|
6-10(Y) |
11-20(Y) |
>20(Y) |
Mean±SD (Range) (Y) |
Median (Y) |
% (n) |
Smoking |
2 (1) |
7(4) |
11(6) |
13(7) |
21.27±13.54 (2-50) |
20 |
Chewing
Tobacco |
31(17) |
22(12) |
28(15) |
7(4) |
11.05±9.27 (1-50) |
10 |
Alcohol |
9 (5) |
17 (9) |
15 (8) |
6 (3) |
12.62±8.09(2-38) |
10 |
Table 7. Anatomical site distribution of oral lesions (B=Buccal Mucosa which includes buccal mucosa only, buccal and gingiva, and buccal and lip; L=lip; T=Tongue)
Anatomical site |
% (n) |
B |
75 (45) |
B(only) |
65(39) |
|
|
BG |
5(3) |
BL |
5(3) |
L |
11.66(07) |
T |
13.33(8) |
Total |
100(60) |
Table 8. Anatomical site distribution according to oral habit (B=Buccal Mucosa which includes buccal mucosa only, buccal and gingiva, and buccal and lip; L=lip; T=Tongue)
Anatomical site |
With habit %(n) |
No HROH %(n) |
p-value |
B |
81 (44) |
17 (1) |
0.0007* |
L |
13(7) |
0(0) |
0.3513 |
T |
6(3) |
83(5) |
<0.0001* |
Total |
100(54) |
100(6) |
|
Clinical appearance: Out of 60 OL patients, 44 (73.33%) cases had homogenous OL patch and only 16 (26.67%) had non homogenous OL patch (Figure 1). Among the anatomical sites, the proportion of homogenous leukoplakia were as follows: buccal mucosa 75.55%, lips 57.14% and tongue 62.5% and the proportion of non-homogenous leukoplakia were as follows: buccal mucosa 24.44%, Lips 42.85% and tongue 37.5% (Table 9).Homogenous appearance of OL lesion was slightly higher in non-addiction group compared to the addiction group, however this difference was not statistically significant (p=0.5; Table 10).
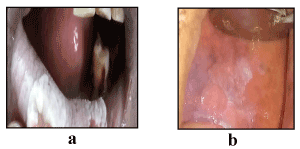
Figure 1. Homogeneous (a) and non-homogeneous oral leukoplakia.
Table 9. Proportion of homogenous and non-homogenous patches in OL patients at different anatomical sites. (B=Buccal Mucosa which includes buccal mucosa only, buccal and gingiva, and buccal and lip; L=lip; T=Tongue)
Appearance of patch |
Total %(n) |
B
%(45) |
L
%(7) |
T
%(8) |
%Homogenous |
73.33(44) |
75.55(34) |
57.14(4) |
62.5(5) |
%Non-homogenous |
26.67(16) |
24.44(11) |
42.85(3) |
37.5(3) |
Total |
100(60) |
100(45) |
100(7) |
100(8) |
Table 10. Appearance of oral leukoplakia lesion in patients with and without high risk habits
Appearance of lesion |
With habit %(n) |
Without habit %(n) |
Homogenous |
72%(39) |
83% (05) |
Non homogenous |
28%(15) |
17%(01) |
Total |
100(54) |
100(6) |
Association of occurrence of non-homogeneous OL with duration of habits: As non-homogeneous OL have more potential for malignant transformation, we determined the proportion of non-homogeneous OL with relation to different habit and duration (Table 11). Duration of habits is segregated into less than and more than 10 years. Occurrence of non-homogeneous OL was significantly higherin patients exposed to risky behavior (of chewing raw tobacco and alcohol consumption) for >10 years than those exposed for <10years (P£0.04). However, corresponding mean age differences were not statistically significant excluding the possibility of contribution of age differencein the two groups. Surprisingly, in patients having habits of Gutkha (a processed form of tobacco) chewing and smoking tobacco, no statistical associations were observed with duration of habit and occurrence of non-homogeneous OL (Table 11).
Table 11. Distribution of non homogenious OL and age among different oral habit groups according to duration of the habit (* statistically significant)
Oral habit |
Duration <10Year |
Duration >10Year |
P value |
Raw Tobaco Chewing |
NH(%) |
18%
|
47% |
0.02* |
Age
Mean±SD |
44±14
|
46±9 |
0.57 |
Gutkha Chewing |
NH
(%) |
24% |
36% |
0.39 |
Age
Mean±SD |
45.3±13.6 |
42±8 |
0.44 |
Smoking Tobaco |
NH
(%) |
25% |
33% |
0.56 |
Age
Mean±SD |
41.7±11.7 |
52.46±11 |
0.003 |
Alcohol Drinking |
NH
(%) |
20% |
47% |
0.04* |
Age
Mean±SD |
44.±14 |
45.5±8 |
0.69 |
No habite |
NH
(%) |
22% |
Age
Mean±SD |
37±13 |
Patient follow-up and compliance: During the study period, only 36 patients were qualified for one-year follow-up. We categorized the patients in 3 groups based on compliance to follow up: (1) Patents visited physically for follow up; (2) Patients respondedto the questions over the phone but were not willing to visit for follow up; (3) No response or contactcould be made.We observed that out of 36 OL patients, 11 patients complied through follow-up visits, 13 complied through phone conversations and 12 did not comply (Table 12).
Table 12. Correlation of patient compliance with literacy rate
Education |
Total Follow up %(n) |
%Compliance by visit %(n) |
%Compliance by phone %(n) |
%Non-compliance %(n) |
Illiterate |
18 (6) |
0(0) |
15.3(2) |
33.3(4) |
<10th class |
47 (16) |
54.5(6) |
38.5(5) |
41.6(5) |
>10thclass |
41 (14) |
45.5(5) |
46.1(6) |
25(3) |
Total |
100(36) |
100(11) |
100(13) |
100(12) |
Illiteracy rates in OL patients complying through follow-up visits, complying through phone and non-complying were 0%, 15.3% and 33.3%, respectively. In OL patients, educated up to 10th standard, the compliance rates for physical follow-up, over the phone and non-compliance were 54.5%, 38.5% and 41.6%, respectively, whereas for higher education (>10th class) group these rates were 45.5%, 46.1%and 25%, respectively. Out of 24 follow-up patients, 70.83% (17/24) reported improvement in OL patch while 29.16% (7/24) reported no improvement (Table 13). Interestingly, when we analyzed response rate in habit and no habit group, at 1 year follow up, 60% (3/5) of no habit patients reported no improvement in contrast to 21% (4/19) in oral habit group (Table 13) (Figure 2). However, the difference was not statistically significant (p=0.09).
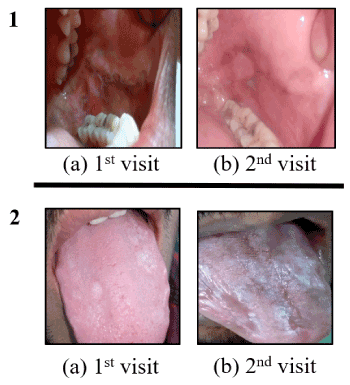
Figure 2. One year follow up change in oral leukoplakia with (Panel-1) or without (panel 2) improvement. Panel-1 : OL with oral high-risk habit; (a)1st visit; (b)2nd visit showing improvement; Panel-2 : OL without oral high-risk habit; (a)1st visit; (b)2nd visit showing no improvement
Table 13. Clinical outcome at one-year follow-up
Clinical outcome |
Total
%(n) |
Without Habit%(n) |
With Habit%(n) |
P value |
Improvement |
70.8(17) |
40 (2) |
79(15) |
0.09 |
No improvement |
29.2(7) |
60(3) |
21(4) |
Total |
100 (24) |
100(5) |
100(19) |
We conducted the hospital-based pilot study on OL patients in a local population in India. As it is the oldest and apex medical college, it has a wide catchment area of patients over the state of Chhattisgarh. We evaluated various clinical, etiological as well as socio-demographical parameters among OL patients and compared those with the background general population of Chhattisgarh to understand how those factors may influence the pathogenesis of OL and disease outcome.
In the current study, among 60 newly diagnosed OL patients, majority of patients had the age group of 31-40yrs. Although, this result is in contrast to a report from UP by Sharma et al. in which they found greater proportion of cases in the older age group of > 41yrs [24], it is similar to other reports from UP and Kerala [25,26]. In our study,the difference of mean age of presentation of OL in maleand female patients was not statistically significant. However earlier study showed that females had more age of incidence [27].
In our study, the occurrence of OLwasobserved higher in male than female (6.5:1). The similar observation was also reported earlier fromotherparts of the country like Karnataka, Uttar Pradesh where male female ratio was 7.2:1 and 8.1:1, respectively [24,27]. In contrast reports from western countries like Croatia and Netherland showed a small female preponderance [28,29]. This difference may be due to differential oral habits,lifestyle and/or socio-cultural differences between different geographical areas. Additionally, there might be gender-specific susceptibility and/or protection which may operate along with the lifestyle factors.
In the present study most common high-risk oral habits in OL patients were, chewing tobacco, alcohol, smoking, pan and areca nut. Out of all high-risk habits in OL, tobacco chewing was the most common addiction (80%) in our study.However, in other parts of India, smoking was found to be predominant habit followed by tobacco chewing [24,27]. In our study, we observed that only 6.6% of the patients have the habit of areca nut chewing. This is also similar to the earlier report from Bangalore by Sujata et al. [27]. However, it varied from region to region in India. The earlier study showed high percentage of areca nut among the OL patients in UP (69.6%) [23] and Maharashtra [30].
As previously reported [31-33], we also observed that high risk habits were more common in male (94.3%) as compared to female (62.5%) OL patients. Habit of smoking, chewing tobacco and alcohol significantly increased the risk of male OL patients but within the female OL group,only chewing tobacco showed the significant risk association (Table 3).However, in a meta-analysis, Rodriguez-Archillaet et al. observed that overall tobacco consumption increased much lower risk (OR=3.49, p<0.001) of OL and could not find any association with alcohol consumption [34]. Interestingly, among the OL group having the habit of chewing tobacco the male factor over female was 5-fold higher (P = 0.0006). This observation is also much higher than previously calculated (P<0.001) “male factor” as a risk for OL [24]. However, in our analysis we only considered impact of male factor within the tobacco-chewing group. This suggests that even though there is increased percentage of chewing tobacco habit in female population in this region, the incidence of OL in female population is low. This supports the hypothesis that there might be gender-specific protection in female population and/or males might be more susceptible to high risk habits, at least in this region. There is a report suggestingthat estrogen may have protective role in occurrence of OL and oral cancer in young females [18,35]. However, we did not observeany statistically significant difference in mean age of occurrence of OL in female verses male patients.
Interestingly, in the group with no risky habit, the male:female ratio was similar to that in general population. This strengthens the hypothesis that differential chewing habit and gender-specific susceptibility to the high-risk habit may interplay in out-numbering male over female patients.
As the mean age of OL patients was 43.4±12y and average addiction period of tobacco-chewing was 11.05±9.27 years in OL patients, the awareness program should start as early as 20 year of age. This age group is the most vulnerable for catching new addiction habit in India. Moreover,this may provide enough opportunity to spread awareness and required information within general population.
Literacy rate among the OL patients was only slightly higher as compared to general population. Only 31.7% of OL patients had education level more than 10th class. Faize et al. [25] noticed that overall 57.1% OPMD patients were illiterate/educated up to primary level. Similarly, Sujatha et al. [27] observed overall 45.8% educated till high school level and only 18% had higher educated (degree or diploma holder).
In our study, buccal mucosa was the most frequent (75%) site of the leukoplakia patch followed by 13.11% in dorsum of tongue and only 11.66% patch found in lip area. In majority of the previous findings worldwide, including India,the most common site for leukoplakia patch was buccal mucosa [3,25,36,37]. Interestingly, when we segregate the OL patients in groups with or without high risk habits, we found that in no habit group, predominant site of OL was tongue, which is also the common site for malignant transformation [22,23]. Similar finding was also observed by Schepman et al. [38]. This clearly signifies the different etiology and pathogenesis in causation of OL in these two groups. Interestingly at 1 year follow up also 60% of no habit group shows no improvement of the lesion compared to only 21% of those with high risk habit group. The high rate of improvement in high risk habit group may be combined effect of abstinence of high-risk oral habit along with surgical or non-surgical treatment. The increase rate of OL at high risk site and less proportion of improvement in patients having no oral habit, support the earlier views in the line of increase rate of malignant transformation in no habit OL group [39-41]. Though it needs to be confirmed in larger sample size, we can say that patients with no high-risk habit need more frequent and careful surveillance. It would be reasonable to look at other etiological factors like HPV for OL patients having no history of high-risk oral habits.
Though malignant transformation rate in non-homogeneous OL of tongue is more common [22,23,41], we observed that non-homogenous OL in lip was more frequent than the non-homogenous leukoplakia of tongue (Table 12). One of the informative observations from our study was characterization of high-risk oral habit in occurrence of non-homogenous OL. In our study patients having habit of raw tobacco chewing (Khaini: a form of chewing tobacco used in India, containing slaked lime) and alcohol consumption more than 10 years shows more occurrence of non-homogeneous OL than patients with habit for less than 10 years. As most of the malignancy or pre malignant conditions occur frequently in higher age group, so effect of long duration of oral habit may be affected by higher age. However, difference of mean age between the shorter and longer duration of the habits were not statistically significant. Though Gutkha chewing and smoking are the well-known risk factors for OL and submucous fibrosis in reports from other part of India [42-45], in our study, we did not find any statistically significant increase in the occurrence of non-homogeneous OL in groups having more than 10 years of habits. This may be due to low consumption or lack of multiple interacting habits. However, this finding should be verified in larger sample size.
As management of OL needspatient compliance to long-term follow-up, we evaluated effect of literacy rate on patient compliance. We observed 0% illiteracy rate in patents who show compliance for physical follow-up, whereas 75% of non complyingpatients are either illiterate or have <10th class education. This suggests more attention is required to patients having low educational level (illiterate or <10th class education) during the first visit; multiple steps should be taken to ensure that they adhere to health surveillance system. Higher literacy rate will give the better compliance for physical follow-up. By increasing coverage of literacy and higher education among general population will benefit the people to come into the health surveillance process.
OL in state of Chhattisgarhis predominantly associated with habit of chewing tobacco in both males and females, however, within high risk oral habit groupthe incidence is much higher in malesrelative to females. Awareness program against habit of tobacco-chewing with special focus on males at 20-year age group will help to reduce occurrence of OL and oral cancer in this region of India. OL patients with no history of oral habit should be followed up more frequently. Increased literacy rate may help in improving patient compliance to follow up health program. For future study, it will be interesting from preventive/therapeutic point to identify any factor/s responsible for gender-related susceptibility or protection to high risk oral habits in the population. For that type of study, population from this region would be a model population. Larger longitudinal study isrequiredto evaluate malignant transformation rate in different forms of OL and to identify preventable etiological factor/s in causation of OL.
This work was supported by the Department of Health Research, Ministry of health, India. We also acknowledge Department of Oral Medicine & Radiology, Government Dental College, Raipur for all necessary support and advice.
- Warnakulasuriya S (2009) Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 45: 309-316. [Crossref]
- More Y, D'Cruz AK (2013) Oral cancer: review of current management strategies. Natl Med J India 26: 152-158. [Crossref]
- Waldron CA, Shafer WG (1975) Leukoplakia revisited. A clinicopathologic study 3256 oral leukoplakias. Cancer 36: 1386-1392. [Crossref]
- Patil PB, Bathi R, Chaudhari S (2013) Prevalence of oral mucosal lesions in dental patients with tobacco smoking, chewing, and mixed habits: A cross-sectional study in South India. J Family Community Med 20: 130-135.
- Sridharan G (2014) Epidemiology, control and prevention of tobacco induced oral mucosal lesions in India. Indian J Cancer 51: 80-85.
- Kumar S, Debnath N, Ismail MB, Kumar A, Kumar A, Badiyani BK, et al. (2015) Prevalence and risk factors for oral potentially malignant disorders in Indian population. Adv Prev Med.
- Hashibe M, Sankaranarayanan R, Thomas G, Kuruvilla B, Mathew B, et al. (2000) Alcohol drinking, body mass index and the risk of oral leukoplakia in an Indian population. Int J Cancer 88: 129-134. [Crossref]
- Warnakulasuriya S, Ariyawardana A (2015) Malignant transformation of oral leukoplakia: A systematic review of observational studies. J Oral Pathol Med.
- Dost F, Le Cao K, Ford PJ, Ades C, Farah CS (2014) Malignant transformation of oral epithelial dysplasia: a real-world evaluation of histopathologic grading. Oral Surg Oral Med Oral Pathol Oral Radiol 117: 343-352. [Crossref]
- van der Waal I, Schepman KP, van der Meij EH, Smeele LE (1997) Oral leukoplakia: A clinicopathological review. Oral Oncol 33: 291-301. [Crossref]
- Mehta FS, Pindborg JJ, Gupta PC, Daftary DK (1969) Epidemiologic and histologic study of oral cancer and leukoplakia among 50,915 villagers in India. Cancer 24: 832-849. [Crossref]
- Chadda R, Sengupta S (2002) Tobacco use by Indian adolescents. Tob Induc Dis 1: 111-119. [Crossref]
- Ray JG, Ganguly M, Rao BS, Mukherjee S, Mahato B, Chaudhuri K, et al. (2013) Clinico-epidemiological profile of oral potentially malignant and malignant conditions among areca nut, tobacco and alcohol users in Eastern India: A hospital-based study. J Oral MaxillofacPathol 17: 45-50.
- Mondal K, Mandal R, Sarkar BC, Das V (2017) An inter-correlative study on clinico-pathological profile and different predisposing factors of oral leukoplakia among the ethnics of Darjeeling, India. J Orofac Sci 9: 34-42
- Speight PM, Khurram SA, Kujan O (2018) Oral potentially malignant disorders: risk of progression to malignancy. Oral Surg Oral Med 125: 612-627. [Crossref]
- Vatanasapt P, Suwanrungruang K, Kamsa-ard, Promthet S, Parkin MD, et al. (2011) Epidemiology of Oral and Pharyngeal Cancers in KhonKaen, Thailand: a High Incidence in Females Asian Pacific. J Cancer Prev 12: 2505-2508.
- Muscat JE, Richie JP Jr, Thompson S, Wynder EL (1996) Gender differences in smoking and risk for oral cancer. Cancer Res 56: 5192-5197. [Crossref]
- Hashim D, Sartori S, La Vecchia C, Serraino D, Maso LD, et al. (2017) Hormone factors play a favorable role in female head and neck cancer risk. Cancer Med 6: 1998-2007. [Crossref]
- Suba Z (2007) Gender-related hormonal risk factors for oral cancer. Pathol Oncol Res 13: 195-202. [Crossref]
- National Family Health Survey(NFHS)– 4, 2015 -16; Ministry of Health and Family Welfare, Govt of India. Annual Health Survey 2010-2011 Fact Sheet, Chhattisgarh. Office of the Registrar General & Census Commissioner, India.http://www.censusindia.gov.in/vital_statistics/AHSBulletins/AHS_Baseline_Factsheets/Chhattisgarh.pdf
- Barnes L, Eveson JW, Reichart P, Sidransky D, edn (2005) World Health Organization. World Health Organization Classification of Tumours. In: Pathology and Genetics. Head and Neck Tumours. Lyon: International Agency for Research on Cancer (IARC) IARC Press 177-179.
- Amagasa T, Fujii E, Suzuki T, Yamashiro M, Ogura I, Miyakura T, et al. (1999) Clinical characteristics of precancerous lesions and early squamous cell carcinoma in the oral cavity. J Japan Society Oral Tumors 11: 357–363
- Sharma, Preeti, Aggarwal, Pooja Reddy, Vandana, et al. (2014) Assessment of Clinical Risk Factors of Oral Leukoplakia in UP Population of India: An Institutional Study. International Journal of Oral-Medical Sciences 12: 230-234.
- Faiz SM, Agarwal E, Bhargava A, Varshney P, Patigaroo AR, Rizvi D, et al. (2018) Spectrum of premalignant oral lesions in rural North Indian population at a tertiary care hospital. Int J Otorhinolaryngol Head Neck Surg 4: 1452-1457.
- PindorgJJ, Mehta FS, Daftary DK (1975) Incidence of oral cancer 30000 vill`agers in India in a 7-year follow-up study of oral precancerous lesions. Community Dent Orial Epidemiol 3: 86-88
- Sujatha, D Hebbar, Pragati, Pai, Anuradha, et al. (2012) Prevalence and Correlation of Oral Lesions among Tobacco Smokers, Tobacco Chewers, Areca Nut and Alcohol Users. Asian Pacific journal of cancer prevention: APJCP 13. 1633-1637.
- Brzak BL, Mravak-Stipetia M, Canjuga I, Baricevia M, Balicevia D, et al. (2012) The frequency and malignant transformation rate of oral lichen planus and leukoplakia--a retrospective study. Coll Antropol 36: 773-777. [Crossref]
- Hogewind WFC, van der Waal (1988) Prevalence study of oral leukoplakia in a selected population of 1,000 patients from Netherlands. Community Dent Oral Epidemiology 16: 302-305.
- Kawatra Abhishek, Lathi Aniket, Kamble Suchit V, Sharma Panchsheel, Parhar Gaurav (2012) oral premalignant lesions associated withareca nut and tobacco chewing among the tobacco industry workers in area of rural Maharashtra. National Journal of Community Medicine 3: 333-338.
- Jaber MA, Porter SR, Gilthorpe MS, Bedi R, Scully C (1999) Risk factors for oral epithelial dysplasia--the role of smoking and alcohol. Oral Oncol 35: 151-156. [Crossref]
- Rani M, BonuS, Jha P. Tobacco use in India: Prevalence and predictor of smoking and chewing in national cross-sectional household survey. Tob Control 12: e4.
- Saraswathi TR, Ranganathan K, Shanmugam S, Sowmya R, Narasimhan PD, et al. (2006) Prevalence of oral lesions in relation to habits: Cross-sectional study in South India. Indian J Dent Res 17: 121-125. [Crossref]
- Rodriguez-Archilla A, Garcia-Gamez MT. Risk Factors for Oral Leukoplakia: A Meta-Analysis. J Res Notes 1: 1001.
- Dietrich T, Reichart PA, Scheifele C (2004) Clinical risk factors of oral leukoplakia in a representative sample of the US population. Oral Oncol 40: 158-163. [Crossref]
- Mishra M, Mohanty J, Sengupta S, Tripathy S (2005) Epidemiological and clinicopathological study of oral leukoplakia. Indian J Dermatol Venereol Leprol 71: 161-165. [Crossref]
- Neville BW, Damm DD, Allen CM, et al. Oral and maxillofacial pathology. 2nd ed. Phila., PA: Saunders 337-369.
- Schepman KP, Bezemer PD, van der Meij EH, Smeele LE, van der Waal I (2001) Tobacco usage in relation to the anatomical site of oral leukoplakia. Oral Dis 7: 25-27. [Crossref]
- Silverman S Jr, Gorsky M, Lozada F (1984) Oral leukoplakia and malignant transformation. A follow-up study of 257 patients. Cancer 53: 563-568. [Crossref]
- [Crossref] Silverman S Jr, Gorsky M, Lozada F (1984) Oral leukoplakia and malignant transformation. A follow-up study of 257 patients. Cancer 53: 563-568.
- Ho PS, Chen PL, Warnakulasuriya S, Shieh TY, Chen YK, Huang IY (2005) Malignant transformation of oral potentially malignant disorders in males: a retrospective cohort study. BMC Cancer 9: 260.
- Schepman KP (1998) Malignant transformation of oral leukoplakia: a follow-up study of a hospital-based population of 166 patients with oral leukoplakia from The Netherlands. Oral Oncol 34: 270–275.
- Mondal K, Mandal R, Sarkar BC, Das V (2017) An inter-correlative study on clinico-pathological profile and different predisposing factors of oral leukoplakia among the ethnics of Darjeeling, India. J Orofac Sci 9: 34-42.
- Gupta S, Singh R, Gupta O P, Tripathi A. Prevalence of oral cancer and pre-cancerous lesions and the association with numerous risk factors in North India: A hospital-based study. Natl J Maxillofac Surg 5: 142-148.
- Urvish Joshi, Bhavesh Modi (2010) A study on prevalence of chewing form of tobacco and existing quitting patterns in urban population of Jamnagar, Gujarat. Indian J Community Med 35: 105-108.
- Abhishek K, Aniket L, Suchit KV, Panchsheel S (2012) Oral premalignant lesions associated with areca nut and tobacco chewing among the tobacco industry workers in area of rural Maharashtra. National Journal of Community Medicine 3: 333-338.


