A liposome with stimuli-responsive properties, would enable us to address several systemic and intracellular delivery barriers. The aim of this study was to combine the thermoresponsive polymer C12H25-poly(N-isopropylacrylamide)-COOH (C12H25-PNIPAM-COOH) with the phospholipid L-α-phosphatidylcholine hydrogenated (Soy) (HSPC) into chimeric/mixed vesicles and to investigate the resultant structures by light scattering techniques and differential scanning calorimetry (DSC). Liposome thermo-responsiveness occurs due to the PNIPAM segment exhibiting a lower critical solution temperature (LCST) at approximately 32°C, in aqueous solutions, which alters its hydrophilic-to-hydrophobic balance. After the physicochemical study, it was deduced that the biomaterials’ molar ratio was determinant for colloidal stabilization of the lyotropic nanosystems. Also, thermodynamic findings are in line with the physicochemical results, since the presence of polymer resulted in different thermodynamic content, which corresponds to a different thermotropic behavior, compared with pure lipid bilayers. With greater understanding of these differences, a wide range of innovative liposomal nanocarriers could be developed in the future, for drug and gene delivery.
chimericliposome, lipid, polymer, thermoresponsiveliposome
Liposomes are nanovesicles, composed of at least one lipid bilayer. Their building blocks are amphiphilic lipids, usually phospholipids, which have been characterized as liquid crystals, an intermediate phase that exhibits properties between those of solid crystals and isotropic liquids. Phospholipids become hydrated in aqueous forms and create lamellar phases. The Lα mesophase refers to liquid crystal phase, while Lβ is a gel or solid crystal phase. The transition Lβ’ → Lα occurs when temperature is ascending, so the aligning forces become weaker and lipids accelerate [1-3].
Thermoresponsive polymers are macromolecules that exhibit an excessive and reversible change in their physicochemical properties, when temperature is altered [4]. The key characteristic of thermoresponsive polymersis their ability to change their hydrophilicity around certain temperature values. They exhibit a two-phase region, in which the polymer is found in both hydrophilic and hydrophobic form. Depending on whether that region is found at high or low temperatures, an upper or lower critical solution temperature (UCST or LCST) exists, respectively. Research mainly focuses on polymers that exhibit LCST in aqueous solution, for medical reasons [5,6].
Development of chimeric/mixed liposomes, which arise from the combination of different in nature biomaterials, such as phospholipids and polymers, gives the opportunity to generate advanced drug delivery nanosystems (aDDnSs) [7]. These complex self-assembled nanostructures act as facilitators for the creation of innovative therapeutic nanosystems and respond to the requirements of recent trends in nanomedicine, as indicated by systems pharmacology [8-13].
The aim of this study is to design and develop thermoresponsive chimeric liposomes, based on a phospholipid and a thermoresponsive homopolymer and to investigate their thermotropic and physicochemical characteristics. Such systems can use temperature fluctuations as a trigger to rearrange and release the drug molecule, an ability that makes them more efficient in site-specific drug delivery than conventional liposomes.
Materials
The phospholipid used for liposomal formulations was L-α-phosphatidylcholine, hydrogenated (Soy) (HSPC) (Figure 1a). It was purchased from Avanti Polar Lipids Inc., (Albaster, AL, USA) and used without further purification. Chloroform and all other reagents used were of analytical grade and purchased from Sigma–Aldrich Chemical Co.C12H25-PNIPAM-COOH was synthesized by RAFT polymerization using 2-(dodecylthiocarbonothioylthio)-2-methylpropionic acid as the RAFT agent, and AIBN as the radical initiator in 1,4-dioxane. The molecular weight of the synthesized homopolymer was 20,000 and had narrow molecular weight distribution (Figure 1b). The structures of the components of bio-inspired nanostructures are presented in Figure 1.
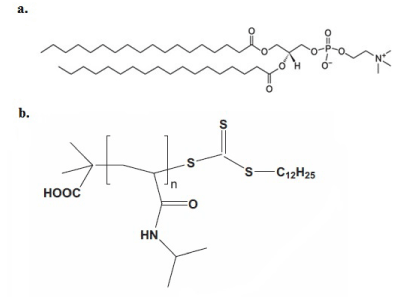
Figure 1: Chemical structures of (a) HSPC lipid and (b)C12H25-poly(N-isopropylacrylamide)-COOH.
Preparation of liposomes
Conventional and chimeric liposomal drug nanocarriers were prepared by the thin-film hydration method. Briefly, appropriate amounts of HSPC and HSPC:C12H25-PNIPAM-COOH (9:0.0, 9:0.02, 9:0.05, 9:0.1, 9:0.2 and 9:0.5 molar ratios) mixtures were dissolved in chloroform and then transferred into a round flask connected to a rotary evaporator. Vacuum was applied and the mixed phospholipid/polymer thin film was formed by slow removal of the solvent at 50°C. The film was maintained under vacuum, for at least 24 h, in a desiccator, to remove traces of solvent and subsequently, it was hydrated in phosphate buffer saline (PBS) (pH=7.4). Lipid films were hydrated in HPLC-grade water, by slowly stirring for 1 h, in a water bath, above the phase transition of lipids (53°C). The resultant multilamellar vesicles (MLVs) were subjected to three 10 min sonication cycles (amplitude 70, cycle 0.5), interrupted by a 10 min resting period, in water bath, using a probe sonicator (UP 200S, Dr. Hielsher GmbH, Berlin, Germany). The resultant vesicles were allowed to anneal for 30 min.
Mean size, size distribution and zeta potential
The effect of PNIPAM on the liposomal lipid bilayers was evaluated by measuring the size (hydrodynamic diameter, Dh), polydispersity index (PDI) and zeta potential (ζ-pot) of liposomes. The Dh, PDI and ζ-pot of the particles were investigated by dynamic and electrophoretic light scattering (DLS and ELS) and used for the characterization of the liposomal dispersions immediately after preparation (t=0 days), as well as for the monitoring of their physical stability over time (t=31 days, at 4ºC, in liquid form). For each measurement, 100 μL aliquots were 30-fold diluted in HPLC-grade water. Measurements were performed at a detection angle of 90° and at 25°C, in a photon correlation spectrometer (Zetasizer 3000 HSA, Malvern, UK) and analyzed by CONTIN method (MALVERN software).
Preparation of pure and chimeric lipid bilayers
Pure lipid and chimeric bilayers were prepared by mixing the appropriate amounts of HSPC and C12H25-PNIPAM in chloroform/methanol (9:1 v/v) solutions and the subsequent evaporation of the solvents under vacuum and heat. Briefly, stock solutions were prepared by dissolving the homopolymer in chloroform/methanol (9:1 v/v). Appropriate amounts of these solutions were mixed with 30.0 mg of HSPC, in order to obtain the desirable molar ratios (9:0.02, 9:0.05, 9:0.1, 9:0.2 and 9:0.5) and the solutions were transferred into vials. Then, the vials were placed into a vacuum machine (TechneDri-Block DB-3 Thermostat Teche Sample Concentrator). Chimeric phospholipid/homopolymer films were formed by removing the solvent at 50°C. The films were maintained under vacuum for 2 h and then, in a desiccator, for at least 24 h, in order to remove traces of solvent. The obtained laminated bilayers were hydrated into the appropriate aqueous medium and then studied by DSC.
Differential scanning calorimetry (DSC)
DSC experiments were performed on an 822eMettler-Toledo (Schwerzenbach, Switzerland) calorimeter calibrated with pure indium (Tm=156.6°C). Sealed aluminum 40 μl crucibles were used as sample holders. The samples investigated were HSPC and HSPC:C12H25-PNIPAM (9:0.02; 9:0.05, 9:0.1, 9:0.2 and 9:0.5 molar ratios) lipid bilayers with a 10 mg/ml concentration (with reference to the whole dispersion) for the overall lipid content. An empty aluminum crucible was used as reference. Prior to measurements the crucibles were subjected to a temperature over the transition of HSPC (53.6°C) to ensure equilibration. All samples were scanned repeatedly until identical thermograms were obtained. Two cooling-heating cycles were performed; 10 to 60°C at 2°C/min scanning rate. The second heating run was taken into account. Enthalpy changes and characteristic transition temperatures were calculated with Mettler-Toledo STARe software.
Statistical analysis
Results are shown as mean value ± standard deviation (S.D.) of three independent measurements. Statistical analysis was performed using Student’s t-test and multiple comparisons were done using one-way ANOVA. P-values<0.05 were considered statistically significant. All statistical analyses were performed using “EXCELL”.
Physicochemical characterization of HSPC:C12H25-PNIPAM-COOH liposomes
Physicochemical characteristics of conventional HSPC liposomes and of thermoresponsive polymer-grafted HSPC:C12H25-PNIPAM-COOH liposomes in different molar ratios (9:0.0, 9:0.02, 9:0.05, 9:0.1, 9:0.2 and 9:0.5 molar ratios) are presented in Table 1. It should be noted that PBS was chosen as the dispersion medium because the pH (∼7.4) and the ionic strength of PBS resemble the conditions met within the human body.
Table 1. The physicochemical characteristics of chimeric HSPC:C12H25-PNIPAM-COOH liposomes.
System |
Molarratio |
Dh1 (nm) |
SD2 |
PDI3 |
SD |
ζ-pot4 (mV) |
SD |
HSPC |
- |
100.7 |
1.4 |
0.466 |
0.006 |
2.2 |
0.4 |
HSPC : C12H25-PNIPAM-COOH |
9:0.02 |
378.9 |
25.1 |
1.000 |
0.000 |
-6.0 |
1.4 |
HSPC : C12H25-PNIPAM-COOH |
9:0.05 |
418.7 |
25.2 |
1.000 |
0.000 |
-6.5 |
1.0 |
HSPC : C12H25-PNIPAM-COOH |
9:0.1 |
198.6 |
7.1 |
0.664 |
0.063 |
-8.7 |
0.2 |
HSPC : C12H25-PNIPAM-COOH |
9:0.2 |
221.4 |
3.0 |
0.608 |
0.033 |
-9.8 |
0.8 |
HSPC : C12H25-PNIPAM-COOH |
9:0.5 |
242.1 |
37.5 |
0.781 |
0.191 |
-5.8 |
2.1 |
1Hydrodynamic Diameter; 2Standard Deviation, 3Polydispersity Index; 4ζ-potential
The size of HSPC liposomes in PBS was around 100 nm, which is in agreement with previous publications. Their ζ-potential was close to zero, because of the absence of net charge on the nanocarrier surface [14].
Conventional HSPC liposomes (reference system) exhibit the smaller size and polydispersity than the other prepared systems. The C12H25-PNIPAM-COOH has a small hydrophobic alkyl chain, which is grafted into the liposomal. After the incorporation of the polymer at the lowest ratio, a great increase of Dh was observed compared with pure HSPC liposomes. For molar ratios 9:0.02 and 9:0.05,Dh was quadrupled and PDI had the maximum value (PDI=1), indicating the heterogeneity of nanoassemblies. By increasing the amount of C12H25-PNIPAM-COOH, better cooperativity between biomaterials was noticed. Specifically, in chimeric system 9:0.1, Dh was 198.6 nm, which is the smallest size in chimeric liposomes, while higher molar ratios led to larger sizes, proportionally to PNIPAM’s concentration. The last three colloidal systems (9:0.1, 9:0.2, 9:0,5 molar ratios) were of low size polydispersity, so their PDI followed the same trend with their Dh (initial increase, but following acute decrease), as the percentage of C12H25-PNIPAM-COOH was increased.
Liposomal size is strongly dependent on polymer concentration, since HSPC phospholipids are lyotropic liquid crystals and modify their formation when temperature and/or concentration change. Even a small amount of C12H25-PNIPAM-COOH can cause membrane curvature, as long as it improves lateral pressure between lipids and due to repulsive forces among them and polymer chains. However, the higher the quantity of polymer, the easier for a liposomal membrane to curve [15].
Zeta potential is another important parameter that gives an indication, concerning the surface charge of liposomal nanovectors. The ζ-potential of HSPC liposomes was found positive, but near zero, because of the absence of net charge on the liposomal membrane. After the incorporation of C12H25-PNIPAM-COOH, a reversal of ζ-potential from positive to negative values was observed. As the molar ratio of the homopolymer was increased, the absolute value of ζ-potential was increased too. This phenomenon was expected because, after the hydration of the HSPC:C12H25-PNIPAM-COOH nanosystems, the terminal –COOH group was ionized, since PBS has pH=7.4 and the pKa of –COOH is around 4.5. These observations indicate that the polymeric chains induce some structural perturbations when included in liposomal membrane bilayers. However, the negative charge of –COOH group (–COO-) was so small, that the ζ-potential remained below -30 mV. This means that ζ-potential cannot be considered as a remarkable factor of stability for these chimeric liposomal colloidal dispersions, but in any case, the experimental observations of this parameter indicate formation of chimeric lipid/polymer liposomes with the polymers chains in the periphery.
Stability assessment of thermoresponsive polymer-grafted liposomes
The physical stability over time of all conventional and chimeric nanosystems was assessed by measuring the size and size distribution overall one-month period. As shown in Figures 2 and 3, all liposomal formulations in PBS were found to roughly retain their original size, but most of them reached high PDI values at the end of the assessment (data not shown).
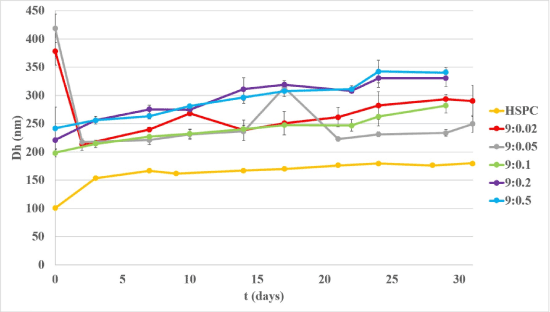
Figure 2: Stability studies in conventional and chimeric nanoassemblies, concerning their size (ZAve).
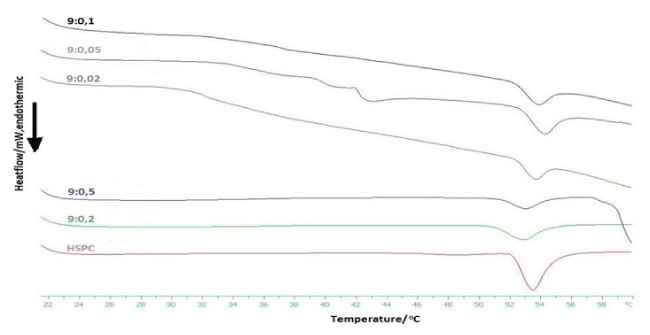
Figure 3: DSC heating scan of HSPC:C12H25-PNIPAM-COOH chimeric bilayers in PBS (pH=7.4) (Heating).
The smaller size was exhibited in conventional liposomes, which is maintained through weeks. Chimeric structures were separated in two groups. Lower molar ratios 9:0.02 and 9:0.05 belong to the first group and display the highest Dh, approximately 400 nm, immediately after preparation, while the third day their size already reduced by half and then remained around so. The second group consists of the 9:0.1, 9:0.2 and 9:0.5 chimeric liposomes, exhibiting the minimum size during their preparation day (t=0days), which is increased slightly, as days passed. As a consequence, it is concluded that 9:0.02 and 9:0.05 chimeric systems need longer relaxation time, in order to achieve better cooperativity among their biomaterials, while higher molar ratios develop adequate cooperativity during annealing time (30 min), right after sonication [16]. The lipid chains of HSPC are saturated and the C12H25– is a small hydrophobic segment. These two conditions may explain the classification of chimeric liposomes in two categories, since the increase in the number of the incorporated chains within the liposome bilayers may result in spatial constrains and gradual morphological changes.
The HSPC:C12H25-PNIPAM-COOH chimeric liposomes were found to retain their original physicochemical characteristics, at least for the time period that they were studied. It is obvious that C12H25-PNIPAM-COOH provides colloidal stability to the systems, through various biophysical ways. Its hydrophilic tails (–COOH) develop repulsive interactions, due to various effects, which keep the nanoassemblies in long distance. Steric stabilization includes the enthalpic process, during which the water molecules between opposing chains are driven away, leading to enthalpy increase and subsequent repulsion [17]. Furthermore, hydrated polymer chains prevent liposome aggregation, due to their stereochemical conformation. Both repulsive forces are responsible for keeping the chimeric nanovectors far enough, thus avoiding the van der Waals attraction of the DLVO theory, since there is an absence of significant electrostatic repulsion, which usually applies on colloidal dispersion systems [18].
Thermotropic behavior investigation of HSPC:C12H25-PNIPAM-COOH chimeric bilayers
The DSC heating and cooling profiles of the prepared pure HSPC bilayers, as well as of the C12H25-PNIPAM-COOH-grafted HSPC bilayers in PBS are presented in Figure 4 and 5 respectively. The values of the corresponding thermodynamic parameters for the system phase transitions are shown in Tables 2 and 3. The effect of the presence and amount of the homopolymer upon the lipid membrane’s thermotropic/phase behavior is discussed, in terms of self-assembled organization, fluidity and cooperativity.
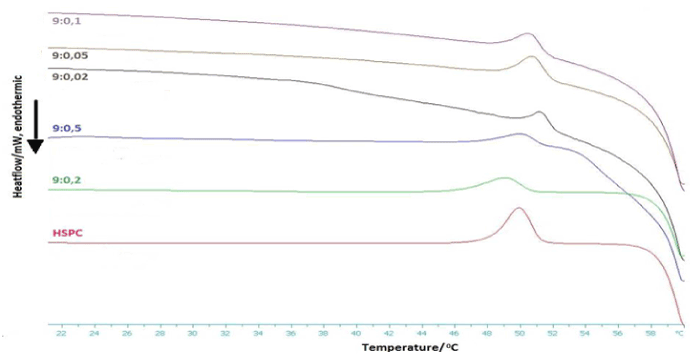
Figure 4: DSC cooling scan of HSPC:C12H25-PNIPAM-COOH chimeric bilayers in PBS (pH=7.4) (Cooling).
The neat HSPC bilayers exhibited two phase transitions, as the temperature was rising. The first was a wide pretransition, with negligible enthalpy change, from gel (Lβ’) to ripple phase (Pβ’), centered at 48.9°C. The second one was a major sharp endothermic phase transition, from rippled (Pβ’) to liquid crystalline phase (Lα), with peak at 53.6°C. These thermotropic results are in line with past studies [19].
The pretransition peak, from gel (Lβ<2021 Copyright OAT. All rights reservase (Pβ’), vanished when polymer chains, regardless of their concentration, were entrapped into the lipid bilayers. As a consequence, it is suggested that the existence of C12H25-PNIPAM-COOH on the liposome outer surface affects significantly the polar head group behavior.
As far as DSC results are concerned, we have likewise noticed two groups. Systems with lower molar ratios, 9:0.02, 9:0.05 and 9:0.1, kept absorbing energy above 32°C, which is the polymer’s lower critical solution temperature (LCST). The polymer chains turn hydrophobic, over 32°C, due to their thermo-responsiveness, perturbate the lipid membrane and set the thermotropic parameters unable to be measured. This happens because of the hydrophobic tail C12H25–, which penetrates into the bilayer and causes membrane disruption and phase separation. As a result, they exhibit properties similar to those of ordinary (isotropic) liquids [14,20]. However, a transition peak around 54°C was observed for each thermogram, which is the main transition temperature for HSPC lipids, something which is expected, as long as there is still conformation among phospholipids.
Molar ratios 9:0.2 and 9:0.5 belong to the second group. The thermoresponsive polymer altered the systems’ thermotropic behavior and modified their main transition, compared to the one of pure HSPC lipid bilayers. More specifically, the specific enthalpy (ΔΗm) was substantially decreased, while the onset (Tonset,m) and peak (Tm) temperatures of the main transition remained almost unaffected, undergoing a downshift of less than 1°C. Finally, the width at half peak height (ΔT1/2) slightly increased. These alterations prove that polymer chains cooperate well with HSPC lipids and that they do not induce significant fluidization [21].
After analyzing all of the thermograms, we conclude that lower molar ratios may not present an adequate thermotropic behavior, because they need longer relaxation time so as to achieve better cooperativity between polymer chains and phospholipids, a fact that is confirmed by their physicochemical studies.
Finally, the slight hysteresis in the cooling diagrams of both conventional and chimeric systems, around 3°C, possibly suggests the formation of a partial interdigitated phase [20].
Conventional liposomes can be modified to produce advanced drug delivery nanosystems (aDDnSs), after the incorporation of stimuli-sensitive polymers, such as the thermoresponsive homopolymer C12H25-PNIPAM-COOH. The polymer guest decreases the membrane tension and prevents liposomal fusion at the same time. The present study aims to highlight that the different ratios of the polymer guest affect the properties and stability of these lyotropic liposomal dispersions in different ways. Regarding HSPC:C12H25-PNIPAM-COOHmixed liposomes, physicochemical studies prove that the most stable systems are 9:0.1 and 9:0.2 molar ratios. These systems not only maintain their hydrodynamic diameter in the long run of one month, but they were also found to be homogeneous enough, due to steric/enthalpic stabilization. Thermodynamic findings have also indicated that better cooperativity was developed with higher concentrations of C12H25-PNIPAM-COOH, where the polymer slightly affected the thermotropic behavior of the lipid bilayer. These results may be taken as a trigger for developing innovative liposomal formulations, incorporating drugs improving their pharmacokinetic profile and consequently their effectiveness, taking into consideration the nanocarriers’ stimuli responsiveness.
- Lagerwall JPF, Scalia G, Nguyen TQ, Wu JJ, Doan V, et al. (2012) A new era for liquid crystal research: Applications of liquid crystals in soft matter nano-, bio- and microtechnology. Curr Appl Phys 12: 252-268.
- Shashi K, Satinder K, Bharat P (2012) A complete review on: Liposomes. Int J Pharm 7:10-16.
- Gin DL, Pecinovsky CS, Bara JE, Kerr RL (2008) Functional Lyotropic Liquid Crystal Materials. Struct Bond 128: 181-222.
- Hoffman AS (1995) "Intelligent" polymers in medicine and biotechnology. Artif Organs 19: 458-467. [Crossref]
- Ward MA, Georgiou TK (2011) Thermoresponsive Polymers for Biomedical Applications. Polymers 3: 1215-1242.
- Roy D, Brooks WL, Sumerlin BS (2013) New directions in thermoresponsive polymers. Chem Soc Rev 42: 7214-7243. [Crossref]
- Demetzos C, Pippa N (2014) Advanced drug delivery nanosystems (aDDnSs): a mini-review. Drug Deliv 21: 250-257. [Crossref]
- Kono K, Nakai R, Morimoto K, Takagishi T (1999) Thermosensitive polymer-modified liposomes that release contents around physiological temperature. Biochim Biophys Acta 1416: 239-250. [Crossref]
- Pippa N, Meristoudi A, Pispas S, Demetzos C (2015) Temperature-dependent drug release from DPPC:C12H25-PNIPAM-COOH liposomes: Control of the drug loading/release by modulation of the nanocarriers’ components. Int J Pharm 485: 374-382. [Crossref]
- Joglekar M, Trewyn BG (2013) Polymer-based stimuli-responsive nanosystems for biomedical applications. Biotechnol J 8: 931-945. [Crossref]
- Mura S, Nicolas J, Couvreur P (2013) Stimuli-responsive nanocarriers for drug delivery. Nat Mater 12: 991-1003. [Crossref]
- Hadinoto K, Sundaresan A, Cheow WS (2013) Lipid-polymer hybrid nanoparticles as new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm 85: 427-443. [Crossref]
- Ganta S, Devalapally H, Shahiwala A, Amiji M (2008) A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release 126: 187-204. [Crossref]
- Naziris N, Pippa N, Meristoudi A, Pispas S, et al. (2017) Design and development of pH-responsive HSPC:C12H25-PAA chimeric liposomes. J Liposome Res 27: 108-117. [Crossref]
- Tribet C, Vial F (2008) Flexible macromolecules attached to lipid bilayers: impact on fluidity, curvature, permeability and stability of the membranes. Soft Matter 4: 68-81.
- Lutz JF, Akdemir O, Hoth A (2006) Point by point comparison of two thermosensitive polymers exhibiting a similar LCST: is the age of poly(NIPAM) over? J Am Chem Soc 128: 13046-13047. [Crossref]
- Immordino ML, Dossio F, Cattel L (2006) Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine 1: 297-315. [Crossref]
- Derjaguin B, Landau L (1993) Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Prog Surf Sci 1: 30-59.
- Koynova R, Caffrey M (1998) Phases and phase transitions of the phosphatidylcholines. Biochim Biophys Acta 1376: 91-145. [Crossref]
- Demetzos C (2008) Differential Scanning Calorimetry (DSC): A Tool to Study the Thermal Behavior of Lipid Bilayers and Liposomal Stability. J Liposome Res 18: 159-173. [Crossref]
- Kolman I, Pippa N, Meristoudi A, Pispas S, Demetzos C (2016) A dual-stimuli-responsive polymer into phospholipid membranes-A thermotropic approach. J Therm Anal Calorim 123: 2257-2271.




