Background: The objective of this report is to demonstrate a functional retinal rescue in a patient with retinitis pigmentosa (RP) after the intravitreal use of bone marrow mononuclear fraction containing CD34+ stem cells.
Case presentation: This report presents the case of a 38-year-old female patient with RP and macular hole, who underwent treatment with the intravitreal injection of bone marrow mononuclear fraction containing CD34+ stem cells and showed an impressive improvement in the visual field after the procedure.
Conclusions: Bone marrow mononuclear fraction containing CD34 + stem cells can lead to the functional improvement of retina via the paracrine effect. Studies with a larger number of cases and longer follow-up times are required for the further analysis of the safety and efficacy of this therapeutic possibility.
retinitis pigmentosa, macular hole, stem cell, bone marrow mononuclear fraction, CD34 + stem cells
RPE: retinal pigment epithelium; RP: Retinitis pigmentosa; IPS: induced pluripotent stem; MNCs: mononuclear cells
Retinitis pigmentosa (RP) is a group of inherited diseases characterized by a progressive loss of peripheral vision and difficulties in night vision (nictalopia), further leading to the loss of central vision [1,2]. No pharmacological therapy has been clearly proven or established to prevent the development and progression of RP or the restoration of vision [2]. Most pharmacological agents try to slow down the progression of the disease via neuroprotection and preserve the useful vision of affected individuals throughout their lives. Such strategies do not correct the underlying causes of PR, but aim to provide supportive and conservative treatment. Auxiliary treatments are also available for treating the secondary complications of PR, such as cystoid macular edema and cataracts [1,2]. Several techniques using stem cells are being tested in both experimental models and clinical studies to treat the degenerative retinal diseases [3-11].
Three clinical studies were conducted in Brazil wherein the mononuclear fraction of the bone marrow containing CD34 + stem cells were used in patients with RP, macular degeneration, and ischemic retinopathy. The researchers observed the signs of activity of these cells [12-15].
On this occasion, this report presents a case of a 39-year-old female patient with a history of RP and a macular hole in the left eye considered to be old (eight years ago). The visual acuities of the right and left eyes were 20/25 and 20/400, respectively, along with the intraocular pressure of 14 mmHg in both the eyes.
Surgery was not indicated in this case because of the older occurrence of macular hole along with the involvement of the RP in the foveal region. The patient was clarified about the clinical studies of cell therapy for RP, and she selected a therapeutic approach. After signing the informed consent form according to the authorization of the ethics and research committee of the Beneficência Portuguesa Hospital of São José do Rio Preto, the patient went through bone marrow collection process via iliac crest puncture, which was performed by the hematology team under local anesthesia. The bone marrow sample was processed in Biosafe Sepax (GE Healthcare, USA).
Initially, the patient underwent the following baseline tests: multifocal electroretinogram, optical coherence tomography (OCT), color and fluorescent retinography, autofluorescence, and automated perimeter background by using the Compass system (Centerview, Padova, Italy).
The patient underwent the intravitreal injection of 10 × 106 mononuclear cells (MNCs) containing CD34 in the left eye under topical anesthesia. A three-month evaluation reported improvement in the visual quality of the patient. The visual acuity observed in the third month of follow-up were 20/25 and 20/150 in the right and left eyes, respectively. The visual field showed significant changes in the left eye that corroborated with the patient’s report (Figure 1).
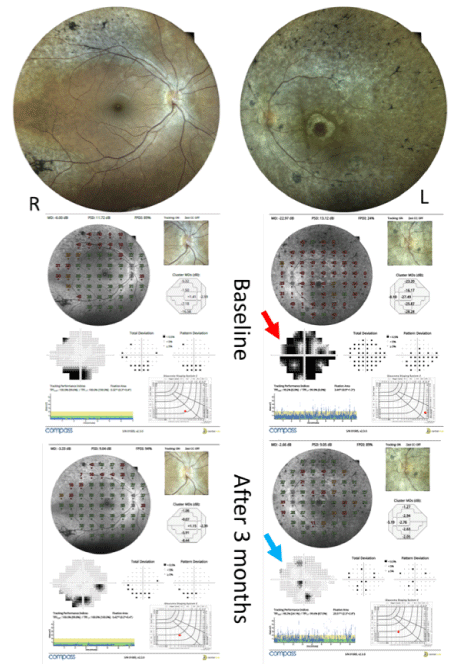
Figure 1. Fundus Automated Perimetry over standard Automated Perimetry showing significant improvement in retinal sensitivity in the left eye, 3 months after cell therapy
The electrical response of the multifocal electroretinogram test also reported improvement in the left eye. There were no changes in the OCT and fluorescent retinography.
In this specific case, unlike previously published protocols, we used the Biosafe Sepax system for cell separation. Most of the published studies use a manual ficoll density separation procedure to enrich the population of MNCs, thus enriching the content of stem cells in the final product of cell therapy. Manual ficoll procedures have certain intrinsic limitations, such as variability in technique, training time, and risk of contamination because of their open nature. The recent technological developments in automated and closed devices have superseded most of the open and manual cell processing procedures [16,17].
This system also allows for a higher concentration of MNCs than the traditional method, possibly increasing the therapeutic effectiveness. Additional benefits of the closed system include the ease of training and a more safer speed of cell separation as it is not an open system (lesser risk of contamination). Additionally, this system also allows the standardization of the technique for a greater reproducibility of clinical studies [17].
In general, stem cells may be able to restore the function of the retina via two pathways: a) Replacement of cells (in this case, it is necessary to use the cells prepared in culture with the retinal pigment epithelium(RPE) cells derived from the embryonic cells and induced pluripotent stem (iPS). b) Rescue therapy is also known as the paracrine effect (consisting of the main mechanism of action of the cells used in this study). The paracrine effect is defined as an action exerted by a substance secreted by a cell in the local cellular environments. Some cell-to-cell communications require a direct cell-to-cell contact. Some cells can form connecting junctions that connect their cytoplasm to the cytoplasm of adjacent cells. In the cardiac muscles, for example, the communicating junctions between the adjacent cells allow the spread of the action potential of the cardiac pacemaker region of the heart to spread and cause, in a coordinated manner, the contraction of the heart [11,18]. Paracrine factors secreted by the cells that are transplanted include cytokines, chemokines, and growth factors, which are involved in orchestrating the repair process controlled by these cells. This mechanism is not yet fully understood, and many ongoing studies are aiming to determine the possibility of only injecting these factors without cells (acellular therapy) [11,18].
The functional improvement observed in the third month corresponds to the previous reports. The duration of the treatment effect is still uncertain. In another study, we observed that there was a decrease in the effect of therapy at the end of the first year [19].
There was no change in the structure of the macular hole, as well as other abnormalities in the thickness of the retina assessed by OCT.
The bone marrow mononuclear fraction containing CD34+ stem cells can lead to the functional improvement of the retina via the paracrine effect. Several clinical studies are under progress, and a more significant number of cases will be necessary to establish which stage of the disease shows the best response to this therapy, provided that the cells are affected in a degenerative process (paracrine effect) and not on the cell replacement as well as identifying the ideal time to reinject the cells in maintaining the therapeutic effect.
"Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal."
Research project approved by National Research Ethics Committee in and it is registered on the: CAAE:66839617.2.0000.5629.
RCS and MR designed the study, PS, LC and TS collected and analyzed the data, and took the writing lead. RCS and MR designed and directed the study. All authors discussed the results and commented on the manuscript.
The authors report no conflicts of interest in this work.
The authors received no funding for this study.
- He Y, Zhang Y, Su G (2015) Recent advances in treatment of retinitis pigmentosa. Curr Stem Cell Res Ther 10: 258-265. [Crossref]
- Menghini M, Cehajic-Kapetanovic J, MacLaren RE (2020) Monitoring progression of retinitis pigmentosa: current recommendations and recent advances. Expert Opin Orphan Drugs 8: 67-78. [Crossref]
- Ludwig PE, Freeman SC, Janot AC (2019) Novel stem cell and gene therapy in diabetic retinopathy, age related macular degeneration, and retinitis pigmentosa. Int J Retina Vitreous 5: 7.
- Terrell D, Comander J (2019) Current stem-cell approaches for the treatment of inherited retinal degenerations. Semin Ophthalmol 34: 287-292. [Crossref]
- Tang Z, Zhang Y, Wang Y, Zhang D, Shen B, et al. (2017) Long-term safety of human retinal progenitor cell transplantation in retinitis pigmentosa patients. J Transl Med 8: 209.
- Mandai M, Kurimoto Y, Takahashi M (2017) Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med 377: 792-793.
- Weiss JN, Levy S (2018) Stem cell ophthalmology Treatment Study: bone marrow derived stem cells in the treatment of Retinitis pigmentosa. Stem Cell Investig 5: 18. [Crossref]
- Comyn O, Lee E, MacLaren RE (2010) Induced pluripotent stem cell therapies for retinal disease. Curr Opin Neurol 23: 4-9.
- Park SS, Bauer G, Abedi M, Pontow S, Panorgias A, et al. (2014) Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findings. Invest Ophthalmol Vis Sci 56: 81-89.
- Cotrim CC, Jorge R, Oliveira MC, Pieroni F, Messias AMV, et al. (2020) Clinical studies using stem cells for treatment of retinal diseases: state of the art. Arq Bras Oftalmol 83: 160-167. [Crossref]
- Park SS, Moisseiev E, Bauer G, Anderson JD, Grant MB, et al. (2017) Advances in bone marrow stem cell therapy for retinal dysfunction. Prog Retin Eye Res 56: 148-165.
- Siqueira RC, Messias A, Voltarelli JC, Messias K, Arcieri RS, et al. (2013) Resolution of macular edema associated with retinitis pigmentosa after intravitreal use of autologous BM-derived hematopoietic stem cell transplantation. Bone Marrow Transplant 48: 612-613.
- Siqueira RC, Messias A, Gurgel VP, Simões BP, Scott IU, et al. (2015) Improvement of ischaemic macular edema after intravitreal injection of autologous bone marrow-derived hematopoietic stem cells. Acta Ophthalmol 93: e174-e176. [Crossref]
- Siqueira RC, Messias A, Voltarelli JC, Scott IU, Jorge R, et al. (2011) Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: a phase I trial. Retina 31: 1207-1214.
- Cotrim CC, Toscano L, Messias A, Jorge R, Siqueira RC, et al. (2017) Intravitreal use of bone marrow mononuclear fraction containing CD34+ stem cells in patients with atrophic age-related macular degeneration. Clin Ophthalmol 11: 931-938.
- Golay J, Pedrini O, Capelli C, Gotti E, Borleri G, et al. (2018) Utility of routine evaluation of sterility of cellular therapy products with or without extensive manipulation: best practices and clinical significance. Cytotherapy 20: 262-270. [Crossref]
- Zinno F, Landi F, Scerpa MC, Aureli V, Lanti A, et al. (2011) Processing of hematopoietic stem cells from peripheral blood before cryopreservation: use of a closed automated system. Transfusion 51: 2656-2663.
- Siqueira RC, Voltarelli JC, Messias AM, Jorge R (2010) Possible mechanisms of retinal function recovery with the use of cell therapy with bone marrow-derived stem cells. Arq Bras Oftalmol 73: 474-479.
- Siqueira RC, Messias A, Messias K, Arcieri RS, Ruiz MA, et al. (2015) Quality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell-clinical trial). Stem Cell Res Ther 6: 29. [Crossref]

