Objectives: The aims of this study were to assess the predictive value of HPV-positivity in cervical lymph node metastases for the detection of a primary tumor (PT), to investigate the influence of HPV-positivity in cervical lymph node metastases on tumor control and survival and to analyse the impact of the 8th edition of the UICC TNM classification on staging and survival.
Methods: Neck dissection specimen of 47 consecutively included patients were analysed by PCR-based HPV DNA-testing and p16-immunostaining and survival analysis by Kaplan-Meier and Cox proportional hazard regression models were performed.
Results: After standard work-up algorithm in patients with suspected CUP 27/47 patients (57%) remained a CUP whereas in 20/47 (43%) a PT was detected (tonsils, lung, parotid gland, skin). 14/20 (70%) with detected PT and 9/27 (30%) with CUP had HPV-positive cervical lymph node metastases (LNM). Sensitivity, specificity, positive predictive value and negative predictive value of HPV-positivity in LNM for diagnosis of a PT in the oropharynx achieved 65%, 95%, 94% and 69%, respectively. In the subgroup of patients with CUP the 5-year overall/disease specific survival (OS/DSS) of patients with HPV-positive compared to HPV-negative LNM was higher (5y-OS 73% vs. 41%, p=0.01, 5y-DSS 73% vs. 53%, p=0.02). The use of the new UICC TNM classification leads to down staging of 65% of patients with detected PT and 33% with CUP. Furthermore, advanced N-category becomes an important negative prognosticator for OS (N≥2: HR 3.05, 95% CI 1.21-7.67, p=0.01).
Conclusion: HPV-positivity in LNM directs the detection of PT in the oropharynx in patients with CUP. One third of LNM in patients with CUP were HPV-positive associated with improved OS and DSS. When applying the 8th edition advanced N-category and UICC stages are negative prognosticators for survival supporting the role for integration of biomarkes in staging systems.
cancer of unknown primary (CUP), human papillomavirus (HPV), diagnosis, survival
Abbreviations
BOT: Base of Tongue; LNM: Cervical Lymph Node Metastasis; CUP: Cancer of Unknown Primary; DSS: Disease Specific Survival; ECS: Extracapsular Spread; EDTA: Ethylene Diamine Tetraacetic Acid; FNAC: Fine Needle Aspiration Cytology; HNSCC: Head and Neck Squamous Cell Carcinomahr: Hazard Ratio; HPV: Human Papillomavirus High Risk Type; LNM: Lymph Node Metastasis; NPV: Negative Predictive Value; OS: Overall Survival; OPSCC: Oropharyngeal Squamous Cell Carcinoma; PET: Positron Emission Tomography; PPV: Positive Predictive Value; PT: Primary Tumor; RT: Radiotherapy; TMA: Tissue Microarrays; TORS: Transoral Robotic Surgery; UICC: Union for International Cancer Control
Cervical lymph node metastasis from clinically undetectable primary squamous cell carcinoma (cancer of unknown primary, CUP) presents a diagnostic and therapeutic challenge. In recent years the rate of CUP decreased as a result of improved imaging techniques, in particular positron emission tomography with contrast-enhanced computerized tomography (PET/CT), as well as the accurate endoscopic examination of the upper aerodigestive tract with biopsy of PET positive areas and routine diagnostic tonsillectomy [1]. Treatment recommendations in CUP range from elective radiotherapy (RT) of the putative mucosal primary sites and bilateral neck to surgery of the neck alone [2-5]. In the era of treatment deintensification the potential gain of RT of putative primary tumor sites has to be weighed against its detrimental effect on toxicity and quality of life. A CUP is associated with a worse prognosis compared to patients with known primary tumors (PT). The literature reports on a vast range of five years overall survival (OS) from 25 to 75% [2,4,6,7]. In the last decade, the role of human papilloma virus (HPV) high risk type infection in the development of head and neck squamous cell carcinoma (HNSCC) has gained evidence [8-14]. HPV is most probably responsible for an increasing incidence of oropharyngeal squamous cell cancer (OPSCC) despite declining prevalence of common risk factors (smoking, alcohol) [15-17]. Patients with HPV-associated OPSCC show a significantly better survival compared to patients with tobacco and alcohol associated HPV-negative tumors [18-21]. Several studies showed a high correlation between HPV-positive lymph node metastases and the detection of the PT in the oropharynx as well as a rising incidence of HPV-positive lymph node metastases with CUP [22-25]. Many HPV-positive tumors present with prominent nodal disease and small or even unknown primaries hidden in palatine and lingual tonsillar crypts difficult to detect even radiologically [26]. On the other hand, PET/CT is plagued by false positives from hypermetabolic oropharyngeal lymphoid tissue [26]. HPV-positivity in lymph node metastases influences the diagnosis and treatment of patients with CUP [27]. The updated 8th edition of the UICC TNM classification of malignant tumors was released in October 2016 and is effective since January 2017. It contains major changes for CUP and OPSCC by integrating HPV-positivity of the PT or the LNM in staging. The aims of this study were (1) to assess the predictive value of HPV-positivity in cervical lymph node metastases for the detection of a PT, in particular in the oropharynx, (2) to investigate the potential impact of HPV-positivity in cervical lymph node metastases on tumor control and survival and (3) to analyse the impact of the 8th edition of the UICC TNM classification on staging and survival.
Patient cohort
All patients referred to the Kantonsspital St. Gallen between 1999 and 2012 for diagnostic work up of suspected CUP were consecutively enrolled in our study. Standard work up algorithm of patients with suspected CUP consisted of sonography and fine needle aspiration cytology (FNAC) of cervical lymph node metastases, radiological work up (CT, MRI and after 01/2009 PET-CT), panendoscopy under general anaesthesia with biopsy of suspicious lesions and ipsilateral diagnostic tonsillectomy.
Based on the findings after complete clinical and radiological work-up, two subgroups were defined. The first group was formed by patients with a detected PT and the second group by patients with a CUP.
Patient charts were analysed for demographic data (age, gender), clinical risk factors (smoking history, alcohol intake), N-category (UICC TNM 7th and 8th edition), characteristics of the primary tumor in case of detection (tumor site and T-category, UICC TNM 7th and 8th edition), treatment modality as well as oncological outcome.
Tissue microarray
Formalin-fixed paraffin-embedded tissues were used to construct three tissue microarrays (TMA) with cores of 47 cervical lymph node metastases and 8 palatine tonsils [28]. Three tissue cores (diameter 2 mm) were punched out of each donor block and transferred to the recipient block. Haematoxylin and eosin staining of TMA sections was performed using standard techniques.
P16 staining
Three-µm tissue sections were deparaffinized followed by antigen retrieval (EDTA pH 9.0, 95° C, 30 minutes). P16 staining (clone E6H4, dilution 1:10, 30 minutes; MTM Laboratories, Heidelberg, Germany) was performed on a Leica BOND MAX instrument (Leica) using the Bond Polymer Refine detection kit (Leica). P16 positivity was defined as ≥ 70% tumor cells with strong nuclear and cytoplasmic staining [29].
HR-HPV detection
Tumor cells were located and marked on H&E stained tissue sections obtained from a representative paraffin block of the primary OPSCC. Tumor cells were micro-dissected from three to five 4 µm thick serial tissue sections of the same paraffin block and genomic DNA was prepared. HPV DNA was detected by PCR amplification using the L1C1/L1C2 consensus primer set as previously described [30]. PCR products were purified using the QIAGEN PCR purification Kit (QIAGEN). Negative and positive controls were included in each HPV PCR run. To assess effective DNA extraction and DNA integrity, beta-globin PCR was performed for samples with negative HPV PCR. Only samples with amplifiable DNA were scored as negative for HPV. HPV typing was performed by direct sequencing of purified PCR products as previously described [31]. A positive HPV-status of the lymph node metastases was defined by immunohistochemical p16 overexpression and HPV DNA detection by PCR [29].
Statistical analysis
All statistical analyses were performed by using the International Business Machines Corporation (IBM) Statistical Package of the Social Sciences (SPSS) Statistics Version 20.0 for Windows. The Kaplan–Meier method provided estimates of overall survival (OS) and disease specific survival (DSS), reported for the entire cohort as well as the two groups (patients with detected PT and CUP) and stratified by clinical risk factors and HPV-positivity. For comparison between the two groups the log-rank (Mantel-Cox) test was used. Cox proportional-hazard models were used to estimate hazard ratios (HR) for different
covariates. We calculated the sensitivity, specificity, positive (PPV) and negative predictive value (NPV) of HPV detection in cervical lymph node metastases using the following definitions.
True positive cases: Patients with HPV-positive lymph node metastases and a PT in the oropharynx. False positive cases: Patients with HPV-positive lymph nodes and a PT outside the ororpharynx or a CUP. True negative cases: Patients with HPV-negative lymph nodes and a PT outside the ororpharynx and patients with a CUP. False negative cases: Patients with HPV-negative lymph nodes and a PT in the oropharynx.
A two-tailed p-value of 0.05 or less was considered statistically significant.
Ethical consideration
The study protocol obtained approval by the local Ethics Committee and all procedures were in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in 1983.
47 patients referred with suspected CUP were enrolled in our study. 34 (72%) were men, 13 (28%) were women. The mean age was 62 years (range: 30 to 89 years). After completion of the standard work-up algorithm a PT was detected in 20/47 patients (43%). 27/47 patients (57%) were finally diagnosed as CUP. In 14/20 patients (70%) with detected PT, the lymph node metastases were defined as positive for HPV based on p16 overexpression and HPV DNA detection by PCR. All the PT of those patients were located in the tonsils. The PT of HPV-negative lymph node metastases were located in the tonsil (1/6), the lung (1/6), parotid gland (2/6) and the skin (2/6).
Only 8/27 (30%) patients with a CUP had HPV-positive lymph node metastases. In 1/27 CUP-patients a PT was initially missed and emerged after 15 months in the base of tongue with HPV-positive lymph node metastases.
The detection of HPV-positivity in lymph node metastases achieved a sensitivity, specificity, positive predictive value and negative predictive value of 65%, 95%, 94% and 69%, respectively, for the diagnosis of a PT in the oropharynx.
Patients with CUP tended to be more often smokers and regular drinkers than patients with detected PT. Based on the 7th edition of the TNM classification advanced N-category and lymph node metastases with extracapsular spread were more frequent in patients with CUP compared to patients with detected PT, but there was no statistically significant difference in UICC tumor stages between the groups (Table 1). Based on the 8th edition of the TNM classification 90% of patients with a detected PT were classified as N1 compared to 30% of patients with CUP. In patients with PT 14/20 (70%) were staged as UICC stage I/II compared to 9/27 (33%) in patients with CUP. This difference was statistically significant (p=0.001) leading to a significant down staging rate of 65% compared of 33% (Table 1). Other factors such as performed treatment, gender and age did not differ between the groups.
Table 1. Patients und tumor characteristics according to detection of primary tumor (n=47).
|
Detected PT (n=20) |
CUP (n=27) |
p-value |
Gender Female Male |
7 (35%) 13 (65%) |
6 (22%) 21 (78%) |
0.33 |
Age Median (range) |
57y (30-89y) |
63y (45-87y) |
0.18 |
Radiological work up None (due to pregnancy) CT/MRI PET/CT |
1 (5%) 8 (40%) 11 (55%) |
- 15 (56%) 12 (44%) |
0.54 |
Treatment Neck dissection Primary RCT Neck dissection plus adjuvant RT Neck dissection plus adjuvant RCT |
3 (15%) 7 (35%) 10 (50%) 0 |
1 (4%) 1 (4%) 8 (29%) 17 (63%) |
0.4 |
HPV Negative Positive |
6 (30%) 14 (70%) |
19 (70%) 8 (30%) |
0.006* |
N-category 7th edition N1 N2 N3 |
6 (30%) 14 (70%) - |
- 24 (89%) 3 (11%) |
0.004* |
ECS Yes No |
6 (30%) 14 (70%) |
15 (56%) 12 (44%) |
0.01* |
N-category 8th edition N1 N2 N3 |
18 (90%) 1 (5%) 1 (5%) |
8 (30%) 7 (26%) 12 (44%) |
0.0001* |
UICC 7th edition Stage I/II Stage III/IV |
1 (5%) 19 (95%) |
- 27 (100%) |
0.24 |
UICC 8th edition Stage I/II Stage III/IV |
14 (70%) 6 (30%) |
9 (33%) 18 (67%) |
0.001* |
Smoking < 10 py > 10py |
14 (70%) 6 (30%) |
12 (44%) 15 (56%) |
0.08 |
Drinking < 3 U/d > 3 U/d |
18 (90%) 2 (10%) |
18 (67%) 9 (33%) |
0.06 |
For survival analysis patients with a PT located outside the upper aerodigestive tract (2 skin cancers, 2 parotid cancers) were excluded resulting in a study group of 43 patients.
The mean followup time of the entire cohort was 64 months (range 5-180 months). The 5-years overall (OS) and disease specific survival (DSS) achieved 64% and 68%, respectively. Analysing the impact of different risk factors on survival, univariate cox proportional hazard regression revealed significantly higher hazard ratios (HR) in OS for patients with CUP as well as a lower HR for HPV-positivity in cervical lymph node metastases, whereas advanced gender, age over 70 years, ECS, alcohol consumption and smoking did not have an impact.
According to 7th edition of the TNM classification advanced N-category (N ≥ 2) and advanced UICC stages (UICC stage III and IV) have no influence on OS, whereas according to the 8th edition of the TNM classification advanced N-category (N ≥ 2) has a statistically significant impact on OS (N ≥ 2: HR 3.05, 95% CI 1.21-7.67, p=0.01). The same results were found for advanced UICC stages (UICC stage I/II vs. III/IV; OS: HR 4.33, 95% CI 1.55-12.08, p=0.005) (Table 2).
Table 2. Univariate hazard ratio for OS of different risk factors (n=43).
Covariate |
Hazard Ratio (95% CI) |
p-Value |
Overall Survival |
|
CUP vs PT |
4.50 (1.18-13.84) |
0.02* |
HPV-positivity |
0.16 (0.05-0.48) |
0.001* |
Alcohol consumption (>3U/d) |
2.30 (0.94-5.67) |
0.06 |
Smoking (>10py) |
1.7 (0.70-4.15) |
0.2 |
Advanced N-category (>N2a) 7th edition |
1.65 (0.63-4.31) |
0.3 |
Advanced N-category (>N2a) 8th edition |
3.05 (1.21-7.67) |
0.01* |
No ECS |
1.68 (0.65-4.06) |
0.2 |
Age > 70 years |
2.24 (0.89-5.63) |
0.08 |
Male Gender |
1.60 (0.53-4.86) |
0.4 |
Disease specific survival |
|
CUP vs PT |
2.62 (0.73-9.41) |
0.1 |
HPV-positivity |
0.18 (0.05-0.67) |
0.01* |
Alcohol consumption (>3U/d) |
2.62 (0.90-7.58) |
0.07 |
Smoking (>10py) |
1.04 (0.36-2.97) |
0.9 |
Advanced N-category (>N2a) 7th edition |
2.66 (0.74-9.59) |
0.9 |
Advanced N-category (>N2a) 8th edition |
2.4 (0.73-8.44) |
0.1 |
No ECS |
1.75 (0. 61-5.07) |
0.3 |
Age > 70 years |
1.53 (0.51-4.58) |
0.4 |
Male Gender |
2.59 (0.58-11.61) |
0.2 |
In DSS only HPV-positivity in cervical lymph node metastases was associated with a statistically higher survival probability. Advanced N-category or UICC stages did not have an influence on DSS irrespective of the edition of the TNM classification.
In multivariate analysis only, HPV-positivity in cervical lymph node metastases kept its statistically significant positive influence on survival (OS: HR 0.17, 95% CI 0.04-0.71, p=0.01, DSS: HR 0.12, 95% CI 0.02-0.69, p=0.02) whereas the other parameters no longer played a statistically significant role.
The comparison of the survival estimates of patients with a CUP (26/43, 63%) and the subgroup of patients with a detected PT (17/43, 37%) showed a statistically significant lower OS but not DSS in patients with CUP (5y OS 87% for primary tumor vs 51% for CUP; p=0.01; Figure 1; 5y DSS 87% for primary tumor vs 58% for CUP; p=0.12, respectively). Since patients with a CUP had a lower rate of HPV-positive lymph node metastases possibly negatively influencing survival outcomes the OS and DSS was analyzed in the subgroup of HPV-positive patients. The 5-year OS and DSS in patients with detected PT was 87% compared to 72% in patients with a CUP and 86% vs 73%, p=0.9 respectively. This difference did not reach statistical significance.
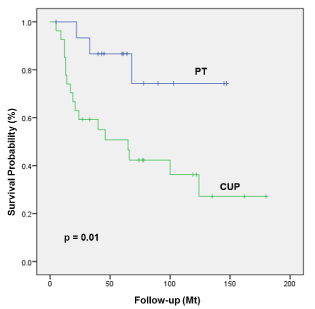
Figure 1. OS of patients with detected primary tumor compared to patients with true CUP.
We also analyzed a potential association between HPV-positivity and survival in the subgroup of patients with CUP. The 5-year OS and DSS of patients with HPV-positive lymph node metastases was higher compared to patients with HPV-negative lymph node metastases (5y OS 73% compared to 41%, p=0.01; Figure 2; 5y DSS 73% compared to 53%, p=0.02; respectively). Other factors such as treatment, tobacco smoking, alcohol intake, ECS, age over 70 years or gender had no impact on survival in patients with CUP. N-category (7th and 8th edition) as well as the UICC staging (7th and 8th edition) had no influence on survival in patients with CUP possibly due to small subgroups of patients.
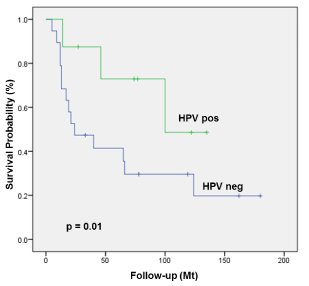
Figure 2. OS of patients with true CUP stratified by HR-HPV-positivity.
In almost half of the patients with cervical lymph node metastases referred to our institution with suspected CUP, the primary carcinoma was identified after the standard work up algorithm consisting of sonography and FNAC of cervical lymph node metastases, radiological work up (CT, MRI and after 01/2009 PET-CT), panendoscopy under general anaesthesia with biopsy of suspicious lesions and ipsilateral diagnostic tonsillectomy. We found a high rate of HPV-positive OPSCC typically presenting with a small PT in the tonsillar crypts and advanced nodal disease. Both HPV DNA detection and p16 overexpression in cervical lymph node metastases have been correlated with a positive HPV-status of the PT [23,24,32,33]. According to Park et al p16 expression was only observed in OPSCC [25]. On the other hand p16 overexpression but not HPV DNA positivity was also detected in cervical lymph node metastases of squamous cell carcinomas of the skin [34]. Therefore, a positive HPV-status of the lymph node metastases in our study was defined by immunohistochemical p16 overexpression and HPV DNA detection as proposed by Smeets et al. [35]. We found a high specificity and a high positive predictive value of HPV-positivity in lymph node metastases and the detection of a PT in the oropharynx. Since HPV-detection and genotyping can be performed on FNAC of metastatic lymph nodes [36], assessing the HPV-status of the cytological materials could serve as a valuable tool in determining the tumor origin in patients with cervical lymph node metastases especially in suspected CUP. In patients with HPV-positive lymph node metastases the diagnostic work up by panendoscopy and multiple biopsies could be no longer warranted since in our cohort none of the primary tumors were located outside the oropharynx or even outside the oropharyngeal lymphatic tissue.
Some recent studies suggest that transoral laser microsurgery (TLM) or transoral robotic surgery (TORS) is more effective for the identification of a primary in the oropharynx especially in the base of tongue (BOT) in patients with suspected CUP [37,38]. Recent case series have reported high rates of detection ranging from 86 to 94% including TLM [38,39] and 72 to 90% including TORS [37,38,40] in the diagnostic work-up of patients with suspected CUP. According to Durmus et al. [41] the PT was identified in 77% of patients (17 of 22) with 80% HPV-positive LNM residing in the palatine tonsil in 59% (13 of 22), and the BOT in 18% (4 of 22). In a study by Channir et al. [42] the PT was identified by TORS in 7/13 patients (54%) at the lingual tonsils with HPV-positive LNM in all of these patients.
In one of our patients with HPV-positive lymph node metastases we initially failed to detect a PT emerging after 15 months in the ipsilateral BOT. This PT would possibly have been diagnosed by routine transoral dissection of the BOT.
Therefore, the resection of the palatine as well as lingual tonsils in particular in case of HPV-positive LNM should be discussed to enhance the detection rate of PT.
Improved identification of the PT site is crucial, since it allows precise definition of the optimal therapeutic approach and targeting of primary or adjuvant RT with or without concomitant chemotherapy. Furthermore, localization of the PT and possible surgical resection possibly allows for the avoidance of adjuvant RT or even chemoradiation to mucosal surfaces depending on the margin status and pathological features of the PT.
The question arises whether ipsilateral palatine and lingual tonsillectomy is sufficient or whether bilateral resection is warranted. According to a recent review a contralateral PT was detected in 6% of BOT and 15% in the contralateral palatine tonsillar specimen [43] comparable to previous reports [44-46].
A further important question is the morbidity of this approach and the exposure to potentially unnecessary surgery with associated risks of perioperative complications. According to the review by Fu et al [43] the overall complication rate of TORS/TLM was low (7%), but not zero. Moreover, a significant decline in multiple QOL domains such as speech, eating, aesthetics, and social disruption has been recently demonstrated up to 12 months after TORS [47].
Identification of the site of PT is important not only to define the optimal management of patients but also for their prognosis. In our cohort the patient group with a detected PT had a 5-year-OS and DSS of 87% and 87%, respectively compared to 51% and 58% in patients with CUP. Large retrospective reviews on patients with CUP reported 5-year OS rates ranging from 43% to 56% [5,7,48-53]. In our cohort CUP patients demonstrated different characteristics known as bad prognosticators such as more HPV-negative lymph node metastases, a higher nodal stage, higher rate of ECS, and a trend to a larger number of patients with the risk factors smoking and alcohol consumption in comparison to patients with a detected primary tumor. But even in the HPV-positive subgroup patients with CUP had a worse oncological outcome compared to patients with a detected PT. Based on the rarity of the disease; only limited information is available on the prevalence, the demographics and the prognostic impact of HPV-positivity in CUP. In our cohort the prevalence of HPV-positivity in patients with CUP was with 30% clearly lower compared to 70% in patients with a detected PT but in line with the literature (18-40%) [54-57]. Only Keller et al. [58] reported with 74% (26/39) on a higher prevalence of p16 expression in lymph node metastases in patients with CUP. Besides the epidemiologic impact of HPV-positivity in OPSCC the HPV-status of a tumor also plays an important prognostic role. Various studies have reported an association between positive HPV-status and survival in primary RT [18-20,59,60] and surgically treated OPSCC patients [61-63]. In line with these findings HPV-positivity of lymph node metastases was an important positive prognostic factor not only in the entire cohort but also in the subgroup of patients with CUP. HPV-positive patients with CUP demonstrated significantly higher survival rates with a 5-year OS of 73% and a 5-year DSS of 73% compared to HPV-negative patients with CUP (5y OS 41% and DSS 53%). In the study by Compton et al [56] the 5-year OS and DSS was with 67% and 67% comparable to our results. In a recent report by Sivars et al. [54] the 5-year OS in HPV-positive patients was with 80% even higher [54]. Keller et al. [58] reported on even more pronounced survival differences in p16 positive compared to p16 negative patients (5y OS 92% vs 30% and 5y DSS 92% vs 60%) [58]. Similar results were demonstrated by Jensen et al. [57], Schroeder et al. [27] and Axelsson et al. [64], whereas Tribius et al. [55] and Dixon et al. [65] did not find any survival difference. In contrast to some [1,2,27,66-68] but in line with other reports [54,56] nodal stage did not have an impact on survival in our cohort. In all but one study [68] demonstrating a prognostic role of the extent of nodal disease in CUP patients the HPV-status of the tumor had not been determined. In the report by Park et al. [68] HPV-positivity was only analysed by in situ-hybridization possibly leading to false-negative results. Other studies failed to demonstrate an association between nodal stage and prognosis in HPV-positive tumors [69]. Only in HPV-negative tumors overall TNM stage and nodal involvement were predictors of survival, whereas in HPV-positive disease these parameters lost their prognostic impact. These findings have been considered in the 8th edition of the UICC TNM classification of malignant tumors: For the first-time biomarkers such as HPV-positivity have been integrated in the staging system of head and neck tumors. When applying this system for patients with detected PT in the oropharynx and for patients with CUP advanced N-category and advanced UICC stages become negative prognosticators for OS. In DSS and subgroup analysis the survival differences were not statistically significant possibly due to the small patient cohort.
Detection of HPV in cervical lymph node metastases can direct the identification of PT especially in the oropharynx in patients evaluated for suspected CUP. One third of lymph node metastases in patients with a CUP are HPV-positive associated with improved OS and DSS. In the 8th edition of the UICC TNM classification of malignant tumors HPV-positivity has been integrated in the staging of OPSCC and CUP. When applying this system, advanced N-category and advanced UICC stages are negative prognosticators for survival supporting the role for integration of biomarkes in staging systems.
The study has been funded by the CTU - Commission cantonal hospital St. Gallen, Switzerland. These results have been presented at the Annual Spring Meeting of the Swiss Society of Oto-Rhino-Laryngology, Head and Neck Surgery, 04.-05.06.2015, Lugano, Switzerland.
2021 Copyright OAT. All rights reserv
- Grau C, Johansen LV, Jakobsen J, Geertsen P, Andersen E, et al. (2000) Cervical lymph node metastases from unknown primary tumours. Results from a national survey by the Danish Society for Head and Neck Oncology. Radiother Oncol 55: 121-129. [Crossref]
- Colletier PJ, Garden AS, Morrison WH, Goepfert H, Geara F, et al. (1998) Postoperative radiation for squamous cell carcinoma metastatic to cervical lymph nodes from an unknown primary site: outcomes and patterns of failure. Head Neck 20: 674-681. [Crossref]
- Reddy SP, Marks JE (1997) Metastatic carcinoma in the cervical lymph nodes from an unknown primary site: results of bilateral neck plus mucosal irradiation vs. ipsilateral neck irradiation. Int J Radiat Oncol Biol Phys 37: 797-802. [Crossref]
- Ligey A, Gentil J, Créhange G, Montbarbon X, Pommier P, et al. (2009) Impact of target volumes and radiation technique on loco-regional control and survival for patients with unilateral cervical lymph node metastases from an unknown primary. Radiother Oncol 93: 483-487. [Crossref]
- Erkal HS, Mendenhall WM, Amdur RJ, Villaret DB, Stringer SP (2001) Squamous cell carcinomas metastatic to cervical lymph nodes from an unknown head-and-neck mucosal site treated with radiation therapy alone or in combination with neck dissection. Int J Radiat Oncol Biol Phys 50: 55-63. [Crossref]
- Beldì D, Jereczek-Fossa BA, D'Onofrio A, Gambaro G, Fiore MR, et al. (2007) Role of radiotherapy in the treatment of cervical lymph node metastases from an unknown primary site: retrospective analysis of 113 patients. Int J Radiat Oncol Biol Phys 69: 1051-1058. [Crossref]
- Issing, WJ, Taleban B, Tauber S (2003) Diagnosis and management of carcinoma of unknown primary in the head and neck. Eur Arch Otorhinolaryngol 260: 436-443.
- Löning T, Ikenberg H, Becker J, Gissmann L, Hoepfer I, et al. (1985) Analysis of oral papillomas, leukoplakias, and invasive carcinomas for human papillomavirus type related DNA. J Invest Dermatol 84: 417-420. [Crossref]
- Koch WM, Lango M, Sewell D, Zahurak M, Sidransky D (1999) Head and neck cancer in nonsmokers: a distinct clinical and molecular entity. Laryngoscope 109: 1544-1551. [Crossref]
- Smith EM, Ritchie JM, Summersgill KF, Hoffman HT, Wang DH, et al. (2004) Human papillomavirus in oral exfoliated cells and risk of head and neck cancer. J Natl Cancer Inst 96: 449-455. [Crossref]
- Kreimer AR, Clifford GM, Boyle P, Franceschi S (2005) Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 14: 467-475. [Crossref]
- Gillison ML, Shah KV (2001) Human papillomavirus-associated head and neck squamous cell carcinoma: mounting evidence for an etiologic role for human papillomavirus in a subset of head and neck cancers. Curr Opin Oncol 13: 183-188. [Crossref]
- D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, et al. (2007) Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 356: 1944-1956. [Crossref]
- Gao X, Chen L (2010) Human papillomavirus and oropharyngeal cancer survival. N Engl J Med 363: 1576. [Crossref]
- Sturgis EM, Ang KK (2011) The epidemic of HPV-associated oropharyngeal cancer is here: is it time to change our treatment paradigms? J Natl Compr Canc Netw 9: 665-673. [Crossref]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69-90. [Crossref]
- Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, et al. (2013) Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol 31: 4550-4559. [Crossref]
- Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA et al. (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100: 261-269. [Crossref]
- Ragin, CC, Taioli E (2007) Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer 121: 1813-1820. [Crossref]
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, et al. (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363: 24-35. [Crossref]
- Benson E, Li R, Eisele D, Fakhry C (2014) The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol 50: 565-574. [Crossref]
- Begum S, Gillison ML, Ansari-Lari MA, Shah K, Westra WH (2003) Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res 9: 6469-6475. [Crossref]
- Begum S, Gillison ML, Nicol TL, Westra WH (2007) Detection of human papillomavirus-16 in fine-needle aspirates to determine tumor origin in patients with metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res 13: 1186-1191. [Crossref]
- Zhang MQ, El-Mofty SK, Dávila RM (2008) Detection of human papillomavirus-related squamous cell carcinoma cytologically and by in situ hybridization in fine-needle aspiration biopsies of cervical metastasis: a tool for identifying the site of an occult head and neck primary. Cancer 114: 118-123. [Crossref]
- Park JM, Jung CK, Choi YJ, Lee KY, Kang JH, et al. (2010) The use of an immunohistochemical diagnostic panel to determine the primary site of cervical lymph node metastases of occult squamous cell carcinoma. Hum Pathol 41: 431-437. [Crossref]
- Graboyes EM, Sinha P, Thorstad WL, Rich JT, Haughey BH (2015) Management of human papillomavirus-related unknown primaries of the head and neck with a transoral surgical approach. Head Neck 37: 1603-1611. [Crossref]
- Schroeder L, Boscolo-Rizzo P, Dal Cin E, Romeo S, Baboci L, et al. (2017) Human papillomavirus as prognostic marker with rising prevalence in neck squamous cell carcinoma of unknown primary: A retrospective multicentre study. Eur J Cancer 74: 73-81. [Crossref]
- Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, et al. (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4: 844-847. [Crossref]
- Grønhøj Larsen C, Gyldenløve M, Jensen DH, Therkildsen MH, Kiss K, et al. (2014) Correlation between human papillomavirus and p16 overexpression in oropharyngeal tumours: a systematic review. Br J Cancer 110: 1587-1594. [Crossref]
- Yoshikawa H, Kawana T, Kitagawa K, Mizuno M, Yoshikura H, et al. (1991) Detection and typing of multiple genital human papillomaviruses by DNA amplification with consensus primers. Jpn J Cancer Res 82: 524-531. [Crossref]
- Broglie MA, Jochum W, Förbs D, Schönegg R, Stoeckli SJ (2015) Brush cytology for the detection of high-risk HPV infection in oropharyngeal squamous cell carcinoma. Cancer Cytopathol 123: 732-738. [Crossref]
- Jakscha J, Zlobec I, Storck C, Obermann EC, Tornillo L, et al. (2013) The clinical impact of p16 status in fine-needle aspirates of cervical lymph node metastasis of head and neck squamous cell carcinomas. Eur Arch Otorhinolaryngol 270: 661-667. [Crossref]
- Yasui T, Morii E, Yamamoto Y, Yoshii T, Takenaka Y, et al. (2014) Human papillomavirus and cystic node metastasis in oropharyngeal cancer and cancer of unknown primary origin. PLoS One 9: e95364. [Crossref]
- Beadle BM, William WN Jr, McLemore MS, Sturgis EM, Williams MD (2013) p16 expression in cutaneous squamous carcinomas with neck metastases: a potential pitfall in identifying unknown primaries of the head and neck. Head Neck 35: 1527-1533. [Crossref]
- Smeets SJ, Hesselink AT, Speel EJ, Haesevoets A, Snijders PJ, et al. (2007) A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer 121: 2465-2472. [Crossref]
- Baldassarri R, Aronberg R, Levi AW, Yarbrough WG, Kowalski D, et al. (2015) Detection and genotype of high-risk human papillomavirus in fine-needle aspirates of patients with metastatic squamous cell carcinoma is helpful in determining tumor origin. Am J Clin Pathol 143: 694-700. [Crossref]
- Patel SA, Magnuson JS, Holsinger FC, Karni RJ, Richmon JD, et al. (2013) Robotic surgery for primary head and neck squamous cell carcinoma of unknown site. JAMA Otolaryngol Head Neck Surg 139: 1203-1211. [Crossref]
- Mehta V, Johnson P, Tassler A, Kim S, Ferris RL, et al. (2013) A new paradigm for the diagnosis and management of unknown primary tumors of the head and neck: a role for transoral robotic surgery. Laryngoscope 123: 146-151. [Crossref]
- Karni RJ, Rich JT, Sinha P, Haughey BH (2011) Transoral laser microsurgery: a new approach for unknown primaries of the head and neck. Laryngoscope 121: 1194-1201. [Crossref]
- Nagel TH, Hinni ML, Hayden RE, Lott DG (2014) Transoral laser microsurgery for the unknown primary: role for lingual tonsillectomy. Head Neck 36: 942-946. [Crossref]
- Durmus K, Rangarajan SV, Old MO, Agrawal A, Teknos TN, et al. (2014) Transoral robotic approach to carcinoma of unknown primary. Head Neck 36: 848-852. [Crossref]
- Channir HI, Rubek N, Nielsen HU, Kiss K, Charabi BW, et al. (2015) Transoral robotic surgery for the management of head and neck squamous cell carcinoma of unknown primary. Acta Otolaryngol 135: 1051-1057. [Crossref]
- Fu TS, Foreman A, Goldstein DP, de Almeida JR (2016) The role of transoral robotic surgery, transoral laser microsurgery, and lingual tonsillectomy in the identification of head and neck squamous cell carcinoma of unknown primary origin: a systematic review. J Otolaryngol Head Neck Surg 45: 28. [Crossref]
- Koch WM, Bhatti N, Williams MF, Eisele DW (2001) Oncologic rationale for bilateral tonsillectomy in head and neck squamous cell carcinoma of unknown primary source. Otolaryngol Head Neck Surg 124: 331-333. [Crossref]
- Lindberg R (1972) Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer 29: 1446-1449. [Crossref]
- Kothari P, Randhawa PS, Farrell R (2008) Role of tonsillectomy in the search for a squamous cell carcinoma from an unknown primary in the head and neck. Br J Oral Maxillofac Surg 46: 283-287. [Crossref]
- Durmus K, Patwa HS, Gokozan HN, Kucur C, Teknos TN, et al. (2014) Functional and quality-of-life outcomes of transoral robotic surgery for carcinoma of unknown primary. Laryngoscope 124: 2089-2095. [Crossref]
- Iganej S, Kagan R, Anderson P, Rao A, Tome M, et al. (2002) Metastatic squamous cell carcinoma of the neck from an unknown primary: management options and patterns of relapse. Head Neck 24: 236-246. [Crossref]
- Christiansen H, Hermann RM, Martin A, Nitsche M, Schmidberger H, et al. (2005) Neck lymph node metastases from an unknown primary tumor retrospective study and review of literature. Strahlenther Onkol 181: 355-362. [Crossref]
- Boscolo-Rizzo P, Gava A, Da Mosto MC (2007) Carcinoma metastatic to cervical lymph nodes from an occult primary tumor: the outcome after combined-modality therapy. Ann Surg Oncol 14: 1575-1582. [Crossref]
- Lu X, Hu C, Ji Q, Shen C, Feng Y (2009) Squamous cell carcinoma metastatic to cervical lymph nodes from an unknown primary site: the impact of radiotherapy. Tumori 95: 185-190. [Crossref]
- Wallace A, Richards GM, Harari PM, Kirwan JM, Morris CG, et al. (2011) Head and neck squamous cell carcinoma from an unknown primary site. Am J Otolaryngol 32: 286-290. [Crossref]
- Balaker AE, Abemayor E, Elashoff D, St John MA (2012) Cancer of unknown primary: does treatment modality make a difference? Laryngoscope 122: 1279-1282. [Crossref]
- Sivars L, Näsman A, Tertipis N, Vlastos A, Ramqvist T, et al. (2014) Human papillomavirus and p53 expression in cancer of unknown primary in the head and neck region in relation to clinical outcome. Cancer Med 3: 376-384. [Crossref]
- Tribius S, Hoffmann AS, Bastrop S, Görögh T, Haag J, et al. (2012) HPV status in patients with head and neck of carcinoma of unknown primary site: HPV, tobacco smoking, and outcome. Oral Oncol 48: 1178-1184. [Crossref]
- Compton AM, Moore-Medlin T, Herman-Ferdinandez L, Clark C, Caldito GC, et al. (2011) Human papillomavirus in metastatic lymph nodes from unknown primary head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg 145: 51-57. [Crossref]
- Jensen DH, Hedback N, Specht L, Høgdall E, Andersen E, et al. (2014) Human papillomavirus in head and neck squamous cell carcinoma of unknown primary is a common event and a strong predictor of survival. PLoS One 9: e110456. [Crossref]
- Keller LM, Galloway TJ, Holdbrook T, Ruth K, Yang D, et al. (2014) p16 status, pathologic and clinical characteristics, biomolecular signature, and long-term outcomes in head and neck squamous cell carcinomas of unknown primary. Head Neck 36: 1677-1684. [Crossref]
- Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM (2001) Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer 92: 805-813. [Crossref]
- Lindquist D, Romanitan M, Hammarstedt L, Näsman A, Dahlstrand H, et al. (2007) Human papillomavirus is a favourable prognostic factor in tonsillar cancer and its oncogenic role is supported by the expression of E6 and E7. Mol Oncol 1: 350-355. [Crossref]
- Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, et al. (2006) High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol 24: 5630-5636. [Crossref]
- Fischer CA, Zlobec I, Green E, Probst S, Storck C, et al. (2010) Is the improved prognosis of p16 positive oropharyngeal squamous cell carcinoma dependent of the treatment modality? Int J Cancer 126: 1256-1262. [Crossref]
- Broglie MA, Soltermann A, Haile SR, Huber GF, Stoeckli SJ (2015) Human papilloma virus and survival of oropharyngeal cancer patients treated with surgery and adjuvant radiotherapy. Eur Arch Otorhinolaryngol 272: 1755-1762. [Crossref]
- Axelsson L, Nyman J, Haugen-Cange H, Bove M, Johansson L, et al. (2017) Prognostic factors for head and neck cancer of unknown primary including the impact of human papilloma virus infection. J Otolaryngol Head Neck Surg 46: 45. [Crossref]
- Dixon PR, Au M, Hosni A, Perez-Ordonez B, Weinreb I, et al. (2016) Impact of p16 expression, nodal status, and smoking on oncologic outcomes of patients with head and neck unknown primary squamous cell carcinoma. Head Neck 38: 1347-1353. [Crossref]
- Issing WJ, Taleban B, Tauber S (2003) Diagnosis and management of squamous cell carcinoma of the head and neck region with unknown primary. A survey of 167 patients. Laryngorhinootologie 82: 659-665. [Crossref]
- Huang CC, Tseng FY, Yeh TH, Wen YH, Hsu CJ, et al. (2008) Prognostic factors of unknown primary head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg 139: 429-435. [Crossref]
- Park GC, Jung JH, Roh JL, Lee JH, Cho KJ, et al. (2014) Prognostic value of metastatic nodal volume and lymph node ratio in patients with cervical lymph node metastases from an unknown primary tumor. Oncology 86: 170-176. [Crossref]
- Keane FK, Chen YH, Neville BA, Tishler RB, Schoenfeld JD, et al. (2015) Changing prognostic significance of tumor stage and nodal stage in patients with squamous cell carcinoma of the oropharynx in the human papillomavirus era. Cancer 121: 2594-2602. [Crossref]


