Abstract
Background: Cardiovascular disease (CVD) is an important problem that threatens the health of all mankind and remains the world's first cause of morbidity and mortality. In this study, we identified the gene characteristic during vascular calcification and explored their potential mechanisms.
Results: Gene expression profiles of GSE65435 were downloaded from GEO database. The GSE65435 dataset contained 18 samples, the 3 SMCs + miR-30e samples and 3 SMCs + ct-miR samples were selected. The gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes pathway (KEGG) enrichment analyses were implemented, and gene signal network and pathway relation network of the differentially expressed genes (DEGs) were analyzed by GCBI. In total, 620 differentially expressed genes were identified in SMCs + miR-30e samples, including 249 up-regulated genes and 371 down-regulated genes. GO analysis summed up that DEGs were significantly concentrated in negative regulation of apoptotic process. KEGG pathway analysis showed that DEGs were enriched in PI3K-Akt signaling pathway.
Conclusions: This study showed that the identified DEGs increased the molecular mechanisms underlying the development of vascular calcification and might be used as new diagnostic and therapeutic strategies for the treatment of vascular calcification.
Keywords
Vascular calcification; miR-30e; differentially expressed genes (DEGs); gene ontology (GO); Kyoto Encyclopedia of Genes and Genomes pathway (KEGG)
Introduction
According to the World Health Organization (WHO) 2011 report, cardiovascular disease (CVD) is the primary problem that threatens the health of all mankind and remains the world's first cause of morbidity and mortality [1]. The number of cardiovascular diseases exceeds cancer, diabetes, chronic respiratory disease, etc. [2]. The data for 2008 showed that 30% of the total number of deaths worldwide are due to cardiovascular diseases, the vast majority of which were coronary heart diseases and stroke, and this trend is expected to continue [3]. In 2030, the number of death due to heart disease and stroke may increase from 18 million to 23.3 million and cardiovascular disease will continue to be the major cause of death [4]. In China, according to the China’s health statistics released by the ministry of health, a total of 27,160,00 people died of cardiovascular diseases in 2007, becoming the first cause of death [5]. CVD pathogenesis has been a hot topic in the field of cardiovascular research, it has been commonly recognized that smooth muscle cell proliferation, inflammatory mediators, vascular endothelial dysfunction and lipid deposition are involved, among which vascular calcification plays an important role in the occurrence and development of a variety of cardiovascular diseases such as coronary heart disease, hypertension, degenerative heart valvular disease and cardiomyopathy [6]. Cardiovascular calcification is similar to the bone formation during the embryonic period, a variety of genes and proteins in minerals and bone metabolism have a certain regulatory role in vascular calcification [7].
MicroRNA (miRNA) is very important for gene regulation. Recent studies have shown that some miRNA can affect the process of bone formation by regulating the target genes, and could also have a regulatory role in the process of vascular calcification [8]. MiR-30e was reported to have the ability to induce adipogenic differentiation and reduce bone formation differentiation in stromal cells by targeting Lrp6 [9]. The article “miR-30e targets IGF2-regulated osteogenesis in bone marrow-derived mesenchymal stem cells, aortic smooth muscle cells, and ApoE2/2 mice” aimed to examine the role of miR-30e in vascular calcification. This article concluded that miR-30e inhibited the osteogenesis in MSCs and SMCs by targeting IGF2 and suppressed their differentiation into adipogenic or smooth muscle lineage [10]. In this study, there is an experiment that SMCs were treated with ct-miR or miR-30e and draw a conclusion that miR-30e inhibited the osteogenesis in MSCs and SMCs and suppressed their differentiation into adipogenic or smooth muscle lineage. The results of gene sequencing of two groups mice were upload to GEO DataSets (GSE65435) [11]. To further show the function of miR-30e of SMCs at the molecular level and explore the possible candidate biomarkers for diagnosis, prognosis, and drug targets, we are analyzing their biological functions and pathways.
Materials and methods
Microarray data
Gene expression profiles of GSE65435 were downloaded from the GEO database. GSE65435, which was based on Affymetrix GPL6246 platform (Affymetrix Mouse Gene 1.0 ST Array), was submitted by Wen, et al. The GSE65435 dataset comprised 18 samples. In this study, we selected the 6 samples, describing the effect of miR-30e in aortic smooth muscle cells differentiation.
Identification of DEGs
The raw data files were uploaded to the website of Gene-Cloud of Biotechnology Information (GCBI) [12]. SMCs + miR-30e samples were used as the test group and the SMCs + ct-miR used as the control group in this data. In the analysis process, we defined P = 0.05, Q = 0.05 and fold change = 2.0.
Gene ontology and pathway enrichment analysis of DEGs and network analysis
All the analysis was completed by the GCBI. In the gene ontology analysis, FDR = 0.05 and P = 0.05. In the pathway enrichment analysis, P = 0.05. Then we analyzed gene signal network to gene ontology analysis and analyzed pathway relation network to pathway enrichment. Then, networks were built by the cytoscape (3.4.0) software.
Results
Identification of DEGs
The samples were composed by 3 test samples and 3 control samples. Based on the P < 0.05 and fold control (FC) > 2.0 standards, a total of 620 genes were identified after the analyses of GSE65435, of which 249 were up-regulated and 371 were down-regulated (Figure 1). The gene Saa3 had a largest difference between SMCs + miR-30e group and SMCs + ct-miR group. The expression of gene Saa3 in SMCs + miR-30e group is 199.97 times in SMCs + ct-miR group. As showed in Table 1, the top ten DEGs were Saa3, Serpine2, Cd34, Grem1, Chi3l1, Hp, Selp, Ch25h, Clmp and Sfrp1.
Table 1. The top 10 mRNAs with high degrees of DEGs.
Gene Symbol |
Accession Number |
Gene Description |
d Score |
Fold Change |
Gene Feature |
Saa3 |
NM_011315 |
Mus musculus serum amyloid A 3 (Saa3), mRNA |
-31.68102 |
-199.976269 |
down |
Serpine2 |
NM_009255 |
Mus musculus serine (or cysteine) peptidase inhibitor, clade E, member 2 (Serpine2), mRNA |
-28.431854 |
-55.253872 |
down |
Cd34 |
NM_001111059 |
Mus musculus CD34 antigen (Cd34), transcript variant 1, mRNA |
-19.894471 |
-12.007931 |
down |
Grem1 |
NM_011824 |
Mus musculus gremlin 1 (Grem1), mRNA |
-19.045846 |
-20.450459 |
down |
Chi3l1 |
NM_007695 |
Mus musculus chitinase 3-like 1 (Chi3l1), mRNA |
-18.246297 |
-13.716394 |
down |
Hp |
NM_017370 |
Mus musculus haptoglobin (Hp), mRNA |
-17.482949 |
-30.85244 |
down |
Selp |
NM_011347 |
Mus musculus selectin, platelet (Selp), mRNA |
-15.500173 |
-8.72038 |
down |
Ch25h |
NM_009890 |
Mus musculus cholesterol 25-hydroxylase (Ch25h), mRNA |
-15.265039 |
-22.893805 |
down |
Clmp |
NM_133733 |
Mus musculus CXADR-like membrane protein (Clmp), mRNA |
-15.021017 |
-11.558135 |
down |
Sfrp1 |
NM_013834 |
Mus musculus secreted frizzled-related protein 1 (Sfrp1), mRNA |
-14.351045 |
-46.482443 |
down |
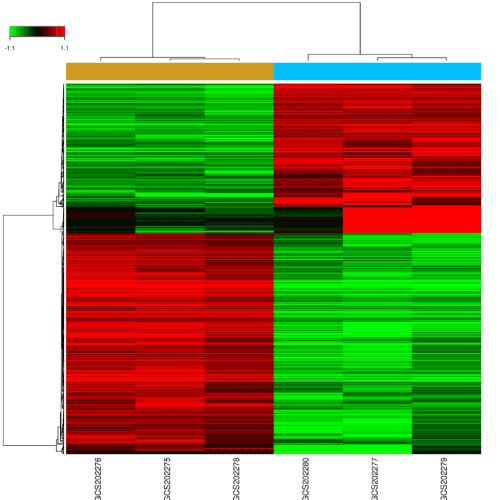
Figure 1. Changes in mRNAs expression profiles in miR-30e treated mice.
GO term enrichment and KEGG pathway analysis
We analyzed DEGs by GCBI online tools which are mainly based on the algorithms of miR and target scan, to identify over represented GO categories and KEGG pathways. GO analysis indicated that up-regulated DEGs were significantly concentrated in biological processes (BP), including the cell cycle, cell division, and cell proliferation.
To identify the biological functions of these genes, GO and pathway enrichment analysis were implemented, respectively. As illustrated in Figure 2, the top ten regulated GOs sensitive to high concentration of miR-30e were positive regulation of angiogenesis, inflammatory response, positive regulation of apoptotic process, negative regulation of cell proliferation, negative regulation of apoptotic process, cell adhesion, positive regulation of transcription from RNA polymerase II promoter, apoptotic process, protein phosphorylation and multicellular organismal development. And the genes of each function were listed in Table 2. GO analysis obviously suggested that high concentration of miR-30e could affect expression of many miRNAs, through many crucial functions such as regulation of angiogenesis, inflammatory response and positive regulation of apoptotic process of the mice with high expression of miR-30e. Combining with the KEGG database, we analyzed the pathways in which the putative target genes were involved. As illustrated in Figure 3, the top ten deregulated pathways sensitive to high concentration of miR-30e were PI3K-Akt signaling pathway, pertussis, cytokine-cytokine receptor interaction, MAPK signaling pathway, pathways in cancer, complement and coagulation cascades, proteoglycans in cancer, metabolic pathways, leishmaniasis and p53 signaling pathway. Table 3 contains the top 10 significantly enriched pathways of the DEGs analyzed by KEGG analysis.
Table 2. The top 10 mRNAs with high degrees of mRNAs-GO-analysis.
GO ID |
GO Name |
Diff Gene Counts in GO |
Gene Amount in GO |
Gene Symbols |
GO:0043066 |
negative regulation of apoptotic process |
38 |
480 |
|Nr3c1|Itga6|Spp1|Dapk1|Dhcr24|Cyr61|Gata6|Gas1|Cxcr7|Sod2|Foxc2|Atf5|Wnt4|Clu|Sgk1|Angptl4|Il6|
Fas|Ier3|Snai2|Pten|Ar|Cth|Aqp1|Serpine1|Mical1|Vnn1|Nes|Cebpb|Timp1|Fgf10|Jak2|Thbs1|Osr1|Btg2|
Gas6|Sfrp1|Ivns1abp| |
GO:0007155 |
cell adhesion |
38 |
500 |
|Cdon|Tgfbi|Pcdh11x|Nrp2|Emb|Tenm3|Mcam|Col6a2|Cdh10|Ptk7|Podxl|Itga6|Pcdh19|Pcdh18|Svep1|
Alcam|Spp1|Cd97|Cdh3|Scarf2|Cd34|Emilin2|Postn|Col6a1|Cdh2|Nuak1|Edil3|Adam12|Tnfaip6|
Ppap2b|Fbln5|Pdpn|Thbs1|Cyr61|Perp|Pcdh9|Cxcr7|Selp| |
GO:0045944 |
positive regulation of transcription from RNA polymerase II promoter |
43 |
745 |
|Nfatc4|Tlr4|Cebpb|Itga6|Ablim1|Ankrd1|Zbtb38|Gata2|Il6|Btg2|Id4|Igf2|Ar|Egr1|Runx1|Foxg1|Fgf10|
Jag1|Foxf2|Nr4a1|Glis3|Prl2c2|Grem1|Osr1|Lif|Mecom|Fos|Sdpr|Foxc2|Tlr2|Cebpd|Hipk2|Rarb|Il33|
Nr3c1|Myd88|Tnip1|Cdon|Gata6|Cyr61|Fgfr2|Il1a|Pbx1| |
GO:0006954 |
inflammatory response |
25 |
214 |
|S1pr3|Cd14|Cxcl1|Nfkbiz|Lipa|Vnn1|Lxn|Ccl2|Selp|Il1a|Tlr4|Tnip1|Ccl7|Mapkapk2|Il6|Tlr2|C3|Cxcl5|
Il8|Thbs1|Myd88|Nos2|Bdkrb1|Mecom|Ptgs2| |
GO:0043065 |
positive regulation of apoptotic process |
26 |
258 |
|Igfbp3|Bnip3|Dusp6|Uaca|Gata6|Ptgs2|Itga6|Nupr1|Sept4|Dapk1|Map3k5|Nfatc4|Jak2|Cyr61|Tlr4|Fosl1|
Lpar1|Ier3|Sfrp1|Nr4a1|Pten|Nox4|Rarb|Ankrd1|Fas|Nos2| |
GO:0008285 |
negative regulation of cell proliferation |
28 |
315 |
|Jak2|Ptch1|Ptgs2|Sod2|Fgf10|Dhcr24|Cth|Il6|Rarb|Btg2|Fosl1|Rerg|Ereg|Pten|Igfbp3|Adarb1|Il1a|Lif|
Serpine2|Cav1|Slc9a3r1|Tlr2|Fgfr2|Sfrp1|Nox4|Il1rl1|Pkp2|Slit3| |
GO:0007275 |
multicellular organismal development |
41 |
954 |
|Sema3c|Enc1|Ano1|Sfrp1|Tmem2|Nxn|Atoh8|Prrx1|Fzd3|Mgp|Pdpn|Nrp2|Zfp521|Wls|Fhl1|Smoc1|
Snai2|Sema3e|Pdgfd|Fzd4|Slc7a5|Ebf1|Foxc2|Jag1|Serpine2|Ereg|Eid2|Olfml3|Pdgfra|Ppap2b|Nes|
Gadd45g|Wnt4|Cxcr7|Foxg1|Fyn|Edil3|Sema3a|Pak3|Mecom|Slit3| |
GO:0006468 |
protein phosphorylation |
33 |
611 |
|Cpne3|Sgk1|Rps6ka6|Nuak1|Trib3|Trib2|Map3k5|Pdk1|Pdgfra|Fgfr2|Gas6|Tgfbr3|Jak2|Hipk2|Pdk4|
Mapkapk2|Alpk1|Stk17b|Ksr1|Mlkl|Map4k4|Dapk1|Ptk7|Map3k8|Bmp2k|Prkar2b|Pak3|Mark1|Nek6|
Ccne1|Trib1|Fyn|Igfbp3| |
GO:0006915 |
apoptotic process |
30 |
540 |
|Peg10|Ank2|Slc40a1|Gulp1|Map3k5|Nek6|Dapk1|Perp|Lcn2|Hipk2|Fgfr2|Casp4|Mecom|Nod1|Stk17b|
Prune2|Sgk1|Trib3|Chac1|Phlpp1|Gadd45g|Slc9a3r1|Bnip3|Rnf130|Pten|Crip1|Serpina3g|Fas|Nr4a1|
Sema3a| |
GO:0045766 |
positive regulation of angiogenesis |
15 |
92 |
|Cd34|F3|Aqp1|C3ar1|Prl2c2|Chi3l1|Gata2|Grem1|C3|Il1a|Hipk2|Serpine1|Runx1|Thbs1|Gata6| |
Table 3. The top 10 mRNAs with high degrees of mRNAs-pathway-analysis.
Pathway ID |
Pathway Name |
Diff Gene Counts in Pathway |
Gene Amount in Pathway |
Gene Symbols |
4151 |
PI3K-Akt signaling pathway |
25 |
356 |
|Pten|Nr4a1|Ccne1|Jak2|Pdgfra|Itga6|Fgfr2|Il4ra|Fgf23|Osmr|Ghr|Tlr2|Fgf10|Il6|Pdgfd|Tlr4|Col6a2|
Lpar1|Spp1|Thbs1|Sgk1|Lpar4|Col6a1|Col3a1|Phlpp1| |
5133 |
Pertussis |
14 |
74 |
|C4bp|Gm5077|Cd14|Il1a|Cxcl5|C3|Serping1|Nos2|Tlr4|Nod1|C1s|Fos|Il6|Myd88| |
4060 |
Cytokine-cytokine receptor interaction |
21 |
266 |
|Il6|Lifr|Osmr|Il8|Pdgfd|Il4ra|Pdgfra|Il1a|Lif|Ppbp|Il13ra1|Ccl2|Cxcl1|Tnfrsf9|Il18rap|Cxcl5|Ghr|
LOC100861978|Fas|Cxcr7|Ccl7| |
4010 |
MAPK signaling pathway |
20 |
259 |
|Pdgfra|Fgf23|Il1a|Fas|Dusp4|Map3k8|Nfatc4|Gadd45g|Fos|Map3k5|Mapkapk2|Rps6ka6|Dusp6|
Mecom|Cacna1c|Nr4a1|Fgf10|Fgfr2|Map4k4|Cd14| |
5200 |
Pathways in cancer |
22 |
326 |
|Fang|Rarb|Fgf23|Fgf10|Wnt4|Dapk1|Slc2a1|Mecom|Fzd3|Fgfr2|Ptgs2|Ar|Fos|Runx1|Pten|Ccne1|
Ptch1|Nos2|Itga6|Il6|Pdgfra|Fzd4| |
4610 |
Complement and coagulation cascades |
11 |
77 |
|Serpine1|Thbd|C3|F3|C4bp|Serping1|Cfh|Bdkrb1|C1s|C3ar1|Gm5077| |
5205 |
Proteoglycans in cancer |
16 |
230 |
|Tlr4|Fgf23|Thbs1|Wnt4|Sdc4|Pdk1|Fgf10|Cav1|Tlr2|Fzd4|Igf2|Ptch1|Ank2|Fas|Ank3|Fzd3| |
1100 |
Metabolic pathways |
35 |
1242 |
|Uprt|Ivd|Pon3|Idi1|Acot1|Gda|Ldhb|Glce|B3galt1|Gcnt2|B3gnt3|Crls1|Ctps|Mtm1|Mboat2|Isyna1|
Dhcr24|Eno3|Asah1|Nos2|Alpl|Hk2|Acot2|Dhrs3|Ppap2b|Pcx|Aldh3a1|A4galt|Adh7|Enpp3|Cth|Gcnt4|
Sqle|Ptgs2|H6pd| |
5140 |
Leishmaniasis |
9 |
66 |
|Tlr4|Jak2|Nos2|Il1a|Myd88|Fos|Tlr2|Ptgs2|C3| |
4115 |
p53 signaling pathway |
9 |
69 |
2021 Copyright OAT. All rights reserv
|Thbs1|Fas|Ccne1|Serpine1|Pten|Gadd45g| |
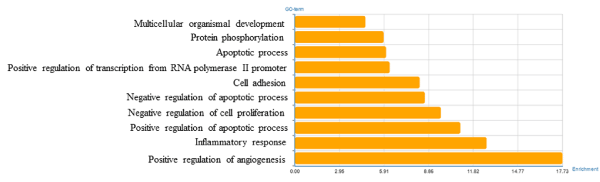
Figure 2. Significantly changed GOs of predicted target genes.
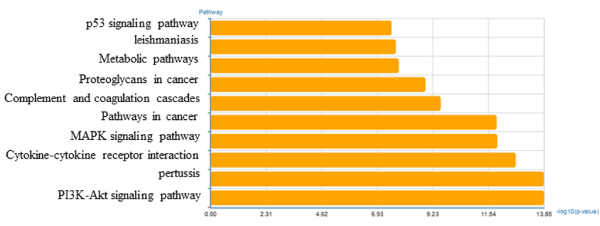
Figure 3. Significantly changed pathways of predicted target gene.
Gene Signal Network and pathway Relation Network
We performed pathway relation network analysis to draw a reciprocity network covering 35 significantly changed pathways (Figure 4). Among them, MAPK signaling pathway (degree = 16), apoptosis (degree = 15), pathways in cancer (degree = 15) and cell cycle (degree = 12) showed highest degree, suggesting that these four pathways might play an important role in apoptosis induced by miR-30e treatment. The top 10 significantly changed pathways were listed in Table 4. Based on the obvious regulated GOs and pathways, we selected intersected genes and further constructed mRNAs-GO-networks to screen the key regulatory functions of the identified mRNAs and their target genes, respectively. As shown in Figure 5 and Table 5, the top rated 10 mRNAs including IL-6, Myd88, Fos, Ppap2b, Tlr2, Tlr4, Hk2, Wnt4, C3 and Gstk1. Apart from the gene Tlr4, the other mRNAs were down-regulated by miR-30e treatment in mice.
Table 4. The top 10 mRNAs with high degrees of mRNAs-GO-network.
Pathway ID |
Pathway Name |
Degree |
Pathway Feature |
4010 |
MAPK signaling pathway |
16 |
down/up |
4210 |
Apoptosis |
15 |
down |
5200 |
Pathways in cancer |
15 |
up/down |
4510 |
Focal adhesion |
12 |
up/down |
4115 |
p53 signaling pathway |
9 |
down/up |
4060 |
Cytokine-cytokine receptor interaction |
9 |
down/up |
4310 |
Wnt signaling pathway |
8 |
down |
10 |
Glycolysis / Gluconeogenesis |
7 |
Up/down |
4620 |
Toll-like receptor signaling pathway |
6 |
down/up |
4630 |
Jak-STAT signaling pathway |
6 |
up/down |
Table 5. The top 10 mRNAs with high degrees of mRNAs-pathway-network.
Gene Symbol |
Gene Feature |
Biotype |
Gene Description |
Degree |
Il6 |
down |
coding |
"Mus musculus interleukin 6 (Il6), mRNA." |
20 |
Myd88 |
down |
coding |
"Mus musculus myeloid differentiation primary response gene 88 (Myd88), mRNA." |
10.5 |
Fes |
down |
coding |
"Mus musculus FBJ osteosarcoma oncogene (Fos), mRNA." |
7.5 |
Ppap2b |
down |
coding |
"Mus musculus phosphorous acid phosphatase type 2B (Ppap2b), mRNA." |
6 |
Tlr2 |
down |
coding |
"Mus musculus toll-like receptor 2 (Tlr2), mRNA." |
4.5 |
Tlr4 |
up |
coding |
"Mus musculus toll-like receptor 4 (Tlr4), mRNA." |
4.5 |
Hk2 |
down |
coding |
"Mus musculus hexokinase 2 (Hk2), mRNA." |
2 |
Wnt4 |
down |
coding |
"Mus musculus wingless-related MMTV integration site 4 (Wnt4), mRNA." |
2 |
C3 |
down |
coding |
"Mus musculus complement component 3 (C3), mRNA." |
1 |
Gstk1 |
down |
coding |
"Mus musculus glutathione S-transferase kappa 1 (Gstk1), nuclear gene encoding mitochondrial protein, mRNA." |
1 |
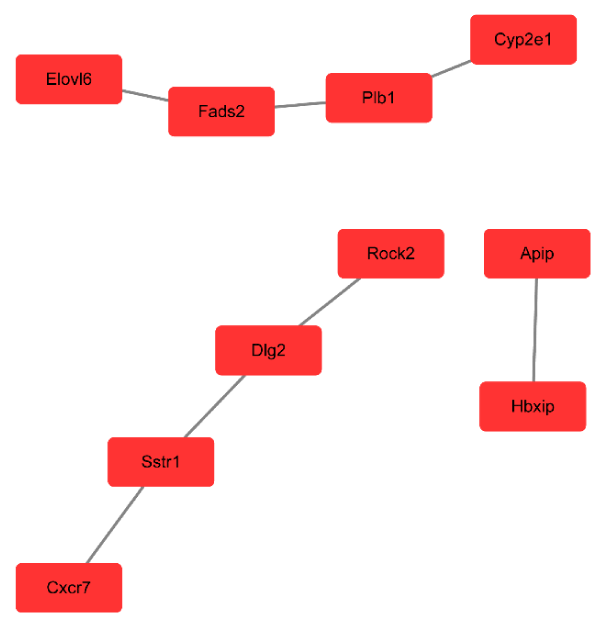
Figure 4. Pathway network (Path-net). Significantly changed pathways were connected in a Path-net to show the interaction network among these pathways.
Figure 5. mRNAs gene network. According to the interactions between miRNAs and the intersected target genes, miRNAs-gene-network was constructed to illustrate the key regulatory functions of the identified miRNAs and their target genes.
Discussion
Vascular calcification is a complex biological process associated with aging and degenerative changes [13]. Studies have shown that vascular calcification is an active process that similar to bone formation and it is regulated by multiple factors involving vascular smooth muscle cells, macrophages, endothelial cells, fibroblasts, multiple signal molecules (AKT, KLFS, Smads etc.). Atherosclerosis risk factors include dyslipidemia, hypertension, diabetes, renal failure and this can promote the occurrence and development of arterial calcification [14,15]. The pathogenesis of vascular calcification is currently considered to be related with intravascular bone formation [16]. From the article “miR-30e targets IGF2-regulated osteogenesis in bone marrow-derived mesenchymal stem cells, aortic smooth muscle cells, and ApoE2/2 mice”, we known that miR-30e inhibited the osteogenesis in SMCs by targeting IGF2 and suppressed their differentiation into adipogenic or smooth muscle lineage [10].
In this study, gene expression data of 3 SMCs + miR-30e samples and 3 SMCs + ct-miR samples were recovered from the GEO dataset under the accession number GSE65435. The study analyzed 620 DEGs between SMCs + miR-30e samples and SMCs + ct-miR samples, among which 249 genes were up-regulated and 371 were down-regulated. Interestingly, the top 10 DEGs were all down-regulated. So, we speculated that the down-regulated genes were related with osteogenesis. For purpose of better concluded the reciprocity of DEGs, we further analyzed GO analysis and KEGG pathway analysis.
The GO term analysis indicated that DEGs were mainly concluded negative regulation of apoptotic process, cell adhesion, and positive regulation of transcription from RNA polymerase II promoter. It is suggested that miR-30e may promote the process of osteoblast apoptosis, and promote the activation of adipocyte cells, leading to the differentiation of adipocytes in SMCs. Furthermore, enriched KEGG pathways of DEGs including PI3K-Akt signaling pathway, pertussis, cytokine-cytokine receptor interaction. Previous studies showed that PI3K-Akt signaling pathway is a key pathway for osteoclast activation [17,18]. MiR-30e promoted osteoclast activation by active this pathway, and then leaded to osteoblasts reduction. The pertussis toxin-insensitive CCR5 signaling in macrophage pathway was inhibited in the SMCs + miR-30e group and inhibited calcium transport into cells [19]. This pathway also contributed to the reduction of osteogenesis in the SMCs cells. The cytokine-cytokine receptor interaction pathway targets on cells and promotes adipogenic differentiation [20,21].
We also analyzed the mRNAs pathway network with DEGs and list the top degree genes: IL-6, Myd88, Fos, Ppap2b, Tlr2, Tlr4, Hk2, Wnt4, C3 and Gstk1. Among them, IL-6 expression in the highest degree. Interleukin 6 (IL-6) is an interleukin that acts as both a pro-inflammatory cytokine and an anti-inflammatory myokine [22,23]. T cells and macrophages secreted the interleukin 6 and stimulate the immune response. In addition, IL-6 also secreted by the osteoblasts to stimulate osteoclast formation [24]. Smooth muscle cells of many blood vessels also secreted IL-6 as a pro-inflammatory cytokine indicating that miR-30e acts in the regulation of IL-6 [25]. The second gene myeloid differentiation primary response gene 88 (MYD88) is a key linker in the Toll-like receptor (TLR) signaling pathway and plays an important role in the transmission of upstream information and disease progression [26]. Toll-like receptors (TLRs) are a class of proteins that play an important role in the innate immune system [27]. From this, we speculate that SMC differentiation is related to the immune system. Ppap2b lets it to regulate vascular and embryonic development by inhibited LPA signaling, which is associated with many human diseases, including cardiovascular disease and cancer, as well as developmental defects [28]. Tlr2 also belong to Toll-like receptor (TLR) signaling pathway. The above results indicated that multiple factors in the body affected the osteogenic differentiation of SMC.
Module analysis of the mRNAs GO network showed that the SMC osteogenesis differentiation was associated with MAPK signaling pathway, apoptosis, and pathways in cancer. The MAPK pathway communicated a signal from a receptor on the surface of the cell to the DNA in the nucleus of the cell. MAPK signaling pathway also promoted the differentiation of osteoclasts, and related with calcification in SMCs [29]. Studies have also showed that MAPK pathway is associated with cancer [30]. The results showed that calcification in SMCs also related with pathways in cancer. We hypothesized that when a cancer-related pathway is activated, it causes a series of cellular responses, including vascular calcification.
Conclusion
In this study, we provide an integrated bioinformatics analysis of DEGs, which may be included in the progress of differentiation of SMCS. The study showed a serious of important targets for future research into the molecular mechanisms and biomarkers. We hope that these genetic analyzes contribute to the study of vascular calcification.
Availability of data and material
miR-30e targets IGF2-regulated osteogenesis in bone marrow-derived mesenchymal stem cells, aortic smooth muscle cells, and ApoE2/2 mice.
Competing interests
The authors have no conflicts of interest to disclose.
Funding
None.
Authors' contributions
Zhi X., Chen X. and Su J.C. designed this study. Zhi X. analyzed the data. All authors read and approved the final manuscript.
Acknowledgements
We are indebted to the authors of the primary studies and clear Med-Trans studio for language polishing.
References
- Mendis S, Lindholm LH, Mancia G, Whitworth J, Alderman M, et al. (2007) World Health Organization (WHO) and International Society of Hypertension (ISH) risk prediction charts: assessment of cardiovascular risk for prevention and control of cardiovascular disease in low and middle-income countries. J Hypertens 25: 1578-1582. [Crossref]
- Mascolo A, Sessa M, Scavone C, De Angelis A, Vitale C, et al. (2017) New and old roles of the peripheral and brain renin-angiotensin-aldosterone system (RAAS): Focus on cardiovascular and neurological diseases. Int J Cardiol 227: 734-742. [Crossref]
- Feigin VL, Mensah GA, Norrving B, Murray CJ, Roth GA; GBD 2013 Stroke Panel Experts Group (2015) Atlas of the Global Burden of Stroke (1990-2013): The GBD 2013 Study. Neuroepidemiology 45: 230-236. [Crossref]
- Mathers CD, Loncar D (2006) Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3: e442. [Crossref]
- Gong WW, Wei XL, Liang YJ, Zou GY, Hu RY, et al. (2016) Urban and Rural Differences of Acute Cardiovascular Disease Events: A Study from the Population-Based Real-Time Surveillance System in Zhejiang, China in 2012. Plos One 2016, 11: e0165647. [Crossref]
- Rosset R, Surowska A, Tappy L (2016) Pathogenesis of Cardiovascular and Metabolic Diseases: Are Fructose-Containing Sugars More Involved Than Other Dietary Calories? Curr Hypertens Rep 18: 44. [Crossref]
- Karwowski W, Naumnik B, Szczepanski M, Mysliwiec M (2012) The mechanism of vascular calcification - a systematic review. Med Sci Monit 18: RA1-RA11. [Crossref]
- Roy S (2016) miRNA in Macrophage Development and Function. Antioxid Redox Signal 25: 795-804. [Crossref]
- Wang J, Guan X, Guo F, Zhou J, Chang A, et al. (2013) miR-30e reciprocally regulates the differentiation of adipocytes and osteoblasts by directly targeting low-density lipoprotein receptor-related protein 6. Cell Death Dis 4: e845. [Crossref]
- Ding W (2016) miR-30e targets IGF2-regulated osteogenesis in bone marrow-derived mesenchymal stem cells, aortic smooth muscle cells, and ApoE (-/-) mice. Cardiovasc Res 109: 373-373.
- GEO database G: https://www.ncbi.nlm.nih.gov/gds/?term=GSE65435
- Gene-Cloud of Biotechnology Information https://www.gcbi.com.cn/gclib/html/index
- Bostrom KI (2016) Where do we stand on vascular calcification? Vascular Pharmacology 84: 8-14.
- Vassalle C, Mazzone A (2016) Bone loss and vascular calcification: A bi-directional interplay? Vascular Pharmacology 86: 77-86.
- Johansson S (2016) A Case of Osteogenesis imperfecta with widespread Vascular Calcification. Acta Radiologica 57: 17-U23.
- Alcantara EH, Shin MY, Beattie JH, Kwun IS (2011) Zn deficiency induces calcification by regulating apoptosis rather than osteogenesis in vascular smooth muscle cells. Faseb Journal p. 25.
- Chang L, Graham PH, Ni J, Hao JL, Bucci J, et al. (2015) Targeting PI3K/Akt/mTOR signaling pathway in the treatment of prostate cancer radioresistance. Crit Rev Oncol Hematol 96: 507-517. [Crossref]
- Asati V, Mahapatra DK, Bharti SK (2016) PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. Eur J Med Chem 109: 314-341. [Crossref]
- Del Corno M, Liu QH, Schols D, Clercq EC, Gessani S, et al. (2001) HIV-1 gp120 and chemokine activation of Pyk2 and mitogen-activated protein kinases in primary macrophages mediated by calcium-dependent, pertussis toxin-insensitive chemokine receptor signaling. Blood 98: 2909-2916. [Crossref]
- Turrin NP, Plata-Salaman CR (2000) Cytokine-cytokine interactions and the brain. Brain Res Bull 51: 3-9. [Crossref]
- Bao Y, Cao XT (2016) Epigenetic Control of B Cell Development and B-Cell-Related Immune Disorders. Clin Rev Allergy Immunol 50: 301-311. [Crossref]
- Slaats J, ten Oever J, van de Veerdonk FL, Netea MG (2016) IL-1 beta/IL-6/CRP and IL-18/ferritin: Distinct Inflammatory Programs in Infections. PLoS Pathog 12: e1005973. [Crossref]
- Matthes T, Manfroi B, Huard B (2016) Revisiting IL-6 antagonism in multiple myeloma. Critical Reviews in Oncology Hematology 105:1-4.
- Azuma MM, Samuel RO, Gomes JE, Dezan E, Cintra LTA (2014) The role of IL-6 on apical periodontitis: a systematic review. Int Endod J 47: 615-621. [Crossref]
- Jordan SC, Choi J, Kim I, Wu G, Toyoda M, et al. (2017) Interleukin-6, A Cytokine Critical to Mediation of Inflammation, Autoimmunity and Allograft Rejection: Therapeutic Implications of IL-6 Receptor Blockade. Transplantation 101: 32-44. [Crossref]
- Echizen K, Hirose O, Maeda Y, Oshima M (2016) Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E-2 and Toll-like receptor/MyD88 pathways. Cancer Sci 107: 391-397. [Crossref]
- Xu Q, Zhu GZ, Li J, Cheng K (2017) Development of Antibacterial Drugs by Targeting Toll-Like Receptors. Curr Top Med Chem 17: 270-277. [Crossref]
- Wu CQ, Huang RT, Kuo CH, Kumar S, Kim CW, et al. (2015) Mechanosensitive PPAP2B Regulates Endothelial Responses to Atherorelevant Hemodynamic Forces. Circ Res 117: E41-E53. [Crossref]
- Zhang FF, Wu LL, Qian J, Qu B, Xia SW, et al. (2016) Identification of the long noncoding RNA NEAT1 as a novel inflammatory regulator acting through MAPK pathway in human lupus. J Autoimmun 75: 96-104. [Crossref]
- Masliah-Planchon J, Garinet S, Pasmant E (2016) RAS-MAPK pathway epigenetic activation in cancer: miRNAs in action. Oncotarget 7: 38892-38907. [Crossref]





