Background: Hypocalcemia is a frequent complication in patients undergoing thyroid surgery. It compromises the patient’s quality of life and increases hospitalization time, costs and mortality. The use of predictive factors to diagnose post-surgical hypocalcemia, allows early management, avoids complications and reduces treatment cost.
Methods: The MEDLINE/Pubmed and EMBASE databases were searched on May 2017. Meta-analysis, systematic reviews, observational studies and narrative reviews were included. The search was strengthened by reviewing the list of references of the selected publications and determining the relevant sources to be included manually in this publication.
Results: To assess patients for hypoparathyroidism, intact parathyroid hormone (iPTH), total serum calcium (TSC) and albumin levels, should be measured during the first 24 hours after the surgery. Patients can be classified into three groups: low-risk, medium/indeterminate risk, and high-risk.
Initiating prophylactic oral elemental calcium, the first day after surgery can reduce the incidence of postoperative hypocalcemia, the length of hospital stay and the need for parenteral calcium. need for parenteral calcium. The prescription of vitamina D (VD) is also recommended.
Conclusion: Hypocalcemia secondary to hypoparathyroidism, is a frequent complication after thyroidectomy. Early diagnosis by assessing predictive factors can prevent hypocalcemia and decrease mobility and mortality. Early evaluation of iPTH and corrected serum calcium (CSC) after neck surgery, are the most appropriate tests to diagnose transitory and permanent hypoparathyroidism.
hypoparathyroidism hypocalcemia, total thyroidectomy preoperative evaluation, prevention
Hypocalcemia is a frequent complication in patients undergoing thyroid surgery. It increases the hospitalized time and costs, decreasing the quality of life and the risk of death. Recommendations are given for the prevention, diagnosis and treatment of hypoparathyroidism in patients undergoing total thyroidectomy.
Hypocalcemia is one of the major complications of surgical interventions in the central neck (level VI) due to the small size of the parathyroid glands (PGs), their proximity and firm adherence to the thyroid, and the risk of compromising their blood flow during surgery.
Despite the expertise of surgeons, postsurgical hypocalcemia remains a prevalent complication in patients undergoing total thyroidectomy and / or central lymph node dissection, causing high postoperative morbidity and compromising the quality of life and increasing costs to the health system [1].
Some efforts have been made to find, intra and postoperative hypocalcemia predictors in an attempt to prevent and manage it early. Nevertheless, lack algorithms for its prevention, diagnosis and treatment. These algorithms could reduce the number of post-operative admissions to the emergency room, and improve morbidity.
We present a review of the literature on the prevention and early detection of post-surgical hypocalcemia; and also give some recommendations for the acute management of the patients undergoing thyroidectomy.
Anatomy and Physiology of the parathyroid glands: The PGs are small glands, brown colored, derived from the pharyngeal pouches and usually located on the dorsal side of the upper and lower poles of the thyroid gland. Given its embryonic origin, they may be located anywhere along the migration route of the pharyngeal pouches (carotid sheath, thymus or anywhere in the anterior mediastinum). They are usually (80% population) four in number although between 1-7% of people have 3 and between 3-6% have more than 4 PGs [2].
Irrigation to the superior upper parathyroid glands often depends on the superior thyroid artery (STA), and in some cases from a branch of the anastomosis between the upper and lower thyroid arteries [3]. The inferior PGs irrigation is predominantly given by branches of the inferior thyroid artery, and, less frequently by branches of the STA, depending on its location (when located in the thyrothymic ligament there is no additional supply by the STA). In a few cases the irrigation comes from branches of the internal mammary artery [4-6].
PGs through the production of parathyroid hormone (PTH) play an indispensable role regulating serum calcium, increasing the calcium levels in blood by increasing renal reabsorption of calcium, bone resorption and activation of calcidiol to stimulate intestinal calcium absorption; all this by means of PTH receptors coupled to G proteins present in these tissues [7,8]. Thus, any injury to the PGs leading to the reduction or loss of their function will generate a reduction in serum calcium which, when severe, can be life threatening, or in a lesser extent, affect importantly the quality of life of the patients and increase the days of in hospital care [9,10].
Post-surgical hypoparathyroidism in thyroid surgery: Postsurgical hypoparathyroidism has been defined as the presence of serum levels of iPTH below 15 pg/mL in the postoperative period [11-13], in the presence of CSC values below 8.0 mg/dL (2.0mmol/L), or ionized calcium below 1.1 mmol/L (4.4 mg/dL) with or without symptoms of hipocalcemia [11,13-20].
Anterior central neck compartment surgery is the leading cause of hypoparathyroidism [9, 21-25] and is one of the most common complications in patients undergoing thyroidectomy, with a prevalence of 10 to 46% [9,26,27].
Transient hypoparathyroidism is defined as the resolution of hypocalcemia, without treatment after the first 6-12 months post-surgery [9,11,12,24,26,28]. It has been described in approximately 10% of patients. Permanent hypocalcemia is reported between 0% and 43% of patients; lacking homogeneity among the available papers and including the definition and the duration of the hypocalcemia or hypoparathyroidism [11,12,26].
The British Thyroid Association Guidelines 2014 consider in general, the need for calcium substitution at 6 months subsequent to the thyroidectomy in less than 10% of patients [29].
The main risk factors for postsurgical hypoparathyroidism are: [11,12,25,30,31].
- Large size and weight of the thyroid gland [17,21]
- Retro-sternal extension of the thyroid [32]
- Dissection of the central neck nodal compartment [15,25,31]
- Re-interventions [17]
- Deficit or insufficiency of vitamin D [33]
- Surgeon expertise [26]
- Graves Basedow disease [26,31]
- Extent of surgery [15,34]
- Female sex [15,31]
- Presurgical use of b Blockers [31]
- Less than 2 parathyroid glands identified [15]
- Parathyroid tissue on the final pathology report [35]
Signs and symptoms of hypocalcemia depend on the severity and the acuity of the onset. In acute hypocalcemia the first symptoms described are neurological; with paresthesias in the perioral region, hands and feet and if untreated progressing to cramps, hyperreflexia and muscle spasms. Irritability, depression and psychotic symptoms may be associated findings. In severe cases, angina pectoris, congestive heart failure or syncope, due to changes in contractility or cardiac electrical conduction may occur. Laryngospasm, bronchospasm or epileptic crises can also occur all of which compromising the patient's life [36,37].
In the neurological examination it is important to remember the classical signs of latent tetany with positive Chvostek's (present in 1-25% healthy subjects and in 94% of patients with true hypocalcemia, although it may be absent in chronic hypocalcaemia) and Trousseau´s signs (absent in a third of patients with hypocalcemia) [22,36,38].
Chvostek´s sign consists on the momentarily contraction of the ipsilateral side of the face (nose or lips) when the facial nerve is tapped at the angle of the jaw (the masseter muscle). Trousseau´s sign is considered more sensitive than Chvostek´s sign. It consists on the spasm of the hand and forearm due to the occlusion of the brachial artery when a blood pressure cuff is placed on the arm and inflated to 10 mm Hg above the systolic pressure during at least 2 minutes.
The most frequent electrocardiographic findings are QTc and ST segments prolongation, T wave inversion and in severe cases, AV block or ventricular fibrillation [22,38]
In chronic hypocalcemia symptoms such as dry skin, rough hair or fragile nails are often more subtle. In spite of that, severe complications may appear in chronic cases such as papilledema, parkinsonism, subcapsular cataracts, calcification of the basal ganglia and intracerebral hemorrhages [22,38].
Workup: The diagnosis of post-surgical hypoparathyroidism is made with CSC and iPTH levels. The determination of iPTH in blood sample during the first 24 hours after surgery allow a confident diagnosis of a temporary parathyroid dysfunction [39]; the measurement serum calcium alone cannot predict hypoparathyrodism, because > 50% of patients with iPTH levels of < 10 pg/mL had a CSC of > 8 mg/dL (2 mmol/L) on the first posoperative morning [40].
Other lab tests are important in the evaluation of the patient suspected with this condition: [9,10,22,33,38]
- Serum phosphorus levels: May be increased in hypoparathyroidism, but low in hungry bone syndrome.
- Vitamin 25 hydroxy-D3: Levels in the insufficiency or deficiency ranges contribute to hypocalcaemia.
- Serum magnesium: Low levels compromise management of hypocalcemia, normal levels are required for proper PTH secretion.
- In some cases, with unexpected clinical complications is important to assess acid-base status as the presence of alkalosis increases the binding sites of the albumin to calcium, thus reducing the proportion of free calcium and causing symptoms of hypocalcemia; in these cases, measurement of CSC is not useful, and determination of ionized calcium is highly recommended.
Predictive factors of postsurgical hypoparathyroidism:
- Serum levels of iPTH
Serum iPTH levels take before, during and after thyroidectomy have been evaluated in different studies as a predictive factor for mild to severe post-surgical hypocalcemia and post-surgical hypoparathyroidism.
In a prospective multicentric study, it was found that preoperative iPTH levels equal to or higher than 47,9 pg/mL (5 pmol/L) were a predicting factor for recovery of parathyroid function [11], however, in a meta-analysis including 115 observational studies, the iPTH taken before surgery had no predictive value by itself in the multivariate analysis [31].
The decrease of the postoperative iPTH value compared with the preoperative, has been proven as a predicting factor of transient and permanent hypocalcemia [11,20,31]. Different values of iPTH defined as threshold taken at different latency times which can be as early as 5 minutes after thyroidectomy (intraoperative iPTH), in the first post-surgical hour (peri-operative iPTH) or at 24 hours post-surgical (post-operative iPTH), have been reported. Regarding levels of intraoperative iPTH, values <9.5pg/mL [41], <10pg/mL [42-45], <11,3pg/mL [46], <12pg/mL [47], <18pg/mL [48] have predicted hypocalcaemia postoperatively ; but the most accepted threshold is < 10 pg/mL.
A decrease with respect to the preoperative baseline value of iPTH >62.5% [48] measured at 10 minutes (intraoperative), or >88% in the first hour (perioperative) [11,31], or 41.9% at 24 hours (post-operatively), accurately predicted postoperative hypocalcemia and may predict persistent hypoparathyroidism after 6 months of follow-up in the majority of patients. The possibility that these patients recover their parathyroid function completely is only 10% [11].
Decreased absolute values of iPTH within the first day postoperatively at 4h <10 pg/mL [49,50] or at 24 h <5,8 pg/mL, are correlated with postoperative hypocalcemia [12,24]. Accordingly, levels > 7pg/mL [20], >9.8mpg/mL [12], >15pg/mL [24,28,47], could exclude the development of persistent hypoparathyroidism. However the majority of authors agree with the cutoff point of less than15 pg / ml (24,28,47)
In accordance with the majority of reports we conclude that postoperative iPTH levels < 10 pg/mL are predictors of hypocalcemia with a sensitivity of 72%-97.5%, specificity of 80%-99%, positive predictive value (PPV) of 53%-90% and a negative predictive value (NPV) of 80%-99% [11,16,45,51,52].
b) Serum calcium values: A statistically significant correlation between normal preoperative calcium levels and the presence of post-surgical hypocalcemia has not been found [31]. However, the progressive increase in serum calcium values between 6 and 24 hours after surgery, and the finding of normal postsurgical calcium levels, have a high NPV (80%-100%) ruling out the possibility of permanent hypocalcemia and hypoparathyroidism [13,19,31,45,51].
c) 25-Hydroxi-vitamin D3 levels: Low levels of VD increase the probability of hypocalcemia in the postsurgical period [30,33].
Prevention of postsurgical hypoparathyroidism: As previously described, the insufficiency or deficiency of VD is an independent preoperative predictor (Figure 1), contributing to postsurgical hypocalcemia. Its measurement is suggested routinely as a first step in preventing post-operative hipocalcemia [30,31]. The high cost is decreasing progressively, and its benefit supports its routine use. In cases of low preoperative serum 25-(OH)2 D3 (<30 ng/mL), the substitution with vitamin D3 is indicated to achieve normalization of serum levels in 8 weeks. A dose of 6,000 IU daily of vitamin D3 orally for 8 weeks and continued daily as maintenance with 1000 to 2000 IU to ensure a 25-(OH)2 D3 between 30 and 50 ng/mL, is recommended [31,53,54].
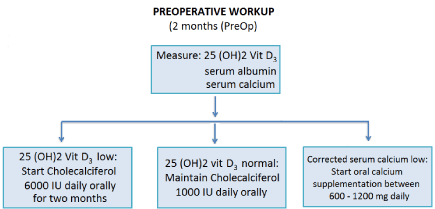
Figure 2. Approach to the acute patient with hypocalcaemia after thyroid surgery. It is recommended to follow up and educational interventions to promote a healthy lifestyle with appropriate diet; improve adherence, and the proper use of drug therapy. PTH: Intact Parathyroid Hormone measured 6 to 24h postoperatively. TCa: Total calcium albumin-corrected value and expressed in mg/dL.
It is a known fact to every surgeon that in order to prevent postoperative hypoparathyroidism while performing thyroid surgeries, the best effort must be made to avoid any kind of damage, either directly to the glands or to the blood supply of the parathyroids. A thorough knowledge of the anatomy and the most frequent variations of the location and blood supply to the glands on the part of the experienced thyroid surgeon is the best tool in preventing damage to the parathyroids and their function [55].
Identifying the location of the PGs and their major vessels (particularly the inferior thyroid artery and its usual bifurcation, its relation to the RLN and its distal branches) and trying to ligate them as distally as possible are the mainstay of a proper surgical technique. Also, a plane of cold capsular dissection, and the use of ultrasound rather than mono or bipolar energy when needed, aid in the objective of preventing vascular compromise to the glands. Nevertheless, even when the surgeon is confident that the PGs are intact and viable at the completion of the procedure, hypocalcaemia may occur. The mechanism of hypoparathyroidism after thyroidectomy is not entirely understood, but the manipulation of the PGs producing transient parathyroid insufficiency or reversible ischemia is commonly cited [56,57].
Based in the findings described above, many groups have developed protocols that include perioperative iPTH and calcium serum levels in order to classify their patients within risk groups and allowing either an early discharge or the establishing of an early in-hospital treatment for thyroidectomized patients using calcium supplements and adjusting surveillance. This has reduced emergency room readmissions as well as prolonged unjustified hospitalizations, improving the quality of life and therefore reducing costs.
Since there are different values in the protocols and articles reported, we present an algorithm based on the literature, adjusted to what is most frequently observed and recommended at our institutions.
Prophylactic supplementation of oral calcium from day 1 postoperative reduces the incidence of postoperative symptomatic hypocalcemia, length of hospital stays and the need for using parenteral calcium in the different schemes [58,59]. The administration of oral calcium 3 gr/day (1gr tid) + calcitriol 1 µg/day (0.5 µg bid) starting in the first postoperative night and extended for two weeks is routine. Once normalized, weekly monitoring of CSC for albumin and phosphorus and titrating doses according to the reports is the generalized use [14,18,57].
Detection, diagnosis and management of hypoparathyrodism: To assess the presence of hypoparathyroidism, the levels of iPTH, serum total calcium and albumin should be measured during the first 24 hours after surgery and the patients should be classified into 3 groups:
Low risk patients: Should iPTH levels be within normal limits (15 – 65 pg/mL) and CSC levels greater than 8 but less than 8,5 mg/dL, the patient may be discharged with an outpatient dose of 600 mg elemental calcium per day orally, and weekly clinical and lab tests surveillance.
Medium/indeterminate risk: For patients with corrected SC >8 mg/dL and iPTH between 5 – 15 pg/mL, they could receive elemental calcium 1200 mg/day and calcitriol 0.5 µg/day in divided doses for discharge. If the patient shows a CSC between 7.5 -8 mg/dL and iPTH between 5-15 pg/mL we recommend elemental calcium of 2400 mg/day and calcitriol 1 µg/day in divided doses. Serum levels of CSC and phosphorus should be monitored and discharged when the calcium reaches levels above 8 mg/dL.
High risk patient: If the values of CSC are below 7.5 mg/dL, iPTH <5 pg/mL it is advisable to start elemental calcium 3000-6000 mg/day and calcitriol 1.5-2 µg/day. If CSC levels is persistently below 7.5 mg/dL despite oral treatment or if patient is severely symptomatic, administer intravenous calcium gluconate in continuous intravenous infusion at an initial dose of 1 mg/kg/hour until achieving values >7.5 mg/dL, and only then it would be possible to switch to oral therapy [16,30,31,60,61] Calcium gluconate (C12H22O14) is available in 10cc ampules (10%) containing 0.232 mmol/L of calcium ion (0.465 mEq/mL). EKG monitoring must be done during calcium infusion. (Figure 2).
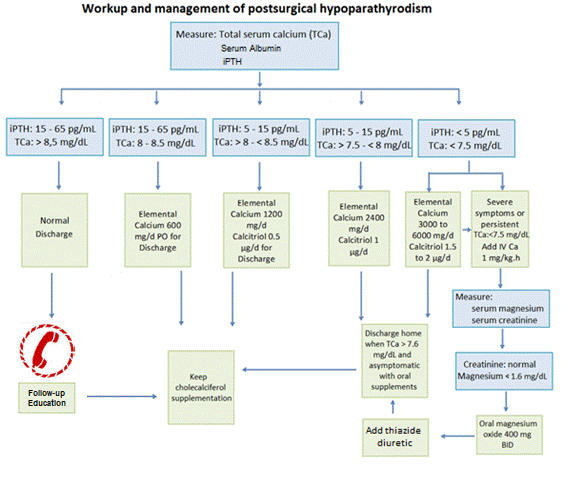
Figure 2. Approach to the acute patient with hypocalcaemia after thyroid surgery. It is recommended to follow up and educational interventions to promote a healthy lifestyle with appropriate diet; improve adherence, and the proper use of drug therapy. PTH: Intact Parathyroid Hormone measured 6 to 24h postoperatively. TCa: Total calcium albumin-corrected value and expressed in mg/dL.
It is also recommended to give vitamin D additional to calcium when the patient does not take vitamin D (cholecalciferol) supplements.
Se muestra la traducción de para evaluar la presencia de hipoparatiroideo
Traducir del para evaluar la presencia de hipoparatiroid
Outpatient management of hypoparathyroidism: Patients who fail to show normal levels of CSC and symptoms of hypocalcemia persist can be used diuretic type thiazides if blood pressure is normal or elevated. Thiazide diuretics lower urine calcium excretion because they enhance renal calcium reabsorption, at the distal tubule [62]. They bind to the chloride site of the sodium/chloride cotransporter at the convoluted distal tubule. This inhibits NaCl resorption, promoting its excretion and decreasing the effective volume. This triggers proximal water and sodium reabsorption, promotes the passive absorption of calcium and enhances the activity of the Na/Ca and increases calcium reabsorption through an active mechanism [63].
The hypocalciuric effect of thiazides is not just secondary to the effective volume depletion but depends upon the levels of PTH (near normal circulating hormone) and producing hypercalcemia due to the calcium release from the bone and probably increasing PTH action in the bone and kidney [64,65]. Follow up with serum levels of calcium, phosphorus and creatinine should be done weekly or monthly during initial dose adjustments. Once the levels are stable, follow up can be done twice a year. A 24-hour urine calcium should be done at least once a year after stable doses of supplements are established, and should be less than 4 mg/kg/24 hours.
New therapy options
Treatment with calcium and vitamin D may be challenging and lead to complications such as calcification in soft tissues, hypercalcemia and hypercalciuria. In response to PTH deficit in these patients, it has been considered since 1929 (with the demonstration of doctor Albright), the utility of bovine PTH in the management of symptomatic hypocalcemia [66]. Many studies have been conducted with 2 molecules of recombinant human PTH, the amino terminal extreme, PTHR (1-34) (Teriparatide) [67] and the complete molecule PTHR (1-84) [68], both proving beneficial in maintaining serum calcium levels and bone mineral density in patients with postsurgical hypoparathyroidism refractory to calcium and VD treatment [69].
Treatment with PTHR (1-84) evidenced an increase in serum calcium levels (variable during the day) with a peak at 6-8 hours post injection, and increased activation of VD, 10 hours post administration [68]. Available PTHR (1-34) with a shorter half life, requires administration every 12 hours, or subcutaneous infusion per pump [70-72], while the molecule (1-84) can be administered every 24 hours [73,74].
In January 2015, the treatment of postsurgical hypoparathyroidism with recombinant PTH (1-84) was approved by the FDA with the brand name Natpara®. Its use as an adjunctive to treatment with VD and calcium in patients with postsurgical hypoparathyroidism was indicated only in patients which cannot be controlled with calcium plus VD or the active molecule of VD (Calcitriol or alfacalcitriol), with the specification to individualize the treatment for each patient, given the evidence of increased risk of osteosarcoma in rats. Its prescription can only be made by qualified and trained personnel in NPS Advantage; with an initial dose of 50 µg/day.
Hypocalcemia secondary to hypoparathyroidism after thyroidectomy is a frequent complication morbidity and mortality. The use of predictive factors allows timely identification of patients at risk and the prevention of complications. Early monitoring of iPTH and corrected or ionized serum calcium levels after neck surgery, are the most appropriate tests used to diagnose transitory and permanent hypoparathyroidism. We present an algorithm for appropriate management of hypocalcemia prevention and treatment.
Gratefulness for collaboration and advice: Dr. Henning Dralle. MD, FRCS FACS, FEBS. Professor of surgery. Department of General, visceral and vascular surgery. Medical Faculty. Martin Luther University Halle-Writtenberg, Germany.
- Rosato L, Avenia N, Bernante P, De Palma M, Gulino G, Nasi PG, et al. (2004) Complications of thyroid surgery: analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J Surg 28: 271-276.
- Potts JPBRMMLCMSJSJ (2015) The Parathyroids. 3rd Edition edn: 946.
- Nobori M, Saiki S, Tanaka N, Harihara Y, Shindo S, Fujimoto Y, et al. (1994) Blood supply of the parathyroid gland from the superior thyroid artery. Surgery 115: 417-423.
- Medina Ruiz BA, Dami Can isa HR, Bogado Yinde LA, Ojeda Fiore H, Rodriguez I, Lezcano H, et al. (2011) ANATOMI´A QUIRU´RGICA DE LAS GLA´NDULAS PARATIROIDES. Revista Argentina de Anatomia 2: 118-125.
- Fancy T, Gallagher D, Hornig JD (2010) Surgical anatomy of the thyroid and parathyroid glands. Otolaryngol Clin North Am 43: 221-227
- Mohebati A, Shaha AR (2012) Anatomy of thyroid and parathyroid glands and neurovascular relations. Clin Anat 25: 19-31. [Crossref]
- Ferre S, Hoenderop JG, Bindels RJ (2012) Sensing mechanisms involved in Ca2+ and Mg2+ homeostasis. Kidney Int 82: 1157-1166. [Crossref]
- Renkema KY, Alexander RT, Bindels RJ, Hoenderop JG (2008) Calcium and phosphate homeostasis: concerted interplay of new regulators. Ann Med 40: 82-91. [Crossref]
- Al-Azem H, Khan AA (2012) Hypoparathyroidism. Best Pract Res Clin Endocrinol Metab 26: 517-522. [Crossref]
- Sergio S Maeda EMF, Ulisses M, Victoria O, Marise B, Lazaretti-Castro (2006) Hypoparathyroidism and Pseudohypoparathyroidism. Arq Bras Endocrinol Metab 50: 664-673.
- Al-Dhahri SF, Mubasher M, Mufarji K, Allam OS, Terkawi AS, et al. (2014) Factors predicting post-thyroidectomy hypoparathyroidism recovery. World J Surg 38: 2304-2310. [Crossref]
- Song CM, Jung JH, Ji YB, Min HJ, Ahn YH, Tae K, et al. (2014) Relationship between hypoparathyroidism and the number of parathyroid glands preserved during thyroidectomy. World J Surg Oncol 12: 1-200.
- Salinger EM, Moore JT (2013) Perioperative indicators of hypocalcemia in total thyroidectomy: the role of vitamin D and parathyroid hormone. Am J Surg 206: 876-881
- Bellantone R, Lombardi CP, Raffaelli M, Boscherini M, Alesina PF, De Crea C, et al. (2002) Is routine supplementation therapy (calcium and vitamin D) useful after total thyroidectomy? Surgery 132: 1109-1112
- Giordano D, Valcavi R, Thompson GB, Pedroni C, Renna L, Gradoni P, et al. (2012) Complications of central neck dissection in patients with papillary thyroid carcinoma: results of a study on 1087 patients and review of the literature. Thyroid 22: 911-917.
- Grodski S, Serpell J (2008) Evidence for the role of perioperative PTH measurement after total thyroidectomy as a predictor of hypocalcemia. World J Surg 32: 1367-1373. [Crossref]
- Lefevre JH, Tresallet C, Leenhardt L, Jublanc C, Chigot JP, et al. (2007) Reoperative surgery for thyroid disease. Langenbecks Arch Surg 392: 685-691. [Crossref]
- Roh JL, Park JY, Park CI (2009) Prevention of postoperative hypocalcemia with routine oral calcium and vitamin D supplements in patients with differentiated papillary thyroid carcinoma undergoing total thyroidectomy plus central neck dissection. Cancer 115: 251-258. [Crossref]
- Tredici P, Grosso E, Gibelli B, Massaro MA, Arrigoni C, Tradati N, et al. (2011) Identification of patients at high risk for hypocalcemia after total thyroidectomy. Acta Otorhinolaryngol Ital 31: 144-148.
- Wang JB, Sun HL, Song CY, Gao L (2015) Association between Decreased Serum Parathyroid Hormone after Total Thyroidectomy and Persistent Hypoparathyroidism. Med Sci Monit 21: 1223-1231.
- Walker Harris V, Jan De Beur S (2009) Postoperative hypoparathyroidism: medical and surgical therapeutic options. Thyroid 19: 967-973.
- De Sanctis V, Soliman A, Fiscina B (2012) Hypoparathyroidism: from diagnosis to treatment. Curr Opin Endocrinol Diabetes Obes 19: 435-442. [Crossref]
- Mitchell DM, Regan S, Cooley MR, Lauter KB, Vrla MC, et al. (2012) Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab 97: 4507-4514. [Crossref]
- Julián MT, Balibrea JM, Granada ML, Moreno P, Alastrué A, et al. (2013) Intact parathyroid hormone measurement at 24 hours after thyroid surgery as predictor of parathyroid function at long term. Am J Surg 206: 783-789. [Crossref]
- Ito Y, Kihara M, Kobayashi K, Miya A, Miyauchi A (2014) Permanent hypoparathyroidism after completion total thyroidectomy as a second surgery: How do we avoid it? Endocr J 61: 403-408. [Crossref]
- Thomusch O, Machens A, Sekulla C, Ukkat J, Brauckhoff M, et al. (2003) The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery 133: 180-185. [Crossref]
- Mehanna HM, Jain A, Randeva H, Watkinson J, Shaha A (2010) Postoperative hypocalcemia--the difference a definition makes. Head Neck 32: 279-283.
- Almquist M, Hallgrimsson P, Nordenström E, Bergenfelz A (2014) Prediction of permanent hypoparathyroidism after total thyroidectomy. World J Surg 38: 2613-2620. [Crossref]
- Perros P, Boelaert K, Colley S, Evans C, Evans RM, et al. (2014) Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 81 Suppl 1: 1-122. [Crossref]
- Khan MI, Waguespack SG, Hu MI. Medical management of postsurgical hypoparathyroidism. Endocr Pract 17: 18-25.
- Edafe O, Antakia R, Laskar N, Uttley L, Balasubramanian SP (2014) Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg 101: 307-320.
- Testini M, Gurrado A, Avenia N, Bellantone R, Biondi A, Brazzarola P, et al. (2011) Does mediastinal extension of the goiter increase morbidity of total thyroidectomy? A multicenter study of 19,662 patients. Ann Surg Oncol 18: 2251-2259.
- Kirkby-Bott J, Markogiannakis H, Skandarajah A, Cowan M, Fleming B, Palazzo F (2011) Preoperative vitamin D deficiency predicts postoperative hypocalcemia after total thyroidectomy. World J Surg 35: 324-330.
- Dralle H, Stang A, Sekulla C, Rusner C, Lorenz K, Machens A, et al. (2014) Surgery for benign goiter in Germany: fewer operations, changed resectional strategy, fewer complications]. Chirurg 85: 236-245.
- Ritter K, Elfenbein D, Schneider DF1, Chen H1, Sippel RS2 (2015) Hypoparathyroidism after total thyroidectomy: incidence and resolution. J Surg Res 197: 348-353. [Crossref]
- Cooney MHyRN (2011) Hypercalcemia and Hypocalcemia. Sixth Edition ed. Elsevier, editor: Elsevier; 3 p.
- Shoback D (2008) Clinical practice. Hypoparathyroidism. N Engl J Med 359: 391-403. [Crossref]
- Fong J, Khan A (2012) Hypocalcemia: updates in diagnosis and management for primary care. Can Fam Physician 58: 158-162. [Crossref]
- Selberherr A, Scheuba C, Riss P, Niederle B (2015) Postoperative hypoparathyroidism after thyroidectomy: efficient and cost-effective diagnosis and treatment. Surgery 157: 349-353. [Crossref]
- Asari R, Passler C, Kaczirek K, Scheuba C, Niederle B. Hypoparathyroidism after total thyroidectomy: a prospective study. Arch Surg 143: 132-137.
- Lang BH, Yih PC, Ng KK (2012) A prospective evaluation of quick intraoperative parathyroid hormone assay at the time of skin closure in predicting clinically relevant hypocalcemia after thyroidectomy. World J Surg 36: 1300-1306.
- BarczyA, ski M, CichoA S, Konturek A, Cicho AW, et al. (2008) Applicability of intraoperative parathyroid hormone assay during total thyroidectomy as a guide for the surgeon to selective parathyroid tissue autotransplantation. World J Surg 32: 822-828. [Crossref]
- Quiros RM, Pesce CE, Wilhelm SM, Djuricin G, Prinz RA, et al. (2005) Intraoperative parathyroid hormone levels in thyroid surgery are predictive of postoperative hypoparathyroidism and need for vitamin D supplementation. Am J Surg 189: 306-309. [Crossref]
- Richards ML, Bingener-Casey J, Pierce D, Strodel WE, Sirinek KR (2003) Intraoperative parathyroid hormone assay: an accurate predictor of symptomatic hypocalcemia following thyroidectomy. Arch Surg 138: 632-635
- Riaz U, Shah SA, Zahoor I, Riaz A, Zubair M (2014) Validity of early parathyroid hormone assay as a diagnostic tool for sub-total thyroidectomy related hypocalcaemia. J Coll Physicians Surg Pak 24: 459-462.
- Gupta S, Chaudhary P, Durga CK, Naskar D (2015) Validation of intra-operative parathyroid hormone and its decline as early predictors of hypoparathyroidism after total thyroidectomy: A prospective cohort study. Int J Surg 18: 150-153.
- Ezzat WF, Fathey H, Fawaz S, El-Ashri A, Youssef T, et al. (2011) Intraoperative parathyroid hormone as an indicator for parathyroid gland preservation in thyroid surgery. Swiss Med Wkly 141: w13299. [Crossref]
- Alía P, Moreno P, Rigo R, Francos JM, Navarro MA (2007) Postresection parathyroid hormone and parathyroid hormone decline accurately predict hypocalcemia after thyroidectomy. Am J Clin Pathol 127: 592-597. [Crossref]
- BarczyA, ski M, CichoA S, Konturek A (2007) Which criterion of intraoperative iPTH assay is the most accurate in prediction of true serum calcium levels after thyroid surgery? Langenbecks Arch Surg 392: 693-698. [Crossref]
- Carr AA, Yen TW, Fareau GG, Cayo AK, Misustin SM, Evans DB, et al. (2014) A single parathyroid hormone level obtained 4 hours after total thyroidectomy predicts the need for postoperative calcium supplementation. J Am Coll Surg 219: 757-764.
- AlQahtani A, Parsyan A, Payne R, Tabah R (2014) Parathyroid hormone levels 1 hour after thyroidectomy: an early predictor of postoperative hypocalcemia. Can J Surg 57: 237-240. [Crossref]
- Kim JP, Park JJ, Son HY, Kim RB, Kim HY, Woo SH, et al. (2013) Effectiveness of an i-PTH measurement in predicting post thyroidectomy hypocalcemia: prospective controlled study. Yonsei Med J 54: 637-642.
- Prof Roger Francis (Chair) DTA (2013) Vitamin D and Bone Health: A Practical Clinical Guideline for Patient Management. The National Osteoporosis Society1: 1-27.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, et al. (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96: 1911-1930. [Crossref]
- Fewins J, Simpson CB, Miller FR. Complications of thyroid and parathyroid surgery. Otolaryngol Clin North Am 36:189-206.
- Lorente-Poch L, Sancho JJ, Ruiz S, Sitges-Serra A (2015) Importance of in situ preservation of parathyroid glands during total thyroidectomy. Br J Surg 102: 359-367.
- Abboud B, Sleilaty G, Zeineddine S, Braidy C, Aouad R, Tohme C, et al. Is therapy with calcium and vitamin D and parathyroid autotransplantation useful in total thyroidectomy for preventing hypocalcemia? Head Neck 30: 1148-1154.
- Kurukahvecioglu O, Karamercan A, Akin M, Tezel E, Ege B, Taneri F, et al. (2007) Potential benefit of oral calcium/vitamin D administration for prevention of symptomatic hypocalcemia after total thyroidectomy. Endocr Regul 41: 35-39.
- Tartaglia F, Giuliani A, Sgueglia M, Biancari F, Juvonen T, et al. (2005) Randomized study on oral administration of calcitriol to prevent symptomatic hypocalcemia after total thyroidectomy. Am J Surg 190: 424-429. [Crossref]
- Wiseman JE, Mossanen M, Ituarte PH, Bath JM, Yeh MW, et al. (2010) An algorithm informed by the parathyroid hormone level reduces hypocalcemic complications of thyroidectomy. World J Surg 34: 532-537.
- Carter Y, Chen H, Sippel RS (2014) An intact parathyroid hormone-based protocol for the prevention and treatment of symptomatic hypocalcemia after thyroidectomy. J Surg Res 186: 23-28.
- Testa A, Fant V, De Rosa A, Fiore GF, Grieco V, et al. (2006) Calcitriol plus hydrochlorothiazide prevents transient post-thyroidectomy hypocalcemia. Horm Metab Res 38: 821-826. [Crossref]
- Desai HV, Gandhi K, Sharma M, Jennine M, Singh P, et al. (2010) Thiazide-induced severe hypercalcemia: a case report and review of literature. Am J Ther 17: e234-236. [Crossref]
- Parfitt AM (1972) The interactions of thiazide diuretics with parathyroid hormone and vitamin D. Studies in patients with hypoparathyroidism. J Clin Invest 51: 1879-1888. [Crossref]
- Popovtzer MM, Subryan VL, Alfrey AC, Reeve EB, Schrier RW (1975) The acute effect of chlorothiazide on serum-ionized calcium. Evidence for a parathyroid hormone-dependent mechanism. J Clin Invest 55: 1295-1302. [crossref]
- Albright F, Ellsworth R (1929) studies on the physiology of the parathyroid glands: i. Calcium and Phosphorus Studies on a Case of Idiopathic Hypoparathyroidism. J Clin Invest 7: 183-201.
- Winer KK, Sinaii N, Reynolds J, Peterson D, Dowdy K, et al. (2010) Long-term treatment of 12 children with chronic hypoparathyroidism: a randomized trial comparing synthetic human parathyroid hormone 1-34 versus calcitriol and calcium. J Clin Endocrinol Metab 95: 2680-2688. [Crossref]
- Sikjaer T, Amstrup AK, Rolighed L, Kjaer SG, Mosekilde L, Rejnmark L (2013) PTH (1-84) replacement therapy in hypoparathyroidism: a randomized controlled trial on pharmacokinetic and dynamic effects after 6 months of treatment. J Bone Miner Res 28: 2232-2243.
- Ramakrishnan Y, Cocks HC (2016) Impact of recombinant PTH on management of hypoparathyroidism: a systematic review. Eur Arch Otorhinolaryngol 273: 827-835. [Crossref]
- Winer KK, Yanovski JA, Sarani B, Cutler GB Jr (1998) A randomized, cross-over trial of once-daily versus twice-daily parathyroid hormone 1-34 in treatment of hypoparathyroidism. J Clin Endocrinol Metab 83: 3480-3486. [Crossref]
- Winer KK, Sinaii N, Peterson D, Sainz B Jr, Cutler GB Jr (2008) Effects of once versus twice-daily parathyroid hormone 1-34 therapy in children with hypoparathyroidism. J Clin Endocrinol Metab 93: 3389-3395. [Crossref]
- Winer KK, Zhang B, Shrader JA, Peterson D, Smith M, et al. (2012) Synthetic human parathyroid hormone 1-34 replacement therapy: a randomized crossover trial comparing pump versus injections in the treatment of chronic hypoparathyroidism. J Clin Endocrinol Metab 97: 391-399. [Crossref]
- Clarke BL, Kay Berg J, Fox J, Cyran JA, Lagast H, et al. (2014) Pharmacokinetics and pharmacodynamics of subcutaneous recombinant parathyroid hormone (1-84) in patients with hypoparathyroidism: an open-label, single-dose, phase I study. Clin Ther 36: 722-736.
- Mannstadt M, Clarke BL, Vokes T, Brandi ML, Ranganath L, Fraser WD, et al. Efficacy and safety of recombinant human parathyroid hormone (1-84) in hypoparathyroidism (REPLACE): a double-blind, placebo-controlled, randomised, phase 3 study. Lancet Diabetes Endocrinol 1: 275-283.


