Abstract
The epidemic of overweight and obesity in youth is increasing the prevalence of prehypertension and hypertension among children and adolescents. The younger the child is at presentation and the more severe the blood pressure abnormality, the more likely a secondary cause of hypertension to be present. Measurement of blood pressure (BP) in children requires adaptation to the age and size of the children. Interpretation must be related to normative values specific to age, gender and height. Evaluation is primarily aimed at identifying secondary causes of hypertension, associated comorbidities, additional risk factors, and evidence of target organ damage. Ambulatory blood pressure monitoring is emerging as a useful tool for evaluation of some patients, particularly for those with suspected white coat hypertension. Uncontrolled and prolonged elevation of blood pressure can lead to a variety of changes in the myocardial structure, coronary vasculature, and conduction system of the heart. These changes in turn can lead to the development of left ventricular hypertrophy, coronary artery disease, various conduction system diseases, and systolic and diastolic dysfunction of the myocardium, cardiac arrhythmias (especially atrial fibrillation), and congestive heart failure. Although these diseases generally develop in response to chronically elevated BP, marked and acute elevation of BP can lead to accentuation of an underlying predisposition to any of the symptoms traditionally associated with chronic hypertension. Management of prehypertension and hypertension is directed at the underlying cause, exacerbating factors, and the magnitude of the blood pressure abnormality. Healthy behavioral changes are a primary management tool for treating hypertension, and more particularly prehypertension and for addressing other cardiovascular risk factors, such as obesity. Pharmacological management is reserved for patients with hypertension who do not respond to behavioral changes, have additional cardiovascular risk factors or diabetes, are symptomatic or have developed target organ damage.
Key Words
hypertension, hypertensive heart disease, children, adolescents
Introduction
Hypertension is a well-recognized cardiovascular risk factor in adults, contributing to morbidity and mortality from myocardial infarction, stroke, congestive heart failure (CHF), peripheral vascular disease, retinopathy, and end-stage renal disease. No study has been of sufficient duration to determine whether hypertension identified in youth is related to cardiovascular disease in adulthood. In addition, manifest atherosclerotic cardiovascular disease is extremely rare in childhood. Nonetheless, evidence to support an association between elevated blood pressure (BP) and atherosclerosis in youth is available from pathology studies and studies of noninvasive markers of atherosclerosis. Blood pressure assessment in youth, either by direct measurement (Bogalusa Heart study) [1] or by inference (Pathobiological Determinants of atherosclerosis in Youth study) [2-4] is independently correlated with the percentage of intimal surface in the coronary arteries and aorta that are affected by early atherosclerotic lesions, including fatty streaks and fibrous plaques. In addition, clustering of elevated blood pressure and other cardiovascular risk factors, as seen with the epidemic of the metabolic syndrome and obesity, is associated with an exponential increase in atherosclerotic vascular involvement [1,5]. These correlations are also evident when noninvasive measures of vascular involvement are used in children and young adults. Ultrasonography has shown that increased blood pressure in children and adolescents is associated with endothelial dysfunction in systemic arteries, increased thickness of the arterial intima–media complex, impaired arterial compliance and distensibility, and increased levels of inflammatory markers [6-8]. Research using ultrafast Computed tomography (CT) has shown a positive correlation between blood pressure and coronary artery calcification in adolescents and young adults [5,9]. These studies provide consistent and compelling evidence that the atherosclerotic process begins in youth, and is accelerated by increased blood pressure.
In addition to accelerated atherosclerosis, there is also evidence of target organ damage-primarily left ventricular hypertrophy (LVH). LVH has been reported in about one third of children and adolescents with mild, untreated hypertension and in a greater proportion of those with persistent hypertension [6,10,11]. LVH can be concentric or eccentric, with concentric being associated with a higher risk of cardiovascular outcomes [5,10]. The risk of LVH increases with the severity of hypertension in adolescents, but the odds of LVH are also increased in those with masked and milder hypertension (but not with ‘white coat’ hypertension), compared with normotensive adolescents [4,12]. Lande et al.. [7] showed that, after matching for body mass index (BMI), children with ‘white coat’ hypertension had greater left ventricular mass index than normotensive controls, but less than patients with persistent hypertension (26% with LVH). Studies have also shown that the presence of concomitant obesity further increases the prevalence of LVH in youths with hypertension [10]. In addition, LVH has been shown to be correlated with increased carotid intima–media thickness an early marker for atherosclerosis- and increasing adiposity in children and adolescents with hypertension [6]. Program on Children and adolescents recommended that the presence of LVH be used to influence therapeutic decisions in patients with hypertension [13]. In this review, I provide a general overview of hypertension, highlighting evaluation and management aspects of this condition that are specific to infants, children, and adolescents.
Definition of hypertension in youth
The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents [13] provides systolic and diastolic blood pressure levels corresponding to the 50th, 90th, 95th, and 99th percentiles based on the child’s sex, age, and height percentile. The height percentile is plotted from normal growth charts [14]. The blood pressure tables and instructions for using them are available in the Report [13] and online [14].
Blood pressure status can be classified on the basis of systolic and diastolic blood pressure percentiles (Table 1) [14]. Measurements below the 90th percentile are considered normal. Prehypertension or hypertension are present when measurements of either systolic or diastolic pressure, or both, are at or above the 90th percentile. Blood pressure should be measured at least twice during the same assessment, and confirmed over at least three separate occasions [15]. Prehypertension is present when the measurement is at or above the 90th percentile, but less than the 95th percentile, as well as when blood pressure reaches or exceeds 120/80 mmHg in an adolescent. Hypertension is present when repeated measurements are at or above the 95th percentile. Hypertension is further classified as either stage 1, in which blood pressure ranges from the 95th to the 99th percentile plus 5 mmHg, or stage 2, where blood pressure is above the 99th percentile plus 5 mmHg. ‘White coat’ hypertension occurs when the patient’s blood pressure remains above the 95th percentile when measured in a clinical setting, but is normal when measured in a familiar setting. If hypertension is confirmed, blood pressure should be measured in both arms and a leg. The classification of blood pressure influences decisions on evaluation and management [16].
Table 1. Classification of hypertension in youth*
|
Category
|
Systolic or diastolic blood pressure
Percentile
|
|
Normal
|
<90th
|
|
Prehypertension
|
90–95th, or if blood pressure exceeds
120/80 mmHg even if <90th up to <95th
|
|
Stage 1 hypertension
|
95–99th plus 5 mmHg
|
|
Stage 2 hypertension
|
>99th plus 5 mmHg
|
*Percentiles are based on normative values related to sex, age, and height percentile.
If systolic and diastolic categories are different, classify by the higher category [14].
Prevalence of hypertension in youth
Population-based studies of blood pressure in children are important in determining the burden of risk, but cohort studies provide important information regarding tracking (the persistence into adulthood of blood pressure abnormalities noted in childhood), which is an evidence component supporting the need for early intervention to reduce blood pressure. Examination of serial cross-sectional data from the National Health and Nutrition Examination Survey in children aged 8-17 years, showed a trend towards an increase in the prevalence of both prehypertension and hypertension in the US [17]. Each survey showed a higher prevalence of prehypertension in boys than in girls, whereas the prevalence of hypertension was similar for both sexes. Overweight and, particularly, obesity increased the likelihood of both prehypertension and hypertension. Ethnic differences were also noted; particularly the prevalence of prehypertension was higher in non-Hispanic black girls compared with other ethnic groups [17]. However, given the impact of sex, ethnicity or race, anthropometry, and the influence of both genetic and environmental factors on blood pressure, together with variations in measurement technique and definitions, comparisons of the prevalence of hypertension in different populations can be problematic.
Longitudinal cohort studies aim to determine the degree to which blood pressure tracks over time, particularly from childhood to adulthood, thus supporting the early identification of high-risk individuals. A meta analysis of 50 cohort studies showed inconsistencies across studies but correlation coefficients for tracking [18], which were higher for systolic than diastolic blood pressure (0.38 and 0.28, respectively). The strength of the tracking correlations increased with older baseline age and decreased with longer periods of follow-up. Although subtle variations were found, tracking correlations were not significantly influenced by race and ethnicity or geographic population. Tracking of diastolic blood pressure was weaker for females and stronger when automated measurement devices were used [16].
Prognosis
High blood pressure is a precursor of heart attacks and strokes, as has been well established in the adult literature. Obese children have approximately a 3-fold higher risk for hypertension than nonobese children [15]. As many as 41% of children with high blood pressure (BP) have left ventricular hypertrophy (LVH) [16]. Almost 60% of children with persistent elevated BP have relative weights greater than 120% of the median for their sex, height, and age. As in adults, in whom abdominal girth correlates to elevated blood pressure, studies show that this measurement is also to be considered in the assessment of a teenager with suspected BP elevation at an early age [17].
Previous
Next: Prognosis
Patient education
Parents, caregivers, and children themselves must be properly advised about restriction of exercise, when appropriate. They must also be informed about the potential adverse effects of medication. Finally, it is vital to educate parents, caregivers, and children about the potential complications of persistent hypertension.
Patho-physiology
BP is determined by the balance between cardiac output and vascular resistance. A rise in either of these variables, in the absence of a compensatory decrease in the other, increases mean BP, which is the driving pressure.
Factors that affect cardiac output include the following [19]:
- Baroreceptors
- Extracellular volume
- Effective circulating volume - Atrial natriuretic hormones, mineralocorticoids, angiotensin
- Sympathetic nervous syndrome
Factors that affect vascular resistance include the following, (19) :
- Pressors - Angiotensin II, calcium (intracellular), catecholamines, sympathetic nervous system, vasopressin
- Depressors - Atrial natriuretic hormones, endothelial relaxing factors, kinins, prostaglandin E2, prostaglandin I2
Changes in electrolyte homeostasis, particularly changes in sodium, calcium, and potassium concentrations, affect some of these factors [20].
Under normal conditions, the amount of sodium excreted in the urine matches the amount ingested, resulting in near constancy of extracellular volume. Retention of sodium results in increased extracellular volume, which is associated with an elevation of BP. By means of various physical and hormonal mechanisms, this elevation triggers changes in both the glomerular filtration rate (GFR) and the tubular reabsorption of sodium, resulting in excretion of excess sodium and restoration of sodium balance [20].
A rise in the intracellular calcium concentration, due to changes in plasma calcium concentration, increases vascular contractility. In addition, calcium stimulates release of renin, synthesis of epinephrine, and sympathetic nervous system activity. Increased potassium intake suppresses production and release of renin and induces natriuresis, decreasing BP [20].
The complexity of the system explains the difficulties often encountered in identifying the mechanism that accounts for hypertension in a particular patient. These difficulties are the main reason why treatment is often designed to affect regulatory factors rather than the cause of the disease. In a child who is obese, hyperinsulinemia may elevate BP by increasing sodium reabsorption and sympathetic tone [5].
Etiology of hypertension in youth
Primary or essential hypertension
Primary or essential hypertension is an epidemic public health problem in adults, but has its origins in childhood. The prevalence of hypertension in adults is age dependent. Overall, hypertension affects about 20% of adults in North America, with a low prevalence of optimal treatment and control, which is of particular concern for patients with diabetes (DM) [21]. For young adult aged18-39 years, isolated systolic hypertension is more prevalent, and has been shown to be associated with male sex, current smoking, obesity, and lower educational level [22]. However, obesity in adults is more likely to be associated with isolated diastolic or combined systolic and diastolic hypertension [22]. Primary or essential hypertension, as well as predisposing factors, can be identified in children and adolescents. The increasing prevalence of increased body weight and obesity in youth has been associated with a greater prevalence of prehypertension and hypertension. Insulin resistance has been shown to be independently associated with hypertension, in addition to the degree distribution of adiposity [23]. Sleep abnormalities are prevalent among obese children and adolescents, and contribute to hypertension in this setting [24,25]. Childhood obesity might accelerate the manifestation and exacerbate primary hypertension in those individuals with a familial predisposition towards developing elevated blood pressure [26]. The genetic basis is likely to be polygenic in nature and reflect multiple pathophysiological mechanisms related to blood pressure homeostasis, although single gene defects associated with hypertension in children are being increasingly defined [27]. A study of dietary and lifestyle factors in children showed that elevated systolic blood pressure was related to increased BMI, carbohydrate intake, and time spent in sedentary pursuits [28]. Primary or essential hypertension is likely to become increasingly prevalent at younger ages, concomitant with the worsening epidemic of obesity in youth [26,29].
Secondary hypertension
By contrast to adult hypertension, high blood pressure in children is likely to have an underlying secondary cause. The younger the child and the greater the degree of blood pressure elevation, the greater the likelihood of a secondary cause. The evaluation of children and adolescents is, therefore, primarily aimed at identifying secondary causes, associated comorbidities, additional risk factors, and evidence of target-organ abnormalities. The prevalence of specific secondary etiologies varies with the age of the patient (Table 2) [16]. Exact etiology-specific prevalence estimates are difficult to obtain, given that etiology is not examined in population-based cohort studies, and referral-based clinical studies might be biased towards a particular etiology. Nonetheless, renal parenchymal abnormalities (polycystic renal disease, multicystic dysplastic renal disease, hydronephrosis, chronic pyelonephritis, glomerulonephritides, and chronic renal failure) are estimated to account for about 75% of secondary hypertension in children, followed by renovascular abnormalities (renal artery stenosis or thrombosis, and renal vein thrombosis) [30]. Both therapeutic and illicit drugs, such as corticosteroids and decongestants, as well as some nutritional substances, such as caffeine, may be iatrogenic causes or contributors to secondary hypertension [16].
Table 2. Prevalent causes of hypertension by age [16]
|
Age group
|
Main causes
|
|
Neonates
|
Renal artery/vein thrombosis
Congenital renal anomalies
Coarctation of the aorta
|
|
<1 year
|
Coarctation of the aorta
Renovascular disease
Renal parenchymal disease
|
|
1–6 years
|
Renal parenchymal disease
Renovascular disease
Coarctation of the aorta
|
|
7–12 years
|
Renal parenchymal disease
Renovascular disease
Primary/essential hypertension
|
|
13–18 years
|
Primary/essential hypertension
Medication or substance use
Renal parenchymal disease
|
Other etiologies are much less prevalent. Wilms tumor, neuroblastoma and, rarely, pheochromocytoma are childhood tumors associated with hypertension. Williams syndrome is associated with renal artery stenosis, and Turner syndrome is associated with coarctation of the aorta. Endocrinopathies associated with hypertension include hypercortisolism, hyperthyroidism, hyperaldosteronism, and diabetes as well as rare metabolic conditions, such as congenital adrenal hyperplasia. These conditions unbalance normal homeostasis. neurocutaneous syndromes, such as tuberous sclerosis and neurofibromatosis, can be associated with various pathophysiological mechanisms contributing to hypertension. Systemic lupus erythematosis can lead to renal parenchymal disease and hypertension [16].
Blood pressure measurement
General screening
Current guidelines recommend that all children over 3 years of age should have their blood pressure assessed as part of routine health maintenance [13]. The setting for assessment should be optimized to reduce distractions and stress, and the patient should be in a relaxed state. Although oscillometric devices are being more frequently used, auscultation and the use of a mercury column sphygmomanometer remains the recommended approach, and the method that was used to derive current normal values of blood pressure upon which cutpoints for decision-making are based [31]. Blood pressure measurements above the 90th percentile from oscillometric assessment should be reassessed with the ausculatory method, since normal values based on this method are not available. Elevated measurements demonstrated by either method should be confirmed by repeated assessment at a minimum of three different time points [16].
For either method, use of an appropriately sized cuff is important, and 80–100% of the inflatable bladder length should encircle the mid-portion of the upper arm. The right arm is preferred, with the child seated (infants can be supine). At least 40% of the arm from the olecranon to the acromion should be covered by the width of the inflatable bladder. Use of a cuff that is too small might falsely elevate readings. Particular care should be taken to ensure the appropriate cuff size when assessing over-weight and obese patients. The head of the stethoscope is placed over the brachial artery pulse, below the lower edge of the cuff. The arm should be relaxed and supported such that the cubital fossa is at the level of the heart. Systolic pressure is taken as the onset of the first Korotkoff sound; disappearance of Korotkoff sounds (the fifth Korotkoff sound) is taken as the diastolic pressure [31]. In some children, Korotkoff sounds are evident down to 0 mmHg. In this instance, the measurement should be repeated with less pressure on the head of the stethoscope; if the low pressure measurement persists, then the fourth Korotkoff sound or muffling may be recorded as the diastolic pressure [16].
Despite the current recommendation for using the fifth Korotkoff sound to indicate diastolic blood pressure, some evidence suggests that the fourth sound might be better. A meta-analysis of 50 cohort studies showed that tracking coefficients tended to be higher for the fourth versus fifth sound, although the difference was not significant [18]. Although tracking might be better for diastolic measurements based on the fourth versus the fifth sound, there are systematic differences in the values derived. An analysis of 129 surveys worldwide showed that diastolic blood pressure measured using the fourth sound was consistently 5 mmHg higher than when using the fifth sound [32]. Successive previous recommendations have advocated use of the fourth sound, followed by use of the fourth sound in infants and young children, and the fifth sound in older children and adolescent [33] followed by the current recommendation for sole use of the fifth sound, based on a growing body of normal values using the fifth sound from the NHANES studies [13,34]. Current normal values and definitions of hypertension are based on the use of the fifth sound, and this should be taken into account if one uses the fourth sound for assessment.
Although sphygmanometric devices with auscultation are currently recommended for blood pressure measurement, automated devices have gained widespread popularity. These devices are easier to use, digit bias is eliminated, observer variation minimized, and they can be used when auscultation is difficult, particularly in very young children. In addition, these devices have been associated with stronger tracking coefficients [18]. However, current normal values and definitions of hypertension are based on the use of sphygmanometry with auscultation, and this should be taken into account when measuring blood pressure with an automated device. Mercury manometers have been banned in some countries and many health-care institutions because of concerns about toxicity. Newer sphygmanometric and oscillometric devices have not yet been fully validated and normal values specific for measurement with these devices are not available [16]. The potential for systematic errors requires further study.
Assessment of young children
For children aged 3 years or younger, blood pressure should be assessed if a secondary condition known to be associated with hypertension is present (Table 3) [13]. Blood pressure measurement can be technically challenging in these patients, and the role of pulse palpation or Doppler devices has not been systematically defined. The use of oscillometric devices might be acceptable. Interpretation of the measurements should take into account the level of cooperation or agitation of the child [16].
Table 3. Conditions under which children < 3 years old should have BP measured
|
Conditions under which children < 3 years old should have BP measured
|
|
■ History of prematurity, very low birthweight, or other neonatal complication
requiring intensive care
■ Congenital heart disease (repaired or
nonrepaired)
■ Recurrent urinary tract infections,
hematuria, or proteinuria
■ Known renal disease or urologic malformations
■ Family history of congenital renal disease
■ Solid organ transplant
■ Malignancy or bone marrow transplant
■ Treatment with drugs known to raise BP
■ Other systemic illnesses associated with hypertension (neurofibromatosis, tuberous sclerosis, etc.)
■ Evidence of elevated intracranial pressure
|
Abbreviation, BP, blood pressure [13].
Measurement in the lower extremity
Blood pressure should be measured in the lower extremities when elevated systolic blood pressure is noted in the upper extremities or if congenital heart disease, particularly aortic coarctation, is suspected. The child should be supine, and comparison made to measurements in the upper extremities also obtained while supine. The cuff can be placed around the child’s thigh, with auscultation or Doppler assessment of the popliteal artery, or around the calf, with assessment of the posterior tibial or dorsalis pedis artery. A large cuff is required and should cover at least 60% of the distance between either the perineum and the knee, or the knee and the ankle. Blood pressure in the upper extremity should not exceed that of the lower extremity. Distal pulse amplification, or a wide pulse pressure, may cause the lower extremity systolic blood pressure to exceed that of the upper extremity by an average of 5 mmHg in older children and adolescents, although these pressures equalize with exercise [35].
Ambulatory blood pressure monitoring
Ambulatory blood pressure monitoring (ABPM) has emerged as a useful modality for assessment of blood pressure in children and has been shown, in adults, to better predict cardiovascular disease and events and the risk of end-organ damage than blood pressure measurements obtained in a clinical setting [36,37]. Recommendations for standard assessment and normative blood pressure values in children and adolescents exist [38]. ABPM is particularly useful in the evaluation of suspected ‘white coat’ hypertension and masked hypertension, to assess blood pressure variability, and to evaluate the effectiveness of drug therapy for hypertension. ABPM is indicated for evaluation of children with conditions associated with hypertension, including diabetes and unrepaired or repaired aortic coarctation, solid-organ transplantation recipients, and carriers of genes associated with polycystic ovary disease, Williams or Turner syndromes, or neurofibromatosis [13]. ABPM should be performed by trained personnel using age-appropriate equipment with the correct cuff size on the nondominant arm. Calibration to resting clinic blood pressure measurements should be performed, and recordings made every 20–30 min while the patient is awake and 30–60 min while asleep. Valid interpretation requires an appropriate number of recordings, including a minimum of one reading per hour with at least 40 to 50 readings in total (representing at least 65% to 75% of all possible readings) over the 24 h period. Values that are considerably outside of the expected range should be deleted. Interpretation should be related to specific pediatric normative values for ABPM. The primary measure is the mean systolic and diastolic ambulatory blood pressure over the entire period, and separately for day time and night time. Additional important measurements include an indicator of blood pressure load, calculated as the percentage of readings that exceed the 95th percentile of normative ambulatory values. Night-time dipping is calculated as the percentage fall of mean night-time values versus mean day-time values [39] and blunted dipping may be associated with nephropathy in patients with diabetes [40,41]. Definitions of hypertension incorporating the contribution of ABPM are shown in table 4 [38].
Table 4. Classification of hypertension in youth incorporating ABP*
|
Category
|
Clinic BP
|
Mean systolic
ABP
|
Systolic BP
load (%)
|
|
Normal
|
<95th percentile
|
<95th percentile
|
<25
|
|
‘White coat’ hypertension
|
>95th percentile
|
<95th percentile
|
<25
|
|
Masked hypertension
|
<95th percentile
|
>95th percentile
|
>25
|
|
Prehypertension
|
>95th percentile
|
<95th percentile
|
25–50
|
|
Ambulatory hypertension
|
>95th percentile
|
>95th percentile
|
25–50
|
|
Severe ambulatory hypertension
|
>95th percentile
|
>95th percentile
|
>50
|
*relates clinic BP to normal values from the Fourth report; relate ambulatory BP to normal values for ambulatory BP. Abbreviations: ABP ambulatory blood pressure; BP, blood pressure. Adapted from [38].
Cardiovascular effects of hypertension
Uncontrolled and prolonged elevation of BP can lead to a variety of changes in the myocardial structure, coronary vasculature, and conduction system of the heart. These changes in turn can lead to the development of LVH, coronary artery disease (CAD), various conduction system diseases, and systolic and diastolic dysfunction of the myocardium, cardiac arrhythmias (especially atrial fibrillation), and CHF [20].
Thus, hypertensive heart disease is a term applied generally to heart diseases, such as LVH (seen in the image below, Figure 1), coronary artery disease, cardiac arrhythmias, and CHF that are caused by the direct or indirect effects of elevated BP. Although these diseases generally develop in response to chronically elevated BP, marked and acute elevation of BP can lead to accentuation of an underlying predisposition to any of the symptoms traditionally associated with chronic hypertension [20].

Figure 1. The left ventricle is markedly thickened with severe hypertension that was untreated for many years. The myocardial fibers have undergone hypertrophy [20].
Differentials
The following conditions should also be considered when evaluating hypertensive heart disease (20):
- Hypertrophic cardiomyopathy
- Congestive heart failure due to other etiologies
- Atrial fibrillation due to other etiologies
- Diastolic dysfunction due to other etiologies
- Sleep apnea
- Athlete's heart (with LVH)
The pathophysiologies of the various cardiac effects of hypertension differ and are described in this section:
Left ventricular hypertrophy
LVH, defined as an increase in the mass of the left ventricle (LV), is caused by the response of myocytes to various stimuli accompanying elevated BP. Myocyte hypertrophy can occur as a compensatory response to increased afterload. Mechanical and neurohormonal stimuli accompanying hypertension can lead to activation of myocardial cell growth, gene expression (of which some occurs primarily in fetal cardiomyocytes), and, thus, to LVH. In addition activation of the renin-angiotensin system, through the action of angiotensin II on angiotensin I receptors, leads to growth of interstitium and cell matrix components. Thus the development of LVH is characterized by myocyte hypertrophy and by an imbalance between the myocytes and the interstitium of the myocardial skeletal structure [41].
Various patterns of LVH have been described, including concentric remodeling, concentric LVH, and eccentric LVH. Concentric LVH is an increase in LV thickness and LV mass with increased LV diastolic pressure and volume, commonly observed in persons with hypertension; this is a marker of poor prognosis in these patients. Compare concentric LVH with eccentric LVH, in which LV thickness is increased not uniformly but at certain sites, such as the septum [41].
Although the development of LVH initially plays a protective role in response to increased wall stress to maintain adequate cardiac output, it later leads to the development of diastolic and, ultimately, systolic myocardial dysfunction [41].
Interestingly, findings from a prospective study [23] also indicate a higher risk of developing systemic hypertension among patients in the higher quartiles of the LV mass at baseline.
Left atrial abnormalities
Frequently underappreciated, structural and functional changes of the left atrium (LA) are very common in patients with hypertension. The increased afterload imposed on the LA by the elevated LV end-diastolic pressure secondary to increased BP leads to impairment of the left atrium and left atrial appendage function, plus increased LA size and thickness. Increased LA size accompanying hypertension in the absence of valvular heart disease or systolic dysfunction usually implies chronicity of hypertension and may correlate with the severity of LV diastolic dysfunction [42].
In addition to LA structural changes, these patients are predisposed to atrial fibrillation. Atrial fibrillation, with loss of atrial contribution in the presence of diastolic dysfunction, may precipitate overt heart failure [42].
Valvular disease
Although valvular disease does not cause hypertensive heart disease, chronic and severe hypertension can cause aortic root dilatation, leading to significant aortic insufficiency. Some degree of hemodynamically insignificant aortic insufficiency is often found in patients with uncontrolled hypertension. An acute rise in BP may accentuate the degree of aortic insufficiency, with return to baseline when the BP is better controlled. In addition to causing aortic regurgitation, hypertension is also thought to accelerate the process of aortic sclerosis and cause mitral regurgitation [19].
Heart failure
Heart failure is a common complication of chronically elevated BP. Patients with hypertension fall into 1 of the following categories [43]:
- Asymptomatic but at risk of developing of heart failure - Stage A or B, per the American College of Cardiology (ACC)/American Heart Association (AHA) classification, depending on whether or not they have developed structural heart disease as a consequence of hypertension
- Suffering from symptomatic heart failure - Stage C or D, per the ACC/AHA classification
Hypertension as a cause of CHF is frequently underrecognized, partly because at the time heart failure develops, the dysfunctioning left ventricle is unable to generate the high BP, thus obscuring the heart failure's etiology. The prevalence of asymptomatic diastolic dysfunction in patients with hypertension and without LVH may be as high as 33%. Chronically elevated afterload and the resulting LVH can adversely affect the active early relaxation phase and the late compliance phase of ventricular diastole [43].
Diastolic dysfunction
Diastolic dysfunction is common in persons with hypertension. It is often, but not invariably, accompanied by LVH. In addition to elevated afterload, other factors that may contribute to the development of diastolic dysfunction include coexistent systolic dysfunction, and structural abnormalities such as fibrosis and LVH. Asymptomatic systolic dysfunction usually follows [43].
Systolic dysfunction
Later in the course of disease, the LVH fails to compensate by increasing cardiac output in the face of elevated BP, and the LV cavity begins to dilate to maintain cardiac output. As the disease enters the end stage, LV systolic function decreases further. This leads to further increases in activation of the neurohormonal and renin-angiotensin systems, leading to increases in salt and water retention and increased peripheral vasoconstriction. Eventually, the already compromised LV is overwhelmed, and the patient progresses to the stage of symptomatic systolic dysfunction [41].
Decompensation
Apoptosis, or programmed cell death, stimulated by myocyte hypertrophy and the imbalance between its stimulants and inhibitors, is considered to play an important part in the transition from compensated to decompensated stage. The patient may become symptomatic during the asymptomatic stages of the LV systolic or diastolic dysfunction, owing to changes in afterload conditions or to the presence of other insults to the myocardium (eg, ischemia, infarction). A sudden increase in BP can lead to acute pulmonary edema without necessarily changing the LV ejection fraction [44].
Generally, development of asymptomatic or symptomatic LV dilatation or dysfunction heralds rapid deterioration in clinical status and a markedly increased risk of death. In addition to LV dysfunction, right ventricular (RV) thickening and diastolic dysfunction also develop as results of septal thickening and LV dysfunction [44].
Cardiac arrhythmias
Cardiac arrhythmias commonly observed in patients with hypertension include atrial fibrillation, premature ventricular contractions (PVCs), and ventricular tachycardia (VT) [45]. The risk of sudden cardiac death is increased [46]. Various mechanisms thought to play a part in the pathogenesis of arrhythmias include altered cellular structure and metabolism, inhomogeneity of the myocardium, poor perfusion, myocardial fibrosis, and fluctuation in afterload. All of these may lead to an increased risk of ventricular tachyarrhythmias [4,45].
Atrial fibrillation (paroxysmal, chronic recurrent, or chronic persistent) is observed frequently in patients with hypertension [47]. In fact, elevated BP is the most common cause of atrial fibrillation in the Western hemisphere. In one study, nearly 50% of patients with atrial fibrillation had hypertension. Although the exact etiology is not known, LA structural abnormalities, associated coronary artery disease, and LVH have been suggested as possible contributing factors. The development of atrial fibrillation can cause decompensation of systolic and, more importantly, diastolic dysfunction, owing to loss of atrial kick, and it also increases the risk of thromboembolic complications, most notably stroke [4,46].
Premature ventricular contractions, ventricular arrhythmias, and sudden cardiac death are observed more often in patients with LVH than in those without LVH [46].
Diagnostic evaluation of hypertension in youth
Clinical evaluation of patients with prehypertension and hypertension begins with a thorough history and physical examination. The focus of this assessment is to detect symptoms and signs that might be associated with a secondary etiology or reveal the presence of other cardiovascular risk factors or high-risk conditions. A detailed family history should focus on cardiovascular risk factors and disease, and inheritable conditions. Past and current medical history should include a review of medications, substances, and dietary supplements. Symptoms and signs specifically related to hypertension are rare in childhood and usually only evident if hypertension is severe. Symptoms and signs in neonates are nonspecific, and include irritability, lethargy, poor weight gain, respiratory distress, congestive heart failure (tachypnea, tachycardia, hepatomegaly), and seizures. Additional symptoms noted in children and adolescents include fatigue, headache, and visual disturbances. A careful and complete review of systems is essential for identification of symptoms that suggest a specific secondary etiology. Lifestyle assessment should also be included, with detailed evaluation of nutritional intake and dietary behaviors (with attention to sodium, potassium, and calcium intake), physical activity habits, time spent in sedentary pursuits, and smoking, or smoke exposure. A sleep history or assessment is particularly important for obese patients [4,16].
Physical examination, in addition to careful assessment of blood pressure, should be focused on assessing cardiovascular risk and detecting signs suggestive of secondary causes. Height and weight should be measured and plotted, and BMI calculated and plotted. Although retinal vasculopathy and papilledema are rare, ophthalmological examination is indicated in patients with severe hypertension. Physical signs of secondary causes can be subtle, such as tachycardia, abdominal bruits, café au lait spots, or thyromegaly, and the assessment should be thorough [4,16].
Laboratory evaluation is aimed at screening and evaluation for secondary causes, assessment for associated cardiovascular risk factors, and identification of target organ damage. Use of testing should be tiered, with some tests included as part of general screening, while more specialized tests are used if a clinical suspicion is present for a specific underlying cause based on history, physical examination, and screening tests. More specialized tests may also be indicated for patients, with stage 1 or 2 hypertension, persistent or worsening hypertension despite treatment, and hypertension in neonates and infants. Particularly with more severe hypertension, the presence of obesity should not preclude more specialized assessment, despite the higher likelihood that the hypertension is primary in nature [16]. A strategy and indication for general assessment versus specific assessment is shown in table 5 [16].
Table 5. Laboratory testing for hypertension in youth
|
Evaluation
|
Main diagnostic utility
|
|
General
|
|
|
Urinalysis
|
Renal disease
|
|
Urine culture
|
Chronic pyelonephritis
|
|
Renal ultrasound
|
Structural renal anomalies, renal scar,
Malignancy
|
|
Blood chemistries (creatinine, urea
nitrogen, uric acid, electrolytes, calcium)
|
Chronic renal failure, endocrinopathies
|
|
Complete blood count
|
Anemia associated with chronic renal failure
|
|
Echocardiography
|
Left ventricular hypertrophy (coarctation of the aorta)
|
|
Fasting lipid profile, glucose
|
Obesity-related metabolic comorbidities
|
|
Etiology-specific
|
|
|
Radionucleide renal scan
|
Renovascular disease
|
|
Renovascular imaging
|
Renovascular disease
|
|
Plasma renin profiling
|
Mineralocorticoid-related disease, genetic disorders of renal sodium handling
|
|
Plasma and urine catecholamines,
Metanephrines
|
Catecholamine-mediated hypertension,
2021 Copyright OAT. All rights reserv
Pheochromocytoma
|
|
Plasma and urine steroids
|
Steroid-mediated hypertension
|
Echocardiography and Ultrasonography
LVH is the main target-organ damage evident in children and adolescents, and echocardiography is the primary modality for assessment. Left ventricular mass is calculated from measurements made from standardized echocardiographic views using the equation of Devereux et al. [47]. Left ventricular mass must be indexed to body size (lean body mass) and height (m2.7) [13]. A cut point of 51 g/m2.7 (99th percentile) has been used to define LVH, although this is somewhat arbitrary, as the relationship to outcomes for children is unknown. Age-specific reference values for left ventricular mass index in children and adolescents have been reported [48].
LVH is symmetric, consisting of equivalent increases in thickness for both the left ventricular portion of the ventricular septum and the left ventricular posterior wall. Left ventricular function must also be assessed (Figures 2 and 3) [48]. Echocardiography is essential in the evaluation of suspected aortic coarctation. The aortic arch and its branches must be examined in precise anatomic detail [48].
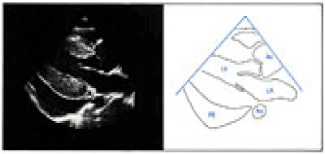
Figure 2. Two dimensional (2D) -parasternal long-axis view showing symmetric left ventricular hypertrophy and a large pericardial effusion [48].
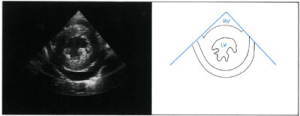
Figure 3. 2D- parasternal short-axis view of the left ventricle during diastole. Left ventricular hypertrophy and a pericardial effusion are present [48].
Abdominal ultrasonography may reveal tumors or structural anomalies of the kidneys or renal vasculature. Renal scarring suggests excessive renin release. Asymmetry in renal size suggests renal dysplasia or renal artery stenosis. Renal or extrarenal masses suggest a Wilms tumor or neuroblastoma, respectively [49].
On Doppler studies, asymmetry in renal artery blood flow suggests renal artery stenosis [49].
Angiography
Angiography may reveal differences in the structure (diameter) of the renal vessels. Sampling of blood from renal arteries, renal veins, and aorta may reveal differences in renin secretion between the kidneys. A renin activity ratio of 3:1 between the kidneys is considered diagnostic of renal vascular hypertension. On digital subtraction arteriography, asymmetry between the 2 renal arteries indicates renal artery stenosis [49].
24-Hour Blood Pressure Monitoring
Monitoring of BP on a 24-hour basis may help in diagnosing so-called white-coat hypertension and provides information about the risk of target end-organ damage. White-coat hypertension is common because most children are uncomfortable at the physicians’ office, fearing invasive examinations, vaccinations, blood draws, and other factors. Use of 24-hour BP monitoring should be considered first in most uncomplicated cases of pediatric stage I hypertension [18,50].
Other Tests
-Cardiac catheterization is not necessary in the evaluation of aortic coarctation [51].
- CT and magnetic resonance imaging (MRI) with angiography can provide further anatomic definition of an aortic coarctation, but neither study is necessary for diagnosis [51].
-Radionuclide imaging may be considered, with or without captopril; asymmetry suggests renal artery stenosis [49].
-Polysomnography helps in identifying sleep disorders associated with hypertension. This test should be considered in obese children with a history of snoring, daytime sleepiness, or any sleep difficulties [25].
Management of hypertension in youth
Management of hypertension in children and adolescents should be directed toward the underlying etiology, exacerbating factors, and the magnitude of the blood pressure abnormality. For both primary and secondary hypertension, exacerbating factors, such as poor dietary and physical activity habits, smoking, stress, sleep disturbance, and obesity, should be addressed and minimized. The goal of management is to reduce blood pressure to within the normal range while preventing or reversing target organ damage. The balance of the risks of therapy versus the benefits should be considered, given that there is no direct evidence to confirm that reducing hypertension in youth affects the patient’s cardiovascular health in adulthood; however, indirect evidence exists and is increasing, and is informing the imperative to treat [52]. The Fourth Report Task Force outlines a general algorithm for management (Figure 4) [13]. Uncomplicated primary prehypertension and hypertension can be effectively evaluated and managed by primary-care providers. Pediatric patients with secondary hypertension usually require evaluation and management by a specialist in the particular underlying etiology. Patients with primary hypertension who require pharmacological management might benefit from consultation with and management by a physician with specific expertise in pediatric hypertension, usually a nephrologist or cardiologist [4].
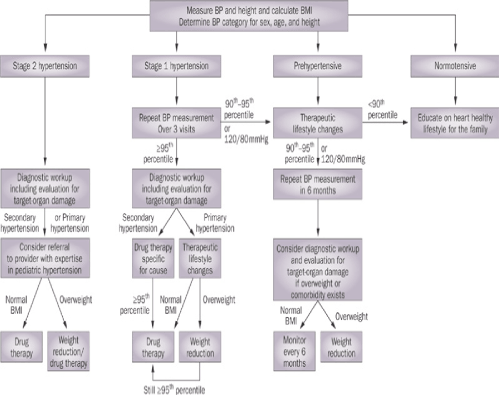
Figure 4. Management algorithm for blood pressure categories in children and adolescents. Abbreviation: BP, blood pressure [13].
Non-pharmacological management
Healthy behavioral changes are the primary management tool for treating hypertension and, particularly, prehypertension. However, the main role of lifestyle changes is to prevent and address other cardiovascular risk factors, particularly those that cluster in association with the metabolic syndrome. Given that overweight and obesity both cause primary hypertension and exacerbate primary and secondary hypertension, weight reduction or maintenance is important. Specific health behaviors that should be targeted include dietary modifications, increasing levels of physical activity while decreasing time spent in sedentary pursuits, and eliminating exposure to tobacco smoke. Measures to address stress and disordered sleep should be incorporated as indicated [16].
Dietary modifications aimed at overweight and obesity could include reduction of cholesterol and fat intake, particularly saturated fat, reducing intake of sweetened rinks and processed and fast foods, and increasing intake of fresh vegetables, fruits, and whole grain products. Eating behaviors to target include limitations on portion sizes and snacking, elimination of meal skipping, and increases in the number of meals eaten as a family. Reduction of sodium intake and increase in potassium intake has been advocated as a strategy for reducing hypertension [53]. Although sodium restriction is difficult to achieve for children, avoiding processed foods, paying attention to sodium content as noted on food labels, and not adding salt to foods is feasible. A meta-analysis of 10 controlled trials, including 966 children, concluded that a modest reduction in salt intake was associated with significant reductions in systolic and diastolic blood pressure [54]. Dietary counseling with a dietician or nutritionist should be family-based and include techniques in motivational interviewing that promote behavioral changes. Online resources, such as “Dietary approaches to stop Hypertension” or DASH from [55] can be useful educational adjuncts. The DASH approach advocates reducing intake of saturated fat and refined sugar, while increasing intake of fruits and vegetables, high fiber foods, and low-fat dairy products. The DASH dietary approach has recently been studied in hypertensive adolescents and been shown to effectively lower systolic blood pressure with improvements in nutritional intake [56].
Physical activity has also been advocated as an important management [13]. A meta-analysis of clinical trials in children and adolescents showed a 1% reduction in systolic blood pressure and 3% reduction in diastolic blood pressure with exercise interventions, although these findings were not statistically significant [57]. Nonetheless, increases in physical activity, both through, aerobic exercise and noncompetitive resistance training, has been recommended for the treatment and prevention of obesity, hypertension, and other cardiovascular risk factors in youth [58]. A caveat is that patients with uncontrolled stage 2 hypertension should be restricted from participation in competitive sports. At least 60 min of moderate to vigorous daily physical activity, together with a reduction in sedentary pursuits to less than 2 h per day, should be the goal [59].
Pharmacological management
Pharmacological management is reserved for patients with stage 1 or 2 hypertension (Table 6) [13]. The presence of other cardiovascular risk factors, such as hyperlipidemia, could be a further indication for pharmacological intervention given the exponential increase in cardiovascular risk associated with multiple risk factors. The goal of pharmacological management is to reduce blood pressure to below the 95th percentile and to prevent target-organ damage. If target-organ damage is present, or the patient has diabetes or chronic renal failure, then the goal is to reduce blood pressure to below the 90th percentile [13,58]. Several classes of drugs are suitable for nonemergent management, including angiotensin-converting-enzyme (ACE) inhibitors, angiotensin-receptor blockers (ARBs), β-blockers, calcium-channel blockers, and diuretics. A review by [58] gives pediatric dosing information for these medications. Many of these drugs used to treat hypertension in adults now have a pediatric indication supported by an appropriate clinical trial [58,60]. However, none of the studies investigating pharmacological management of hypertension in children and adolescents have had a cardiovascular end point, and none have compared different drugs in children. In addition, there have been several negative clinical trials, highlighting the need for careful attention to differences in pharmacology between adults and children, pediatric formulations, and appropriate primary end points [61]. These trials have also been short-term in nature; therefore, long-term and comparative efficacy and safety remain unknown [11]. As a result, guidelines about the use of specific agents to use have been necessarily vague [13] and compliance in practice has been suboptimal [62-64].
Table 6. Indications for pharmacological management [13]
|
Indications for Antihypertensive Drug Therapy in Children
|
|
§Symptomatic hypertension
|
|
§Secondary hypertension
|
|
§Hypertensive target-organ damage
|
|
§Diabetes (types 1 and 2)
|
|
§Persistent hypertension despite non-pharmacologic measures.
|
The choice of initial drug remains at the discretion of the patient’s care provider. In some circumstances, the choice of drug might be directed by the underlying pathophysiological mechanism. An ACE inhibitor or angiotensin-receptor blocker might be recommended for patients with diabetes and microalbuminuria or proteinuric renal diseases. A clinical trial of intensified blood pressure control with ramipril in hypertensive children with chronic renal disease showed a benefit in terms of freedom from worsening renal function [65]. A calcium-channel blocker or β-blocker might be recommended for patients with associated migraine headaches and a β-blocker might be preferred for patients with hypertension after repair of aortic coarctation [58]. Patients with hypertension primarily associated with obesity, particularly those with higher renin levels, may benefit from an ACE inhibitor [58]. Those obese patients with low renin levels may benefit from a diuretic. The chosen agent should be started at the lowest dose and titrated as tolerated on the basis of adverse effects and treatment response (Table 7) [39]. A stepped-care approach individualized to the patient has been recommended [50,58]. Patients who do not respond to their initial drug may be switched to another drug in that class or to a drug in a different class. Some patients require an additional drug from a different class, usually one with a complementary mechanism of action. Ongoing monitoring is important, and should address blood pressure response, adverse effects, and compliance with both pharmacological and nonpharmacological management, assessment for target-organ damage, and resolution or exacerbation of additional cardiovascular risk factors. Hypertensive emergencies are rare in children and are usually related to underlying renal disease or after repair of aortic coarctation, characterized by severe and symptomatic hypertension with blood pressure above the 99th percentile, and should be treated carefully with intravenous antihypertensive medications [66].
Table 7. Pharmacological options for pediatric hypertension
|
Class
|
Drug
|
Dose
|
Interval
|
|
Diuretics
|
Amiloride
|
0.4–0.6 mg/kg per day
|
q.d.
|
|
|
Chlorthalidone
|
0.3 mg/kg per day
|
q.d.
|
|
|
Furosemide
|
0.5–2.0 mg/kg per dose
|
q.d.–b.i.d.
|
|
|
Hydrochlorothiazide
|
0.5–1 mg/kg per day
|
q.d.
|
|
|
Spironolactone
|
1 mg/kg per day
|
q.d.–b.i.d.
|
|
Beta-adrenergic blockers
|
Atenolol
|
0.5–1 mg/kg per day
|
q.d.–b.i.d.
|
|
|
Metoprolol
|
0.5–1.0 mg/kg per day
|
q.d. (ER)
|
|
|
Propanolol
|
1 mg/kg per day
|
b.i.d.–t.i.d.
|
|
Calcium channel blockers
|
Amlodipine
|
0.06–0.3 mg/kg per day
|
q.d.
|
|
|
Felodipinea
|
2.5 mg per day
|
q.d.
|
|
|
Nifedipine
|
0.25–0.5 mg/kg per day
|
q.d.–b.i.d. (ER)
|
|
Angiotensin-converting
enzyme inhibitors
|
Captopril
|
0.3–0.5 mg/kg per dose
|
b.i.d.–t.i.d.
|
|
|
Enalapril
|
0.08–0.6 mg/kg per day
|
q.d.
|
|
|
Fosinopril
|
0.1–0.6 mg/kg per day
|
q.d.
|
|
|
Lisinopril
|
0.08–0.6 mg/kg per day
|
q.d.
|
|
|
Ramiprila
|
2.5–6 mg per day
|
q.d.
|
|
Angiotensin-receptor blockers
|
Candesartan
|
0.16–0.5 mg/kg per day
|
q.d.
|
|
|
Irbesartana
|
75–150 mg per day
|
q.d.
|
|
|
Losartan
|
0.75–1.44 mg/kg per day
|
q.d.
|
|
|
Valsartan
|
2 mg/kg per day
|
q.d.
|
q.d., once daily; b.i.d., twice daily; t.i.d., three times daily; ER, extended release. The maximum recommended adult dose should never be exceeded [39].
Management of Hypertensive Crisis
Hypertensive crises occur as a result of an acute illness (eg, postinfectious glomerulonephritis or acute renal failure), excessive ingestion of drugs or psychogenic substances, or exacerbated moderate hypertension [67].
The clinical manifestations may be those of cerebral edema, seizures, heart failure, pulmonary edema, or renal failure. Accurate assessment of BP in every patient presenting with a seizure is essential, particularly when no seizure disorder has been established in that patient [50,67].
Anticonvulsant drugs are usually ineffective in treatment of a seizure due to a hypertensive crisis. However, seizures due to severe hypertension must be treated with a fast-acting antihypertensive drug [67].
The following drugs are currently used in the treatment of hypertensive emergencies:
- Labetalol, 0.2-1 mg/kg/dose up to 40 mg/dose as an intravenous (IV) bolus or 0.25-3 mg/kg/h IV infusion
- Nicardipine, 1-3 µg/kg/min IV infusion
- Sodium nitroprusside, 0.53-10 µg/kg/min IV infusion to start
Sublingual nifedipine is no longer recommended for the treatment of acute hypertension, because of reports of death from hypotension in the adult population [50,67].
Transcatheter Therapy
Interventional cardiac catheterization procedures can be used to treat coarctation of the aorta. Balloon dilation of a previously untreated coarctation remains controversial. Some pediatric cardiologists recommend this approach and may also place a stent at the coarctation site. The appropriateness of this approach remains to be determined in studies of long-term outcome. Balloon dilation, with or without stent placement, has gained widening acceptance for treatment of recurrent coarctation. Recurrence is most likely to arise when young infants must undergo surgical repair because of the severity of the lesion [51].
Interventional catheterization with balloon dilation can also successfully relieve many instances of discrete renal artery stenosis [49].
Summary& Conclusions
Adult hypertension often begins in childhood. However, prehypertension and hypertension are distinctly different in children and adolescents than they are in adults, and require evaluation and management reflecting differences in the underlying pathophysiology and the developmental aspects of these conditions. The latest recommendations by the National Blood Pressure Education Program aim to clarify diverse concepts ranging from the ideal blood pressure measurement technique in children to the establishment of threshold values for 50th, 95th, and 99th percentiles associated with gender, age, and height. Although manifest cardiovascular disease related to hypertension is rare during childhood, early onset of this condition undoubtedly contributes to an acceleration of cardiovascular disease and target-organ damage, particularly in the presence of the clustering of risk factors associated with obesity. Public health strategies need to be aimed toward childhood obesity and hypertension if a future epidemic of cardiovascular disease is to be prevented. such initiatives should be based on an expanded evidence base derived from longitudinal cohort studies, mechanistic studies, and randomized clinical trials of interventions that utilize noninvasive markers of atherosclerosis as vascular end points.
Recommendations
(1) Hypertension screening should be included in a school health program. Follow up and regular blood pressure measurement should be an important step in school health programs. Waist circumference and BMI should be measured in the school clinic. It could be used in a health promotion program to identify individuals who should seek, and be offered, weight management and those at risk of developing hypertension.
(2) Prevention of cardiovascular risk factors as early as childhood may be an important strategy to prevent noncommunicable diseases in a life course perspective, particularly in settings with scarce resources and limited health care capacity. Programs and policies to limit sedentary behaviours and promote physical activity and healthy nutrition among children are recommended.
Acknowledgement
This work was supported by the National Research Centre, Egypt
References
- Berenson GS, Srinivasan SR, Bao W, Newman WP 3rd, Tracy RE, et al. (1998) Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Eng J Med 338: 1650–1656. [Crossref]
- McGill HC Jr, McMahan CA, Zieske AW, Malcom GT, Tracy RE, et al. (2001) Effects of nonlipid risk factors on atherosclerosis in youth with a favorable lipoprotein profile. Circulation 103: 1546–1550. [Crossref]
- Homma S, Ishii T, Malcom GT, Zieske AW, Strong JP, et al. (2001) Histopathological modifications of early atherosclerotic lesions by risk factors-findings in PDAY subjects. Atherosclerosis 156: 389–399. [Crossref]
- Basiratnia M, Abadi SF, Amirhakimi GH, Karamizadeh Z, Karamifar H (2012) Ambulatory blood pressure monitoring in children and adolescents with type-1 diabetes mellitus and its relation to diabetic control and microalbuminuria. Saudi J Kidney Dis Transpl 23: 311-315. [Crossref]
- Mitsnefes MM (2012) Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol 23: 578-585. [Crossref]
- Sorof JM, Alexandrov AV, Cardwell G, Portman RJ (2003) Carotid artery intimal–medial thickness and left ventricular hypertrophy in children with elevated blood pressure. Pediatrics 111: 61–66. [Crossref]
- Lande MB, Carson NL, Roy J, Meagher CC (2006) Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension 48: 40–44. [Crossref]
- Aggoun Y, Farpour-Lambert NJ, Marchand LM, Golay E, Maggio AB, et al. (2008) Impaired endothelial and smooth muscle functions and arterial stiffness appear before puberty in obese children and are associated with elevated ambulatory blood pressure. Eur Heart J 29: 792–799. [Crossref]
- Mahoney LT, Burns TL, Stanford W, Thompson BH, Witt JD, et al. (1996) Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: the Muscatine Study. J Am Coll Cardiol 27: 277–284. [Crossref]
- Daniels SR, Loggie JM, Khoury P, Kimball TR (1998) Left ventricular geometry and severe left ventricular hypertrophy in children and adolescents with essential hypertension. Circulation 97: 1907–1911. [Crossref]
- Hanevold C, Waller J, Daniels S, Portman R, Sorof J, et al. (2004) The effects of obesity, gender, and ethnic group on left ventricular hypertrophy and geometry in hypertensive children: a collaborative study of the International Pediatric Hypertension Association. Pediatrics 113: 328–333. [Crossref]
- McNiece KL, Gupta-Malhotra M, Samuels J, Bell C, Garcia K, et al. (2007) Left ventricular hypertrophy in hypertensive adolescents: analysis of risk by 2004 National High Blood Pressure Education Program Working Group staging criteria. Hypertension 50: 392–395. [Crossref]
- The National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics 114: 555–576. [Crossref]
- Centers for Disease Control and Prevention (2009) National Center for Health Statistics. CDC Growth Charts. [Online], http://www.cdc.gov/growthcharts.
- Falkner B, Gidding SS, Portman R, Rosner B (2008) Blood pressure variability and classification of prehypertension and hypertension in adolescence. Pediatrics 122: 238–242. [Crossref]
- McCrindlle BW (2010) Assessment and management of hypertension in children and adolescents. Nat Rev Cardiol 7: 155-163. [Crossref]
- Ostchega Y, Prineas RJ, Paulose-Ram R, Grim CM, Willard G, et al. (2003) National Health and Nutrition Examination Survey 1999–2000: effect of observer training and protocol standardization on reducing blood pressure measurement error. J Clin Epidemiol 56: 768–774. [Crossref]
- Chen X, Wang Y, Appel LJ, Mi J (2008) Impacts of measurement protocols on blood pressure tracking from childhood into adulthood: a metaregression analysis. Hypertension 51: 642–649. [Crossref]
- Gruskin AB (1995) Factors affecting blood pressure. In: Drukker A, Gruskin AB (Eds.), Pediatric Nephrology: Pediatric and Adolescent Medicine. (3rdedn.), Basel, Switzerland: Karger; 1097.
- Williams CL, Hayman LL, Daniels SR, Robinson TN, Steinberger J, et al. (2002) Cardiovascular health in childhood: A statement for health professionals from the Committee on Atherosclerosis, Hypertension, and Obesity in the Young (AHOY) of the Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 106:143–60. [Crossref]
- Joffres MR, Hamet P, MacLean DR, L'italien GJ, Fodor G (2001) Distribution of blood pressure and hypertension in Canada and the United States. Am J Hypertens 14: 1099–1105. [Crossref]
- Grebla RC1, Rodriguez CJ, Borrell LN, Pickering TG (2010) Prevalence and determinants of isolated systolic hypertension among young adults: the US National Health and Nutrition Examination Survey. J Hypertens 28: 15-23. [Crossref]
- Maffeis C, Banzato C, Brambilla P, Cerutti F, Corciulo N, et al. (2010) Insulin resistance is a risk factor for high blood pressure regardless of body size and fat distribution in obese children Nutr Metab Cardiovasc Dis 20: 266-73. [Crossref]
- Amin RS, Carroll JL, Jeffries JL, Grone C, Bean JA, et al. (2004) Twenty four hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Respir Crit Care Med 169: 950–956. [Crossref]
- Amin R, Somers VK, McConnell K, Willging P, Myer C, et al. (2008) Activity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension 51: 84–91. [Crossref]
- Robinson RF, Batisky DL, Hayes JR, Nahata MC, Mahan JD (2004) Body mass index in primary and secondary pediatric hypertension. Pediatr Nephrol 19: 1379–1384. [Crossref]
- Ingelfinger JR (2006) The molecular basis of pediatric hypertension. Pediatr Clin North Am 53: 1011-1028, x-xi. [Crossref]
- Sugiyama T, Xie D, Graham-Maar RC, Inoue K, Kobayashi Y, et al. (2007) Dietary and lifestyle factors associated with blood pressure among US adolescents. J Adolesc Health 40: 166–172. [Crossref]
- Flynn JT, Alderman MH (2005) Characteristics of children with primary hypertension seen at a referral center. Pediatr Nephrol 20: 961-966. [Crossref]
- Bartosh SM, Aronson AJ (1999) Childhood hypertension. An update on etiology, diagnosis, and treatment. Pediatr Clin North Am 46: 235-252. [Crossref]
- Ostchega Y, Carroll M, Prineas RJ, McDowell MA, Louis T, et al. (2009) Trends of elevated blood pressure among children and adolescents: data from the National Health and Nutrition Examination Survey 1988–2006. Am J Hypertens 22: 59–67. [Crossref]
- Brotons C, Singh P, Nishio T, Labarthe DR (1989) Blood pressure by age in childhood and adolescence: a review of 129 surveys worldwide. Int J Epidemiol 18: 824–829. [Crossref]
- Report of the Second Task Force on Blood Pressure Control in Children (1987) Task Force on Blood Pressure Control in Children. National Heart, Lung, and Blood Institute, Bethesda, Maryland. Pediatrics 79: 1–25.
- National Heart Lung and Blood Institute (2009) NIH Information for health professionals, interactive tools and resources. [online], http://www.nhlbi.nih.gov/health/prof/other/index.htm
- Knecht SK, Mays WA, Gerdes YM, Claytor RP, Knilans TK (2007) Exercise evaluation of upper- versus lower-extremity blood pressure gradients in pediatric and young-adult participants. Pediatr Exerc Sci 19: 344–348. [Crossref]
- Metoki H, Ohkubo T, Kikuya M, Asayama K, Obara T, et al. (2006) Prognostic significance of night-time, early morning, and daytime blood pressures on the risk of cerebrovascular and cardiovascular mortality: the Ohasama Study. J Hypertens 24: 1841–1848. [Crossref]
- Lin JM1, Hsu KL, Chiang FT, Tseng CD, Tseng YZ (1995) Influence of isolated diastolic hypertension identified by ambulatory blood pressure on target organ damage. Int J Cardiol 48: 311–316. [Crossref]
- Urbina E1, Alpert B, Flynn J, Hayman L, Harshfield GA, et al. (2008) Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension 52: 433–451. [Crossref]
- Lurbe E , Cifkova R, Cruickshank JK, Dillon MJ, Ferreira I, et al. (2009) Management of high blood pressure in children and adolescents: recommendations of the European Society of Hypertension. J Hypertens 27: 1719–1742. [Crossref]
- Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, et al. (2002) Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med 347: 797-805. [Crossref]
- Shimbo D, Muntner P, Mann D, Barr RG, Tang W, et al. (2011) Association of left ventricular hypertrophy with incident hypertension: the multi-ethnic study of atherosclerosis. Am J Epidemiol 173: 898-905. [Crossref]
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, et al. (2001) Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 285: 2370-5. [Crossref]
- Peacock F, Amin A, Granger CB, Pollack CV Jr, Levy P, et al. (2011) Hypertensive heart failure: patient characteristics, treatment, and outcomes. Am J Emerg Med 29: 855-862. [Crossref]
- Gandhi SK, Powers JC, Nomeir AM, Fowle K, Kitzman DW, et al. (2001) The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med 344: 17-22. [Crossref]
- Ghali JK, Kadakia S, Cooper RS, Liao YL (1991) Impact of left ventricular hypertrophy on ventricular arrhythmias in the absence of coronary artery disease. J Am Coll Cardiol 17: 1277-82. [Crossref]
- Jouven X, Desnos M, Guerot C, Ducimetiere P (1999) Predicting sudden death in the population: the Paris Prospective Study I. Circulation 99: 1978-83. [Crossref].
- Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, et al. (1986) Echocardiographic assessment of left ventricular hypertrophy:comparison to necropsy findings. Am J Cardiol 57: 450–458. [Crossref]
- Khoury PR, Mitsnefes M, Daniels SR, Kimball TR (2009) Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr 22: 709–714. [Crossref]
- Arnett DK, Glasser SP, McVeigh G, Prineas R, Finklestein S, et al. (2001) Blood pressure and arterial compliance in young adults: The Minnesota Children's Blood Pressure Study. Am J Hypertens 14: 200–5. [Crossref]
- Singh D, Akingbola O, Yosypiv I, El-Dahr S (2012) Emergency management of hypertension in children. Int J Nephrol 2012: 420247. [Crossref]
- Rocchini AP (2000) Coarctation of the aorta and interrupted aortic arch. In: Moller JH, Hoffmann U (Eds.), Pediatric cardiovascular medicine. New York: Churchill Livingstone; 570.
- Mensah GA (2008) High blood pressure in children and adolescents: to treat or not to treat is not the question. J Clin Hypertens (Greenwich) 10: 889-893. [Crossref]
- Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, et al. (2006) Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 47: 296–308. [Crossref]
- He FJ1, MacGregor GA (2006) Importance of salt in determining blood pressure in children: meta-analysis of controlled trials. Hypertension 48: 861–869. [Crossref]
- National Heart Lung and Blood Institute (2003) NIH. Your guide to lowering blood pressure. Lower your blood pressure by eating right. [Online], http://www.nhlbi.nih.gov/health/public/heart/hbp/hbp_low/hbp_low.pdf.
- Couch SC, Saelens BE, Levin L, Dart K, Falciglia G, et al. (2008) The efficacy of a clinic-based behavioral nutrition intervention emphasizing a DASH-type diet for adolescents with elevated blood pressure. J Pediatr 152: 494–501. [Crossref]
- Kelley GA, Kelley KS, Tran ZV (2003) The effects of exercise on resting blood pressure in children and adolescents: a meta-analysis of randomized controlled trials. Prev Cardiol 6: 8–16. [Crossref]
- Flynn JT, Daniels SR (2006) Pharmacologic treatment of hypertension in children and adolescents. J Pediatr 149: 746-754. [Crossref]
- Strong WB, Malina RM, Blimkie CJ, Daniels SR, Dishman RK, et al. (2005) Evidence based physical activity for school-age youth. J Pediatr 146: 732-737. [Crossref]
- Flynn JT (2008) Pediatric hypertension: recent trends and accomplishments, future challenges. Am J Hypertens 21: 605–612. [Crossref]
- Benjamin DK Jr, Smith PB, Jadhav P, Gobburu JV, Murphy MD, et al. (2008) Pediatric antihypertensive trial failures: analysis of end points and dose range. Hypertension 51: 834–840. [Crossref]
- Boneparth A, Flynn JT (2009) Evaluation and treatment of hypertension in general pediatric practice. Clin Pediatr (Phila) 48: 44-49. [Crossref]
- Yoon EY1, Davis MM, Rocchini A, Kershaw D, Freed GL (2009) Medical management of children with primary hypertension by pediatric subspecialists. Pediatr Nephrol 24: 147–153. [Crossref]
- Sinaiko AR (2008) Antihypertensive therapy in children: implications for future studies. Hypertension 52: 201–202. [Crossref]
- ESCAPE Trial Group, Wühl E, Trivelli A, Picca S, Litwin M, et al. (2009) Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361: 1639-1650. [Crossref]
- Patel HP, Mitsnefes M (2005) Advances in the pathogenesis and management of hypertensive crisis. Curr Opin Pediatr 17: 210-214. [Crossref]
- Adelman RD, Coppo R, Dillon MJ (2000) The emergency management of severe hypertension. Pediatr Nephrol 14: 422–7. [Crossref]




