Acetylsalicylic acid, or aspirin, is one of the most common non-steroidal anti-inflammatory drugs, which has been shown to have anti-cancer effects. However, high doses are needed making it unsuitable as an oncology agent. We have previously reported increased potency in a series of hydroxybenzoate zinc (HBZn) aspirin analogues. Here we show that 3-hydroxybenzoate magnesium (3-HBMg) and 4-hydroxybenzoate magnesium (4-HBMg) aspirin derivatives showed cytotoxic effects at doses as low as 1mM. At these concentrations, 3-HBMg and 4-HBMg aspirin increased the level of caspase-3, p53, Bax and decreased the expression of the anti-anti-apoptotic protein, Bcl-2, in HT-1080 Human fibrosarcoma cells.
hydroxybenzoate magnesium analogues, HT-1080 human cell, caspase-3, p53, Bax, Bcl-2
Hydroxybenzoic acids are a group of phenolic compounds (Figure 1), this family includes the common drug, acetylsalicylic acid or aspirin. Previous research has clearly indicated that aspirin and other salicylates can reduce risk of large bowel cancer [1-2]. In addition, salicylic acid and acetylsalicylic acid, have common side effects e.g. inhibition of platelet aggregation and stomach lining erosion due to their acidity. Therefore, the several attempts were carried out to design derivatives that focus on improving aspirin efficacy. Our previous research indicated that the designed new salicylate analogues reduced the effective dose of aspirin and induced apoptosis in different cell lines, including fibrosarcoma, breast cancer, and primary chronic lymphatic leukaemia (CLL) [3-6]. These compounds include hydroxybenzoate Na, Ca, and Zn, (Figure 1), 4-HBZn was the most effective anti-cancer agent [4,6]. In this respect, zinc 4-HBZn reduced cell viability, arrested cell cycle in G0/G1 and regulated apoptosis via intrinsic pathway [3,7]. In addition, 4-HBZn synergized with the purine nucleoside analogue fludarabine [8]. Table 1 shows the rank order of the results obtained when HT-1080 human fibrosarcoma and primary CLL cells were treated with a range of hydroxybenzoate analogues. The aim of the current research was to investigate in vitro the pharmacological effect of 3-HBMg and 4-HBMg (Figure 1) in HT-1080 Human fibrosarcoma cells.
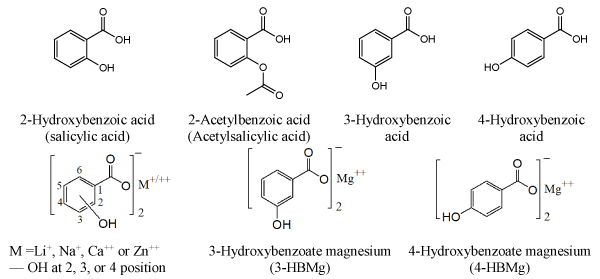
Figure 1. Chemical structures of hydroxybenzoic acid and their metal ion compounds.
Table 1. Summary of pharmacological properties in vitro [4, 9,10,11]
|
Hydroxybenzoate metal compounds |
Results |
Cell viability results |
2-HBNa, 2-HBLi, 2-HBCa and 2-HBZn
3-HBNa, 3-HBLi, 3-HBCa and 3-HBZn
4-HBNa, 4-HBLi, 4-HBCa and 4-HBZn (as shown in Figure 1) |
HBNa and HBLi < HBCa < HBZn |
2-HBM<3-HBM<4-HBM |
Morphology |
Showed apoptotic with no necrotic cell death |
3-HBMg and 4-HBMg was obtained from Cardiff Medical School, UK. Epithelial cancer cell lines HT-1080 were obtained from ATCC (Manassas, VA).
Cell culture
Cell culture for HT-1080 cells were conducted at 37°C in a humidified atmosphere con- taining 5% CO2 [9-11]. Cells were cultured in a RPMI-1640 medium. The medium contained GlutaMAX, 25 mmol/L HEPES buffer (Sigma-Al-drich, UK), 10% foetal bovine serum (FBS) (Sigma- Aldrich, UK) and 1% penicillin (10,000 U/mL; Sigma- Aldrich, UK).
Determination of cell viability by MTT assay
HT-1080 cells were seeded into 96-well plates at a density of 2.6 × 104 cell/well in 90 µL optimized medium. The cells were allowed to settle for 24 h before treated with individual dose of 3-HBMg, and 4-HBMg, i.e., 0, 0.1, 0.15, 0.2, 0.4, 0.6, 0.8 and 1 mM/L. The treated cells were allowed to grow for 48 h. At the end of the incubation period and dose point, 110 µL of 0.22 μm filter-sterilized, 5 mg/mL 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich, UK) was added at a temperature of 37°C. The 96-well plate was kept in the dark for 2 h before the medium containing MTT was removed. 100 µL dimethyl sulphoxide (DMSO) obtained from Ajax Finechem Pty Ltd, Australia, was added to dissolve the formasane crystals. The 96-well plates were shaken for 15 min in the dark to dissolve the formasane crystals. The optical density (OD) of each treatment was measured at 570 nm using LabSystems Multiskan EX Version 3.0 (Thermo Lab systems, Helsinki, Finland). Each experiment was performed in four replicates. The optical densities were normalized according to the control [11].
Determination of apoptotic proteins expression by western blot
HT-1080 cells (6 × 105 cell/mL) were seeded in T-100 flask, cultured and treated with 3-HBMg and 4-HBMg compounds (0.0, 0.2, 0.6 and 1.0mM/L) for 48 hours, as described in the previous section. The medium was removed, and the cells were washed with cold PBS to remove the medium. Subsequently, RIPA buffer (150 mmol/L sodium chloride (NaCl), 2 mmol/L ethylenediaminetetraacetic acid (EDTA), 50 mmol/L Tris-HCl, pH 7.5) and lysis buffer (1% deoxycholic acid and 1% NP-40) were added. Protease inhibitor cocktail tablets (Bio-Rad Laboratories, USA) were also added. The cell lysates were then centrifuged at 12,000 × g for 15 min. The supernatant was further centrifuged at 4°C at 16,000 × g for 5 min to obtain a clear solution of the protein mixture. The protein mixture was used to measure the expression of caspase-3, p53, Bcl-2 and Bax by Western blotting using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal antibody control. Total cell lysate protein doses were determined by assay dye (Bio-Rad Laboratories, USA) and absorption was measured at 595 nm. 60 µg of the extracted protein and GAPDH internal antibody control on sodium dodecyl sulfate (SDS) were loaded to 4%–12% Bistrisacrylamide gel in 3-(N-Morpholino) propanesul- fonic acid (NuPAGE MOPS) running buffer (Invitrogen™ Life Technologies, Scotland, UK). After running the gel at 75 V for 3 hours at room temperature, the resolved proteins were transferred onto a nitrocellulose membrane (Sigma-Aldrich, UK). The membranes were first incubated with an appropriate primary antibody (caspase-3, p53, Bcl-2, Bax, or GAPDH as a loading control and internal standard), followed by peroxidase conjugated anti-mouse IgG antibody (Sigma-Aldrich, UK). The membranes were washed and developed using a chemiluminescent reagent (Amersham, UK) prior to exposure to photographic films. The protein bands’ intensities were scanned and quantified using a densitometer [11].
Determination of apoptotic protein by annexin-V assay
HT-1080 cells (1.37×105) were seeded into 6-well plates containing coverslips (30mm diameter) placed into the centre of 6-well plates. HT-1080 cells were allowed to grow for 48 hours. After 24 hours, the medium was replaced with fresh medium containing the different doses of 3-HBMg and 4-HBMg. (0.0, 0.2, 0.6 and 1mM). After 48 hours, cells were treated with fluorescein isothiocyanate (FITC)-labelled annexin-V (2 ml) for 15 min and then with propidium iodide (2.5 ml) for 5 min in the dark. Slides were then examined using a fluorescence microscope. 3 random fields, each of 100 cells, were counted per slide and the percentage of apoptotic cells was calculated based on number of cells that showed nuclear morphology changes [12].
Data obtained in these experiments represent an average of three replicates which were statistically analysed using Graphpad Prism 5.0 software (Graphpad Software Inc., San Diego, CA, USA)
Both 3-HBMg and 4-HBMg compounds induced dose-dependent growth inhibition in HT-1080 Human fibrosarcoma cells line (Figure 2). Using MTT cell viability assay indicated that the maximum level of cell viability reduction of 3-HBMg and 4-HBMg was 50.5 and 36.3% respectively at 1mM dose. In contrast, the minimum level of reduction in HT-1080 cell viability were % and 20.2% when 0.1mM 3-HBMg and 4-HBMg respectively were incubated for 48 hours.
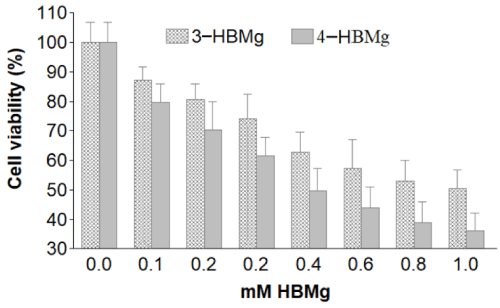
Figure 2. Cell viability of 3-HBMg and 4-HBMg-treated HT-1080 for 48 hours using data derived from MTT viability assay in 4 replicates.
Generally, adding different metal ion to hydroxybenzoic acids improved cytotoxicity between 3 to 5.6-fold in different cell lines, including Hela cells, HT-1080 human fibrosarcoma cells, and Caco-2 cells [12]. Similar results were obtained when 2-, 3-, and 4-hydroxybenzoate calcium were incubated with different cell lines [10-11].
In order to see the effects of different doses of 3-HBMg and 4-HBMg on the manipulation of apoptosis-related proteins, the p53 tumour suppressor, caspase-3 and Bax pro-apoptotic protein, and the anti-apoptotic Bcl-2 proteins were analysed by Western plots electrophoresis assay.
shows the immunoblots of the four analysed apoptosis-related proteins. The results indicate that the treatment of HT-1080 cell line with individual 3-HBMg and 4-HBMg compound increased the level of Bax and reduced the corresponding anti-apoptotic Bcl-2 in dose-dependent manner in HT-1080 cells. Our previous investigation, when hydroxybenzoate calcium and hydroxybenzoate zinc was used similar results were obtained [7,11]. In addition, when HT-1080 cells were treated with different doses of 3-HBMg and 4-HBMg compounds for 48 hours, both caspase-3 and p53 increased which may indicate that the cell death in HT-1080 cells is associated with the intrinsic pathway (Figure 3,4).
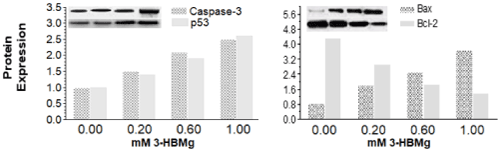
Figure 3. Apoptosis-related expressed proteins in HT-1080 cells due to the treatments with different doses of 3-HBMg for 48 hours.
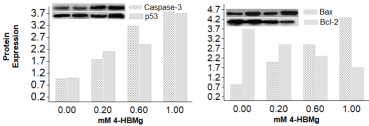
Figure 4. Apoptosis-related expressed proteins in HT-1080 cells due to the treatments with different doses of 4-HBMg for 48 hours.
In order to investigate whether different concentration of 3-HBMg and 4-HBMg induced apoptosis in HT-1080 cells, the immunohistochemical assay was used to label the flag out of the phosphatidylserine. Figure 5 shows the morphological results of apoptotic HT-1080 cells which was labelled by annexin V. These results are in line with the other results in which the induction of apoptosis by 3-HBMg and 4-HBMg in HT-1080 cells increased in dose-dependent manner.
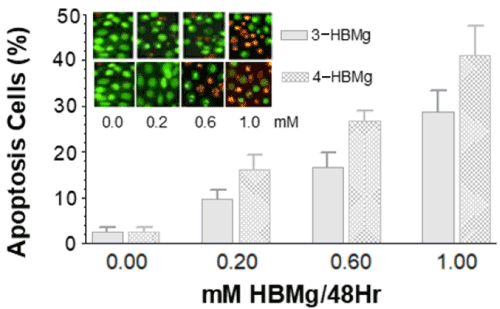
Figure 5. Immunohistochemistry of treated HT-1080 cells with different doses of 3and 4-HBMg. Annexin V was used to label the flagged-out phosphatidylserine when cells undergo apoptotic cell death. Data were derived from 3 slides, in which both labelled apoptotic and viable HT-1080 cells were counted then percentage of apoptotic cells were calculated (%=apoptotic cells/total cells present in the slide). Treatment of HT-1080 cells with 0.2, 0.6 and 1 mM 3-HBMg and 4-HBMg were significantly different from the control sample at p = 0.001. Different doses were significantly different from each other at p =0.05.
The results from the western blot and immunohistochemical assays indicated that 3-HBMg and 4-HBMg induced apoptosis in HT-1080 cells in a dose-dependent manner.
- Elwood PC, Gallagher AM, Duthie GG, Mur LA, Morgan G (2009) Aspirin, salicylates, and cancer. The Lancet 373: 1301-1309. [Crossref]
- Abdelrahim M, Safe S (2005) Cyclooxygenase-2 inhibitors decrease vascular endothelial growth factor expression in colon cancer cells by enhanced degradation of Sp1 and Sp4 proteins. Mol Pharmacol 68: 317-329. [Crossref]
- Wolfe MM, Lichtenstein DR, Singh G (1999) Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med 340: 1888-1899. [Crossref]
- Mahdi JG, Pepper CJ, Alkarrawi MA, Mahdi AJ, Bowen ID (2010) Sub‐millimolar concentration of the novel phenol‐based compound, 2‐hydroxy benzoate zinc, induces apoptosis in human HT‐1080 fibrosarcoma cells. Cell Prolif 43: 95-102. [Crossref]
- Mahdi J, Al-Musayeib N, Mahdi E, Pepper C (2013) Pharmacological importance of simple phenolic compounds on inflammation, cell proliferation and apoptosis with a special reference to β-D-salicin and hydroxybenzoic acid. Eur J Inflamm 11: 327-336.
- Mahdi JG, Mahdi EJ, Al-Hazzaa A, Pepper CJ (2013) The Effect of Hydroxybenzoate Lithium Complexes in Inducing Apoptosis in HT-1080 Human Fibrosarcoma Cells. Journal of Cancer Research 2013: ID 203659.
- Pepper C, Mahdi JG, Buggins AG, Hewamana S, Walsby E, et al. (2011) Two novel aspirin analogues show selective cytotoxicity in primary chronic lymphocytic leukaemia cells that is associated with dual inhibition of Rel A and COX‐2. Cell Prolif 44: 380-390. [Crossref]
- Mahdi JG, Alkarrawi MA, Mahdi AJ, Bowen ID, Humam D (2006) Calcium salicylate‐mediated apoptosis in human HT‐1080 fibrosarcoma cells. Cell Prolif 39: 249-260. [Crossref]
- Mahdi E, Buggins AG, Jones CH, Walsby EJ, Mahdi A, et al. (2014) A novel aspirin analog resensitizes primary human leukemia cells to fludarabine via the selective inhibition of prostaglandin biosynthesis and repression of Nf-κb target genes: 39. British Journal of Haematology 165: 20.
- Mahdi JG, Mahdi AJ, Mahdi EJ, Abdulsatar A, Mahdi AJ, et al. (2015). Morphological modulation of human fibrosarcoma HT-1080 cells by hydroxybenzoate compounds during apoptosis. Adv Mod Oncol Res 1: 68-75.
- Mahdi JG, Al-Musayeib NM, Mahdi EJ, Abdulsatar AJ, Pepper CJ (2014) The anti-proliferation and pro-apoptosis of hydroxybenzoate calcium complexes in HT-1080 human fibrosarcoma cells. European Journal of Oncology 19: 21-33.
- Merghani NM, Al Hazzaa A, Mahdi EJ, Manning AJ, Buggins AG, et al. (2015) The effect of hydroxybenzoate calcium compounds in inducing cell death in epithelial breast cancer cells. Adv Mod Oncol Res 1: 122-131.





