Introduction: Studies of myocardial dissection find a structure with defined planes that allows the physiological movements of narrowing, shortening-torsion, lengthening-detorsion and widening. The slippage between the band segments, when performing both torsion and ventricular detorsion, implies that there should be an antifriction mechanism that avoids dissipating the energy used by the heart. Is there a histology that explains this fact? Do Thebesius and Langer´s venous ducts play a role in this mechanism? Is there an organic lubricant resource?
Materials and methods: Ten bovine hearts (800-1000 g) and seven human hearts (300 g/average) were used. Hearts were divided into two groups: Group I) deployed in their myocardial band (five bovine and three human); Group II) without deploying the band (five bovine and four human). Five transverse cuts were made from the base to the apex. Samples of myocardial tissue were taken in the two groups.
Results: In all the hearts analyzed, hyaluronic acid was found in the cleavage planes between the myocardial bundles, combined with the venous ducts of Thebesius and Langer.
Conclusions: The hyaluronic acid would act as a lubricant and provide great resistance to mechanical pressures in a functional association with the venous ducts of Thebesius and Langer, that would intervene as tributaries of the necessary hydration to said element and thus could counteract the friction of the surfaces by exercising an anti-friction mechanism.
heart, dissection, friction, hyaluronic acid, thebesius-langer veins, intramyocardic sliding
“[The] muscle bundles, one on top of the other but in discordant orientations have been objected in several ways, but a basic objection is that if this were true, the friction between them would be great, generating heat and wasting energy… making necessary an antifriction lubricating mechanism… [there is] a vast system of flat lacunar blood spaces usefully arranged between layers and bands of muscle tissue… that have ample communication with the coronary vessels… and what is much more significant and interesting, with the lumen of both ventricles through the ghostly Thebesian veins”.
Letter sent by Luis Becú to Francisco Torrent Guasp, June 3, 1996 [1].
Classical anatomy of the heart considered that the muscle structure forming the myocardium was homogeneous and compact. Based on this concept, it was described by an external and internal surface limiting a solid, uniform muscle mass. This classical structural notion does not explain cardiac mechanics; hence, it is essential to establish its true internal anatomy. Historically, very little importance was attributed to the spatial arrangement of the muscle bundles forming the myocardium.
Anatomical dissection studies [2] however, revealed a structure with well defined planes allowing the successive and concatenated physiological motions of narrowing, shortening-twisting, lengthening-untwisting and stretching. Sliding between the myocardial band segments during twisting and untwisting implies the need for an antifriction mechanism to avoid dissipating the energy used by the heart. Is there a histological basis that explains this fact? Do the Thebesian and Langer conduits play a role in this mechanism? Is there an organic lubricating source?
In this regard, from the embryonic folding stages where cardiogenesis occurs fusing into a single heart tube, this consists of a loose connective tissue rich in hyaluronic acid (HA) called cardiac jelly [3]. In adult life the heart also contains HA but no explanation was ever given for its presence. Hyaluronic acid, a linear polysaccharide formed by disaccharide units (GAGs) consisting of glucuronic acid and N-acetylglucosamine (NAcGlu) is a component of the extracellular matrix, helping to shape its three-dimensional mesh together with glycoconjugates. The synthesis of HA is very high during the development and growth stages, but decreases in adult tissues. However, most tissues preserve the potential of synthesizing HA, and often produce it rapidly and extensively in response to physiological stimuli. Several types of cells generate HA, but the main source are mesenchymal cells, astrocytes in the central nervous system and fibroblasts in the heart [4].
The main function of HA is support and lubrication in most tissues, but up to now its function in the heart has not been carefully studied. To investigate this subject, we have used hearts dissected from bovine and human specimens, and the histological and histochemical analysis of the anatomical samples.
Ten young bovine hearts (800-1000 g) and seven human hearts (with an average weight of 300 g) were used. Hearts were divided into two groups: Group I with the myocardial band unfolded (five bovine and three human hearts) (Figure 1) and Group II without unfolding the myocardial band (five bovine and four human). In this latter group, five transverse, 2 cm thick sections were made from base to apex to analyze the heart (Figure 2).

Figure 1. Myocardial band unfolded in all its extension. PA: Pulmonary artery; RS: Right segment; LS: Left segment; DS: Descending segment; AS: Ascending segment; A: Aorta (bovine heart)

Figure 2. Transverse section of the left and right ventricles (bovine heart). 1. Interband fibers. 2. Right paraepicardial bundle. 3. Right paraendocardial bundle. 4. Anterior septal band. 5. Posterior septal band. 6. Intraseptal band. 7 Descending segment. 8. Ascending segment. The black arrows indicate the direction of motion of each segment during systole. The yellow arrow indicates the plane of friction between both segments
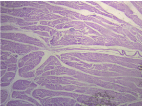
Figure 3. Intramyocardial venous conduits (bovine heart). Hematoxylin-eosin stain (15x)
Figure 4. Longitudinal section of the left ventricle. It shows the descending segment adjacent to the ascending segment. The circle indicates the end of the ascending segment, which runs alone to attach to the cardiac fulcrum. This area is activated in the cardiac suction phase. The histology shows the different orientation of the longitudinal fibers of the ascending segment (AS) in relation to the transverse fibers of the descending segment (DS) (bovine heart)

Figure 5. Interstitial space between cardiomyocytes showing hyaluronic acid (HA), stained in pale blue with Alcian blue stain (adult human heart)

Figure 6. Hyaluronic acid (HA) stained with Alcian blue (15x) (adult human heart)
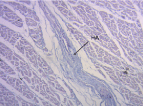
Figure 7. Hyaluronic acid (HA) stained with Alcian blue (15x) (bovine heart)
The hearts examined correspond to material from morgue (human) and slaughterhouses (bovine). The study previously approved by the Institutional Ethics Committee (President Perón Hospital).
Samples of myocardial tissue were collected in both groups; in Group I along the length of the myocardial band and in Group II in the anterior, lateral and posterior points of each transverse section at the left ventricular level. All samples underwent histological and histochemical analysis with Alcian blue staining, a reliable marker to identify the presence of HA and even provide a semiquantitative assessment.
The heart to be unfolded was boiled in water with the addition of acetic acid (15 cc per liter) for approximately two hours. Prior work before unfolding the muscle band consists in separating the atria from the ventricles in a very simple maneuver that demonstrates the different evolutionary origins between both types of chambers. Then, the aorta and the pulmonary artery are cut at three centimeters from their origin, separating the attachment between them, to finally perform a longitudinal incision on the superficial fibers (interband or aberrant fibers) which extend transversally along the anterior wall of the ventricles. Prior boiling of the anatomical piece allows the easy execution of all these steps. Between the atrial and the ventricular walls there is only connective tissue, allowing the easy separation of these chambers due to the denaturation produced by heat.
The key maneuver to unfold the myocardial band consists in entering the anterior interventricular sulcus with a blunt instrument, leaving on the left side of the operator the end of the band corresponding to the pulmonary artery and its continuity with the right ventricular free wall. Next, traction is applied towards the same left side, completely releasing the pulmonary artery from the rest of the myocardial band (Figure 1). Below the aorta we find the cardiac fulcrum, which is the supporting point of the origin and end of the myocardial band [5,6].
In all the hearts investigated we found HA in the cleavage planes between the myocardial bundles associated to Thebesian and Langer venous conduits. These small venous conduits, which originate from intramyocardial veins (Thebesian) or from branching vessels of the great coronary vein (Langer), run across the myocardium and drain into the cardiac cavities (). The descending segment fibers in the anterior wall of the left ventricle course deeply in the mesocardium crossing obliquely and inner to those of the ascending segment located externally. Between both segments is the friction plane (). In addition, among the cardiomyocytes, we have found spaces with a capillary network and plasmatic fluid rich in HA, stained with Alcian blue (). In our study, HA was very scarce in the trigones, aorta and pulmonary artery, but very abundant in the myocardium and papillary muscles. No differences were found between bovine and human hearts ().
Segment sliding: As already developed by Torrent Guasp, the myocardial band segment fibers are oriented to achieve ventricular torsion owing to their spiraling arrangement [2]. This concept of cardiac mechanics was later confirmed both by electrophysiological as by diffusion tensor nuclear magnetic resonance imaging and echocardiographic investigations [7,8].
It is fundamental to assume that this opposite motion of the ascending and descending segments, as well as of the latter against the septal region of the myocardial band (anterior and posterior septal and intraseptal bands), would generate friction between their sliding surfaces during left ventricular twisting (systole) and untwisting (suction) movements ().
Sliding between the internal and external myocardial segments takes opposite directions during the ejective and suction phases of the heart, generating friction, as confirmed by the speckle tracking technique developed by echocardiography [8].
Ventricular twisting-untwisting represents the functional need that makes the heart fibers adopt a helical shape. This is manifested as myocardial strain in its three axes: longitudinal, circumferential and radial. The study of myocardial torsion is a potential marker of myocardial disease severity when it departs from normal values. Ventricular torsion may thus become a more adequate marker of the heart’s condition superior to functional class and ejection fraction in the understanding of heart failure.
Antifriction mechanism: Hyaluronic acid would play its role as a lubricating substance in the antifriction mechanism thus providing resistance to the great mechanical pressures generated by the friction between muscle fibers. A study by Loren, et al. [9] mentions that fibroblasts preserve the extracellular matrix of tissues, synthetizing certain precursors of extracellular components as well as some fibers. Fibrous proteins and an essentially amorphous substance, mainly formed by HA and interstitial plasma, are among the extracellular matrix components. In cardiac muscle fibers, the natural intermyofibrillar space is aligned with HA forming long, thin fibers parallel to the myofibrils and adjacent to the external collagenous tissue. If it breaks, it loses its properties.
The main function of HA is to support and lubricate the joints and fundamentally the skin, but no findings are reported in the literature regarding its function in the heart. However, some articles describe its proinflammatory role in some cardiovascular disorders. Thus, in hypertrophic cardiomyopathy, individual cardiomyocytes are separated by HA and in addition, it is found in large quantity forming patches with big annular structures within the extracellular matrix but with no specific function. Hyaluronic acid can bind water, acting as a lubricant and providing greater resistance to mechanical pressure. Therefore, when it is in great concentrations it may interact with other HA molecules, developing meshes or networks which confer viscoelastic properties to the tissue. Its function as lubricant is well-known in the skin and joints, and this property could be essential to understand cardiac dynamics. In healthy hearts, as shown in our investigations, its function among cardiac fibers could be to allow intramyocardial sliding between the internal and external segments that move in opposite directions generating friction. According to this concept an antifriction mechanism would be necessary to avoid energy dissipation during segment sliding.
During our studies, we considered that the antifriction mechanism could be generated by the loose “spongy” myocardial tissue with transmural blood currents (sinusoids), rudiments of the evolutionary and embryonic heart, in which the veins of Adam Thebesius (German anatomist, 1686-1732) and those described by Karl Langer (Vienna, 1819-1887) would play the fundamental role of hydraulic system ().
These small venous conduits originate from intramyocardial veins (Thebesian) or from branching vessels of the major coronary vein (Langer), run across the myocardium and drain into the cardiac cavities. Evidently, the ones that drain into the left ventricular chamber deoxygenate the blood coming from the lungs. In fact, this results in greater oxygen content in the left atrium than in the corresponding ventricle. Some essential questions arise here: Why would these venous conduits play a counterproductive function for the organism by deoxygenating arterial blood? Which is their true role? Initial studies on these conduits considered that they could oxygenate the myocardium in case of coronary artery occlusion.
In this regard, the presence of HA in the cleavage planes between the myocardial bundles of bovine and human hearts found in our studies, together with Thebesian and Langer venous conduits as branches for the necessary hydration of this element, could clarify this lubricating effect, thus counteracting the abrasion between surfaces as an antifriction mechanism.
Appearing in the first chordate animals, HA is abundant in connective tissues, 50% in the skin and 25% in the bones and joints. It is an excellent lubricant due to its capacity to bind water and provides great resistance to mechanical pressures. Hyaluronic acid helps to develop the extracellular matrix by interacting with proteoglycans or collagen. When it is very concentrated it may interact with itself forming networks or meshes that provide viscoelastic properties to the tissue. During development it helps the morphogenesis of embryonic structures. For example, it may be released from the basal layers of epithelia, curving them due to the great amount of water it attracts.
This functional association between the Thebesian and Langer venous conduits and the substantial amount of HA found in our investigations in bovine and human hearts, added to the knowledge of its lubricating role in the rest of the organism, could be crucial to understand cardiac dynamics.
Accordingly, ventricular torsion is correlated with a lubricating mechanism that facilitates myocardial segment sliding to avoid loss of energy. Therefore, these venous conduits and the helical contraction would continuously drive the plasmatic fluid with HA through a rich capillary network. Effectively, we have found spaces between the cardiomyocytes containing a capillary network and plasmatic fluid rich in HA.
Owing to the importance of this finding, it is necessary to confirm it with further investigations and other markers of HA.
We know that the helical structure of the myocardial band allows the dynamic performance of ventricular twisting and untwisting. Anatomical studies, the progress of ventricular dynamic knowledge through the propagation of the electric impulse and echocardiographic studies of ventricular rotation confirm the structure-function relationship of the heart. Sliding between the internal and external myocardial segments takes opposite directions during the ejective and suction phases of the heart, generating friction. Energy expenditure would be great in the absence of a spongy system in the myocardium, consisting of Thebesian and Langer venous conduits with a lubricating antifriction system.
The histological studies of this spongy mesh and its conduits have shown what could be considered the antifriction effect: HA, which flows throughout the myocardial thickness.
- Trainini J, Herreros J (2019) “El explorador del corazón. Biografía de Francisco Torrent Guasp”. Ed Biblos Buenos Aires pp: 80.
- Torrent Guasp F, Buckberg G, Carmine C, Cox J, Coghlan H, et al. (2001) The structure and function of the helical heart and its buttress wrapping. I. The normal macroscopic structure of the heart. Seminars in Thorac and Cardiovasc Surg 13: 301-19. [Crossref]
- Bignami A, Asher R (1992) Some observations on the localization of hyaluronic acid in adult, newborn and embryonal rat brain. Int J Dev Neurosci 10: 45-57. [Crossref]
- Hong-yan Ding, Ya-nan Xie, Qiang Dong, Koji Kimata, Yoshihiro Nishida, et al. (2019) Roles of hyaluronan in cardiovascular and nervous system disorders. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 20: 428-436. [Crossref]
- Trainini JC, Lowenstein J, Beraudo M, Wernicke M, Trainini A, et al. (2020) Myocardial torsion and cardiac fulcrum (Torsion myocardique et pivot cardiaque). Morphologie. [Crossref]
- Trainini JC, Lowenstein JA, BeraudoM, Trainini A, Mora Llabata V, et al. (2019) “Myocardial Torsion”. Ed. Biblos, Bs. As., pp: 38-61.
- Poveda F, Gil D, Martí E, Andaluz A, Ballester M, et al. (2013) Estudio tractográfico de la anatomía helicoidal del miocardio ventricular mediante resonancia magnética por tensor de difusión. Rev Esp Cardiol 66: 782-790. [Crossref]
- Mora V, Roldán I. Romero E, Saurí A, Romero D, Perez-Gozabo J, et al. (2018) Myocardial contraction during the diastolic isovolumetric period: analysis of longitudinal strain by means of speckle tracking echocardiography. J Cardiovasc Dev Dis 5: 41. [Crossref]
- Lorén C, Dahl C, Do L, Almaas V, Geiran O, et al. (2019) Low molecular mass myocardial hyaluronan in human hypertrophic cardiomyopathy. Cells 8: 97. [Crossref]







