Abstract
Background: The utilization of human umbilical cord-derived mesenchymal stem cells (UC-MSCs) or granulocyte-colony stimulating factor (G-CSF) as standalone therapies for the treatment of endometrial injury has been extensively documented. However, the possibility of using UC-MSCs overexpressing G-CSF for the treatment of endometrial injury remains underreported.
Methods: UC-MSCs were isolated from human umbilical cords and characterized by flow cytometry. Subsequently, UC-MSCs overexpressing G-CSF were established. The rats with endometrial injury were intravenously transplanted with UC-MSCs or UC-MSCs overexpressing G-CSF. The uterine histology was examined through Masson’s trichrome staining and hematoxylin & eosin staining assays. Immunohistochemical assessment of pan-keratin (AE1/AE3) and vascular endothelial growth factor (VEGF) levels was conducted in uterine tissues. Western blotting was employed to ascertain the levels of fibrosis-, angiogenesis-, inflammation-, apoptosis-, and endometrial receptivity-related proteins.
Results: UC-MSCs overexpressing G-CSF effectively mitigated the pathological characteristics of damaged endometrium, enhanced angiogenesis, and promoted epithelial growth. Additionally, treatment with UC-MSCs overexpressing G-CSF significantly modulated the levels of proteins associated with fibrosis, angiogenesis, inflammation, apoptosis, and endometrial receptivity. This highlights the therapeutic potential of UC-MSCs in regulating key processes involved in angiogenesis, fibrosis, inflammation, apoptosis of endometrial stromal cells, and enhancing endometrial receptivity during the progression of endometrial injury.
Conclusion: Transplantation of UC-MSCs overexpressing G-CSF contributed to the repair of endometrial injury, probably through regulating multiple signaling pathways such as fibrosis, angiogenesis, inflammation, apoptosis, and endometrial receptivity. To some extent, these findings provide a theoretical foundation for the use of endometrial injury therapy in clinical settings.
Keywords
umbilical cord mesenchymal stem cells, granulocyte-colony stimulating factor, endometrial injury, pan-keratin, vascular endothelial growth factor
Introduction
Endometrial health is a pivotal determinant of successful pregnancy, and severe endometrial injury in women of reproductive age often leads to the development of thin endometrium and even intrauterine adhesions (IUA). IUA can directly result in amenorrhea, infertility, miscarriage, or other serious symptoms [1-3]. The occurrence of IUA is attributed to diminished viability or apoptosis of endometrial stromal cells. This leads to endometrial atrophy and disrupts its normal functioning [4]. Furthermore, the activation of apoptosis signaling pathways due to endometrial injury inhibits angiogenesis within the endometrium, impeding its regeneration process. During this progression, irreversible damage occurs to the basal lamina accompanied by fibrosis [5,6]. Clinically, successful repair and remodeling of the endometrium depend on the re-growth of epithelial cells and blood vessels on the exposed surface of the uterus [7,8]. However, the intricate nature of the uterine environment and its physiological functions impose limitations on the potential efficacy of treatment strategies [9]. Therefore, it is imperative for gynecology to explore multifunctional therapies that can effectively repair endometrial injury, enhance its microenvironment, and improve tolerance levels to augment pregnancy rates among infertility patients.
Currently, mesenchymal stem cells (MSCs) have been extensively employed in the treatment of diverse tissue and organ injuries [10,11]. Emerging evidence has indicated that transplantation of MSCs from different sources has a positive effect on the repair and regeneration of the damaged endometrium. For example, Hu, et al. confirmed the effects of menstrual blood-derived MSCs (Men-MSCs) on endometrial injury repair, indicating that Men-MSCs have the potential to enhance endometrial thickness in a mouse model of endometrial injury, potentially by upregulating keratin, vimentin, and vascular endothelial growth factor (VEGF) levels [12]. Meanwhile, Wang, et al. [13] reported that transplantation of bone marrow-derived MSCs (BM-MSCs) can effectively repair the damaged endometrium through activating progesterone receptor and estrogen receptor [13]. A recent study conducted by Zhang, et al. [14] established a 95% ethanol-induced endometrial injury rat model and they demonstrated that transplantation of human umbilical cord-derived MSCs (UC-MSCs) contributes to the repair of endometrial injury and restoration of fertility, potentially by attenuating excessive fibrosis and inflammation, promoting endometrial cell proliferation, and facilitating vascular remodeling [14]. However, in the study of MSCs transplantation for repairing endometrial injury, it has been observed that the number of stem cell transplants is extremely limited, with less than 0.1% proportion of detected stem cells in the damaged tissue post-transplantation [15]. Further study has revealed that the paracrine effect of stem cells serves as the primary mechanism for endometrial repair rather than mere cellular replacement [16]. Granulocyte colony-stimulating factor (G-CSF), initially discovered in hematopoietic stem cells, and also plays an immunoregulatory and angiogenic role in MSCs [17-19]. In female reproduction, G-CSF has been demonstrated to participate in multiple processes including follicle growth and development, ovulation, and embryo implantation [20,21]. G-CSF receptors are expressed in ovarian granulosa cells, endometrium, and placenta; moreover, G-CSF significantly influences pregnancy outcomes by affecting embryo implantation and ovarian function while promoting endometrial thickening [22,23]. It can even serve as a remedial measure for cases involving failed embryo implantation [24]. Recently, the therapeutic effect of human UC-MSCs in combination with G-CSF on rats with acute liver failure has been comprehensively elucidated [25], but its effect on endometrial injury is still in the preliminary stage of exploration.
In this study, we present a novel therapeutic approach for the management of endometrial injury by investigating the potential synergistic effects of UC-MSCs combined with G-CSF in mitigating endometrial injury. This study aims to provide a technically efficient, safe, and economically viable treatment modality for clinical application in endometrial injury while establishing a solid theoretical foundation.
Materials and methods
Reagents
The pL6.3-CMV-GFP-IRES-MCS lentivirus vector was from Novobio Scientific (Shanghai, China). Transfection reagent Lipofectamine 3000 was obtained from Invitrogen (Carlsbad, CA, USA). BD Biosciences (Franklin Lakes, NJ, USA) provided monoclonal antibodies (mAbs) CD73 (cat. no. 550741), CD34 (cat.no. 568294), CD90 (cat.no. 555595), CD14 (cat.no. 347493), CD45 (cat.no. 560976), CD105 (cat.no. 562408), CD19 (cat.no. 555415) and HLA-DR (cat.no. 347364). Maxim Biotech (Fuzhou, China) provided us the Masson’s trichrome Staining Kit. From Abcam (Cambridge, UK), hematoxylin & eosin (HE) Staining Kit, primary antibodies VEGF (cat. no. ab32152) and AE1/AE3 (cat. no. ab961) for immunohistochemistry, and primary antibodies p38 (cat. no. ab4822), AKT (cat. no. ab4822), p-AKT (cat. no. ab4822), LIF (cat. no. ab4822), p65 (cat. no. ab4822), PI3K (cat. no. ab4822), Smad3 (cat. no. ab4822), TGFβ-1 (cat. no. ab4822), αγβ3 (cat. no. ab179475) and GAPDH (cat. no. ab8245) for western blotting as well as the corresponding HRP-conjugated secondary antibodies were available. The BCA Kit were manufactured by Thermo Fisher Scientific (Rockford, MD, USA). RIPA lysis buffer was obtained from BOSTER (Wuhan, China). The ECL Kit was got from Tanon (Shanghai, China). The total RNA Extraction Kit was acquired from Promega (Madison, WI, USA), and Qiagen (Dusseldorf, GER) provided First Strand Kit and QuantiFast SYBR® Green PCR Kit.
Isolation and phenotype determination of human UC-MSCs
The human umbilical cords (UC) were procured from full-term cesarean section surgeries conducted at our hospital. The patients had been informed in advance and had given their prior consent to donate. We obtained ethical approvals from the Ethics Committee of Jinhua Maternal & Child Health Care Hospital (approval No. 2021-KY-010) and this research adheres to the principles outlined in the Declaration of Helsinki. The isolated UC was incised, delicately peeled off the surface membrane, and then cleared of blood from the tissues. This was followed by rinsing in PBS three times. The tissue samples were manually divided into sections measuring 1-2 mm3 and subjected to incubation with collagenase type II at a concentration of 0.075% for a duration of 30 min. This was followed by treatment with trypsin at a concentration of 0.125% for another 30 min, while gently agitating the samples at a temperature of 37°C. Subsequently, the sections were evenly distributed onto culture dishes containing L-DMEM, supplemented with 5% FBS and 1% penicillin/streptomycin, and maintained in a humidified atmosphere with an additional presence of 5% CO2 at a temperature of 37°C.
After a period of three days, the non-adherent cells were eliminated. The surface antigen was detected using cells from passage 5 (1 × 106) through a BD LSR II flow cytometer provided by BD Biosciences. For flow cytometry analysis, the UC-MSCs that were isolated underwent incubation with mAbs conjugated with different fluorescent markers: phycoerythrin (PE)-conjugated anti-CD73 and anti-CD34; fluorescein isothiocyanate (FITC)-conjugated anti-CD90, anti-CD14 and anti-CD45; allophycocyanin (APC)-conjugated anti-CD105 and anti-CD19; as well as peridinin-chlorophyll protein complex (perCP)-conjugated anti-HLA-DR.
Lentivirus production and UC-MSCs overexpressing G-CSF establishment
The pL6.3-CMV-GFP-IRES-MCS lentivirus vector was utilized to incorporate the complete cDNA of the human G-CSF-coding gene. Lipofectamine 3000 was employed to transfect HEK293FT cells with lentivirus-G-CSF (LV-G-CSF). After 48 h of transfection, the culture supernatants were collected and subjected to centrifugation for viral particle concentration. UC-MSCs were then infected with these concentrated lentivirus particles at a multiplicity of infection of 50 pfu per cell in order to establish UC-MSCs overexpressing G-CSF. Following a further 48 h post-infection, the UC-MSCs were maintained in DMEM supplemented with 10% FBS.
Rat endometrial injury model establishment
A total of 24 eight-week-old female Sprague-Dawley rats with body weight (230 ± 10) g were purchased from the Experimental Animal Center of Zhejiang Province (Hangzhou, China). All rats were raised in a specific pathogen-free environment for one week, with free access to food and water under a light/dark cycle of 12/12 h at a temperature of 23°C-25°C. After that, the rats were separated into four groups at random: the sham, model, UC-MSCs and LV-G-CSF + UC-MSCs groups, with 6 rats in each group. As previously described [14], a 95% ethanol-induced endometrial injury rat model was established. The rats in the model, UC-MSCs and LV-G-CSF + UC-MSCs groups received pentobarbital sodium (50 mg/kg) for anesthetization and maintained body temperatures at 37°C ± 0.5°C. Under aseptic surgical conditions, the abdominal wall was incised, followed by careful extraction of the uterus. A solution containing 0.3 mL of 95% ethanol was then administered into the uterine cavity, which was subsequently rinsed twice with saline solution. The wound site was covered using moist gauze for protection. In contrast, the sham group did not receive any intervention and instead received an injection of physiological saline in place of 95% ethanol. Eight days later, rats in the UC-MSCs and LV-G-CSF + UC-MSCs groups were intravenously transplanted with UC-MSCs (100 μL) and UC-MSCs overexpressing G-CSF (100 μL) respectively, with a concentration of 1 × 106/mL. Three weeks after intervention, rats were euthanatized via overdose of pentobarbital sodium (200 mg/kg). The uterus was collected for succeeding experiments. All the experiments of this study were conducted by three experienced technicians who were blinded to grouping. We adhered to the protocols outlined in the NIH Guide for the Care and Use of Laboratory Animals when conducting all experiments on rats. Furthermore, the Ethics Committee of Jinhua Maternal & Child Health Care Hospital granted approval for these procedures (approval No. 2021-KY-010).
Histology
The uterine tissues were fixed, dehydrated, and embedded in paraffin. Subsequently, the tissue samples were sliced into sections with a thickness of 4 μm. Following dewaxing and rehydration procedures, the sections underwent HE staining and Masson's trichrome staining assays. Utilizing an OLYMPUS BX53 light microscope (Tokyo, Japan), images were captured at a magnification of 400×.
Immunohistochemistry
The uterine tissue samples were fixed, embedded in paraffin, deparaffinized, and rehydrated. Following antigen retrieval, the samples underwent overnight incubation at 4°C with primary antibodies VEGF (1:1,500) and AE1/AE3 (1:1,500). Subsequently, secondary antibodies conjugated with HRP (1:3,000) was applied for 1 h at 37°C. DAB staining was performed on each slice followed by capturing images using a light microscope (magnifications, 400×).
Total RNA isolation and qRT-PCR
The total RNA from UC-MSCs was isolated via a total RNA Extraction Kit. Subsequently, we utilized a First Strand Kit to synthesize cDNA. For qRT-PCR analysis, we employed the QuantiFast SYBR® Green PCR Kit following the provided instructions. To determine gene expression levels, we applied the 2-ΔΔCt method. Normalization was performed using GAPDH as a reference gene, and the primers used in this study can be found in Table 1.
Western blotting assay
The uterine tissue samples were subjected to lysis using RIPA buffer, followed by determination of protein concentrations utilizing a BCA Protein Kit. Subsequently, the protein products were separated through SDS polyacrylamide gel electrophoresis and transferred onto PVDF membranes. To block the membranes, a solution containing nonfat milk at a concentration of five percent was employed. Following this step, the primary antibodies (p38, AKT, p-AKT, LIF, p65, PI3K, Smad3, TGFβ-1, αγβ3 and GAPDH; all diluted at 1:1,000) were introduced to the membranes for overnight incubation at 4°C. The corresponding secondary antibodies (diluted at 1:3,000) were then added and allowed to incubate for 1 h at room temperature. Visualization of immunoblotting was achieved using an ECL detection Kit.
Statistical analysis
The differences in the data were assessed using Student's t-tests and one-way ANOVA as appropriate. Data analysis was conducted using SPSS software version 22.0. The data were presented as mean ± standard deviation (SD). Statistical significance was defined as P < 0.05.
Results
Characterization of human UC-MSCs
The flow cytometry technique was employed to analyze the surface molecular expression of human UC-MSCs that had undergone five passages for enrichment. As illustrated in Figure 1A-B, the result showed a high expression of CD73/CD90/CD105 (>99%), while a low expression of CD14/CD19/CD34/CD45/HLA-DR (<1%) for the isolated human UC-MSCs. These results conformed with the expression standard of MSCs surface molecular markers [26].
UC-MSCs overexpressing G-CSF ameliorates the pathological features of damaged endometrium
To explore the effects of UC-MSCs overexpressing G-CSF on the injured endometrium in a rat model, a UC-MSC cell model that overexpressed G-CSF was firstly established. As shown in Figure 2A, we found that the expression of G-CSF was remarkably increased in the LV-G-CSF + UC-MSCs group relative to that of UC-MSCs group (P < 0.001), suggesting that UC-MSCs overexpressing G-CSF was established successfully. As illustrated in Figure 2B, the images of HE staining showed that the endometrial structure of the sham group seemed more complete, epithelial cells were arranged closely, and blood vessels and glands were clearly visible. In the model group, the cells of the endometrial intrauterine epithelium were necrosis and abscission, and the number of glands in the functional layer was significantly reduced. The uterus recovered well in both the UC-MSCs and LV-G-CSF + UC-MSCs groups, and the glands and blood vessels were obvious. When the severity of endometrial injury increases, there is a proliferation of interstitial fibrous connective tissue that facilitates the repair process for damaged tissue. Consequently, this ultimately results in the formation of fibrotic lesions. Hence, the degree of endometrial fibrosis serves as a direct indicator for assessing the extent of damage to the endometrium. The results of Masson's trichrome staining revealed notable differences among the various experimental groups (Figure 2C). It was observed that rats in the model group exhibited pronounced fibrosis, with severe adhesion of the injured uterine cavity as well as replacement of myometrium by collagen. However, both UC-MSCs treatment and LV-G-CSF + UC-MSCs treatment demonstrated superior reparative effects when compared to the model group.
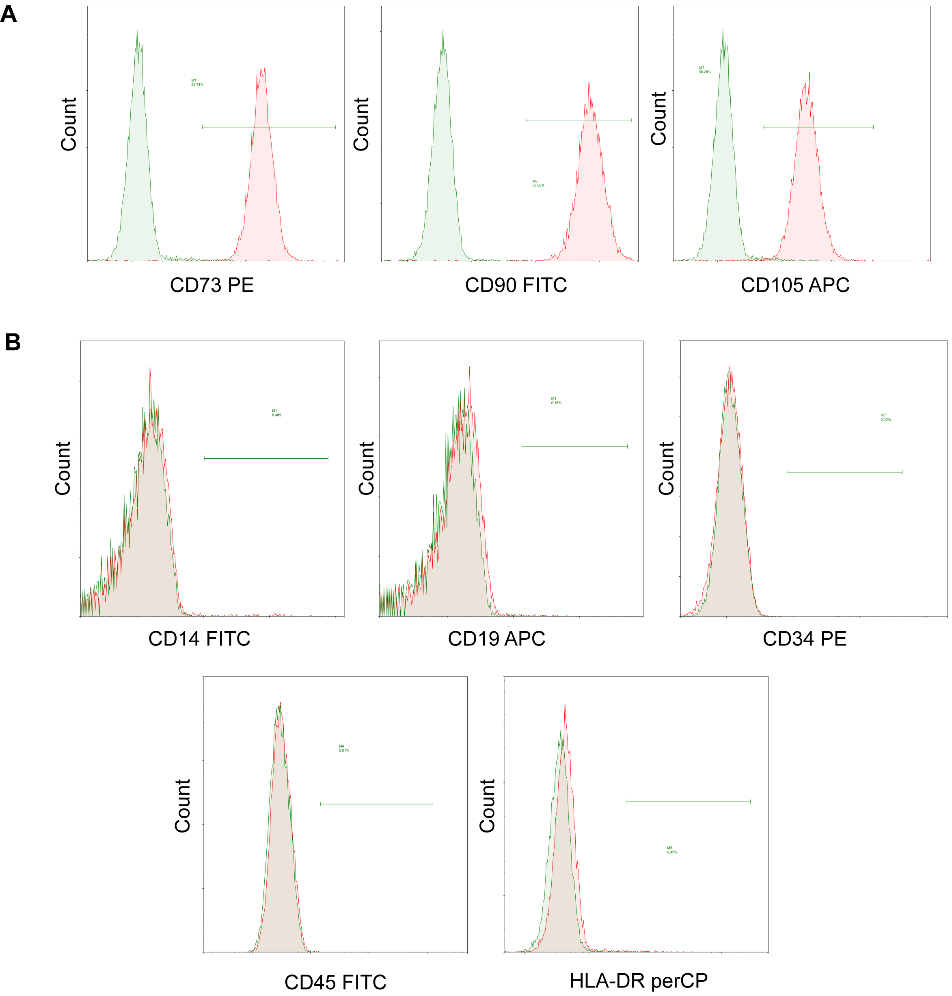
Figure 1. Flow cytometry analysis of cells harvested from human umbilical cords displays MSCs phenotype. MSCs were positive for (A) CD73, CD90 and CD105, and negative for (B) CD14, CD19, CD34, CD45 and HLA-DR
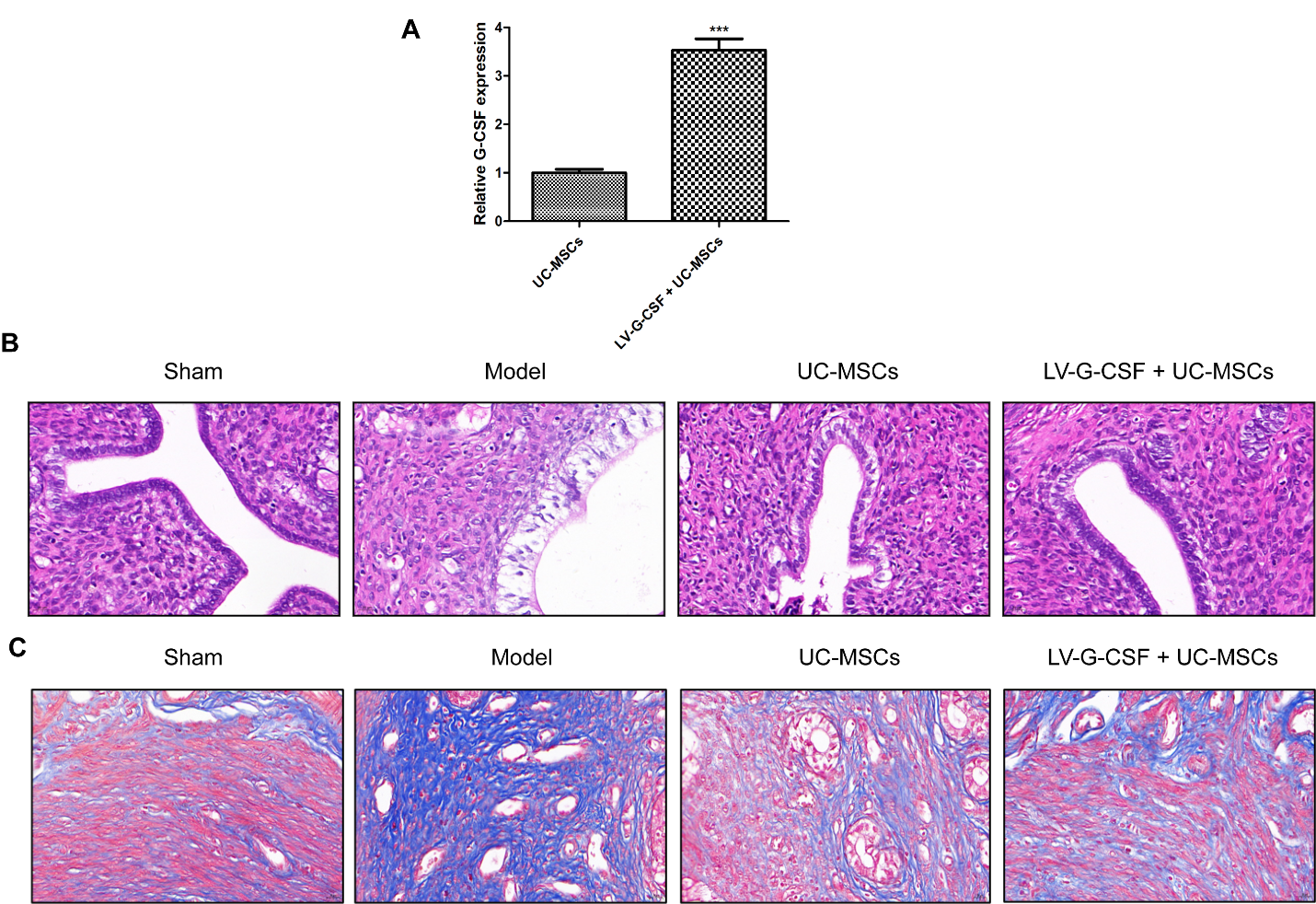
Figure 2. UC-MSCs overexpressing G-CSF ameliorates the pathological features of damaged endometrium. (A) The expression of G-CSF in UC-MSCs following transfection with LV-G-CSF was detected via qRT-PCR. ***P < 0.001 vs. UC-MSCs. The uterine histology was examined through (B) hematoxylin & eosin staining and (C) Masson’s trichrome staining assays. Bar=20 μm
UC-MSCs overexpressing G-CSF promotes angiogenesis and epithelial growth
VEGF is vital for the formation of new vessels and re-epithelialization of the endometrium [27]. Pan‑keratin (AE1/AE3) is a marker of endometrial epithelial cells [28]. Through the results of immunohistochemistry, we demonstrated that the expression of VEGF and AE1/AE3 was dramatically decreased in the model group by contrast to the sham group (Figure 3, P < 0.001). However, UC-MSCs treatment, particularly UC-MSCs overexpressing G-CSF, significantly restored the VEGF and AE1/AE3 levels in injured endometrium (P < 0.05).
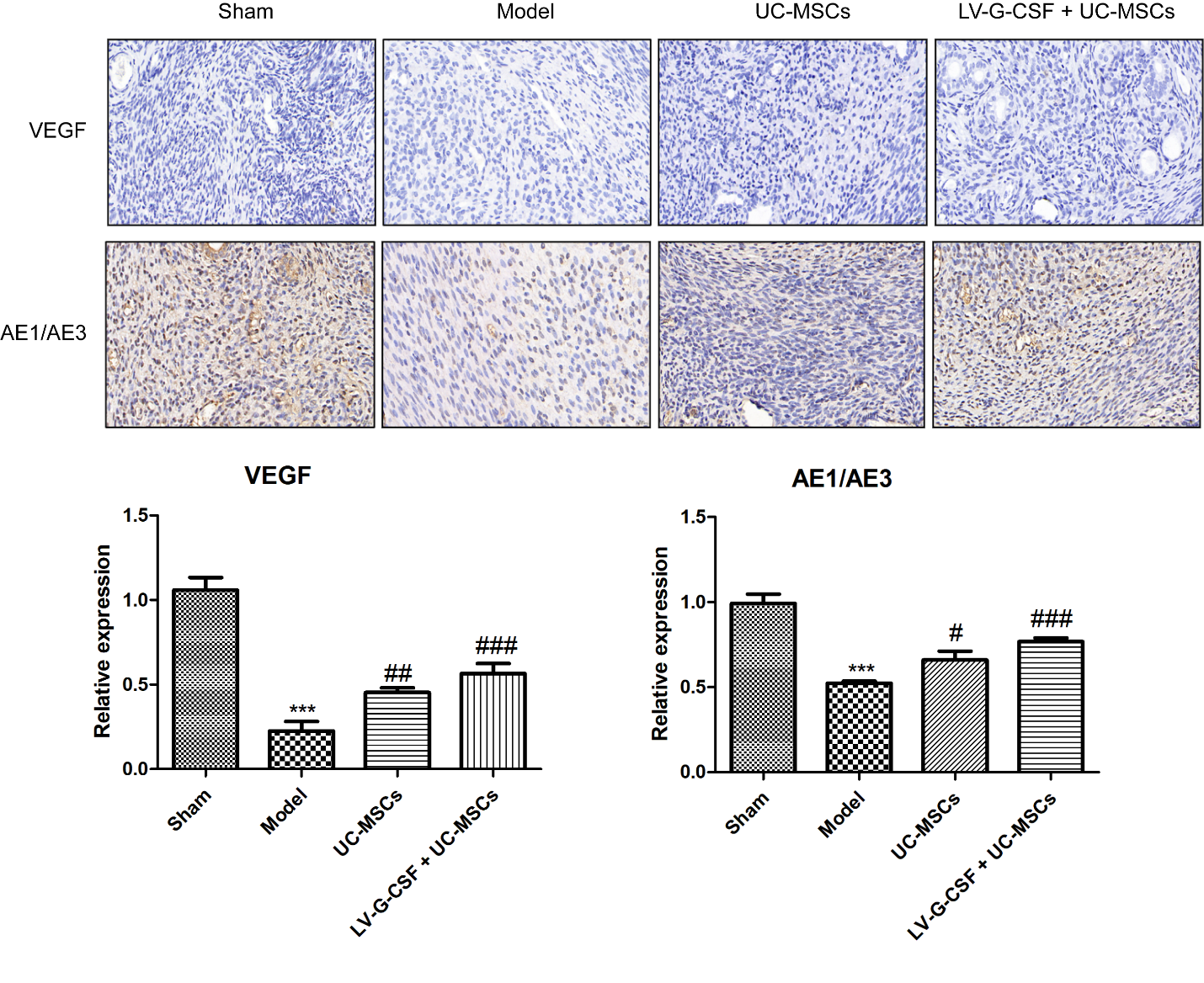
Figure 3. UC-MSCs overexpressing G-CSF promotes angiogenesis and epithelial growth. Immunohistochemical assessment of VEGF and pan-keratin (AE1/AE3) levels was conducted in uterine tissues. Bar=20 μm. ***P < 0.001 vs. sham; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. model
Effects of UC-MSCs overexpressing G-CSF on fibrosis-, angiogenesis-, inflammation-, apoptosis-, and endometrial receptivity-related pathways
We further investigated the effects of UC-MSCs overexpressing G-CSF on fibrosis-, angiogenesis-, inflammation-, apoptosis-, and endometrial receptivity-related pathways using western blotting. As shown in Figure 4, compared to the sham group, pronounced downregulation of p38, LIF, and αγβ3 protein levels (P < 0.001) but upregulated protein levels of AKT, p-AKT, p65, PI3K, Smad3, and TGF-β1 were observed in the model group (P < 0.001). The administration of UC-MSCs overexpressing G-CSF significantly restored the levels of p38, LIF, and αγβ3 proteins (P < 0.001) but inhibited the expression of AKT, p-AKT, p65, PI3K, Smad3, and TGF-β1 (P < 0.01). Additionally, UC-MSCs administration only was found to reduce the levels of AKT, p-AKT, PI3K, Smad3 (P < 0.05), and elevate the levels of LIF and αγβ3 (P < 0.01), while having no significant effect on p38, p65 and TGF-β1 contents.
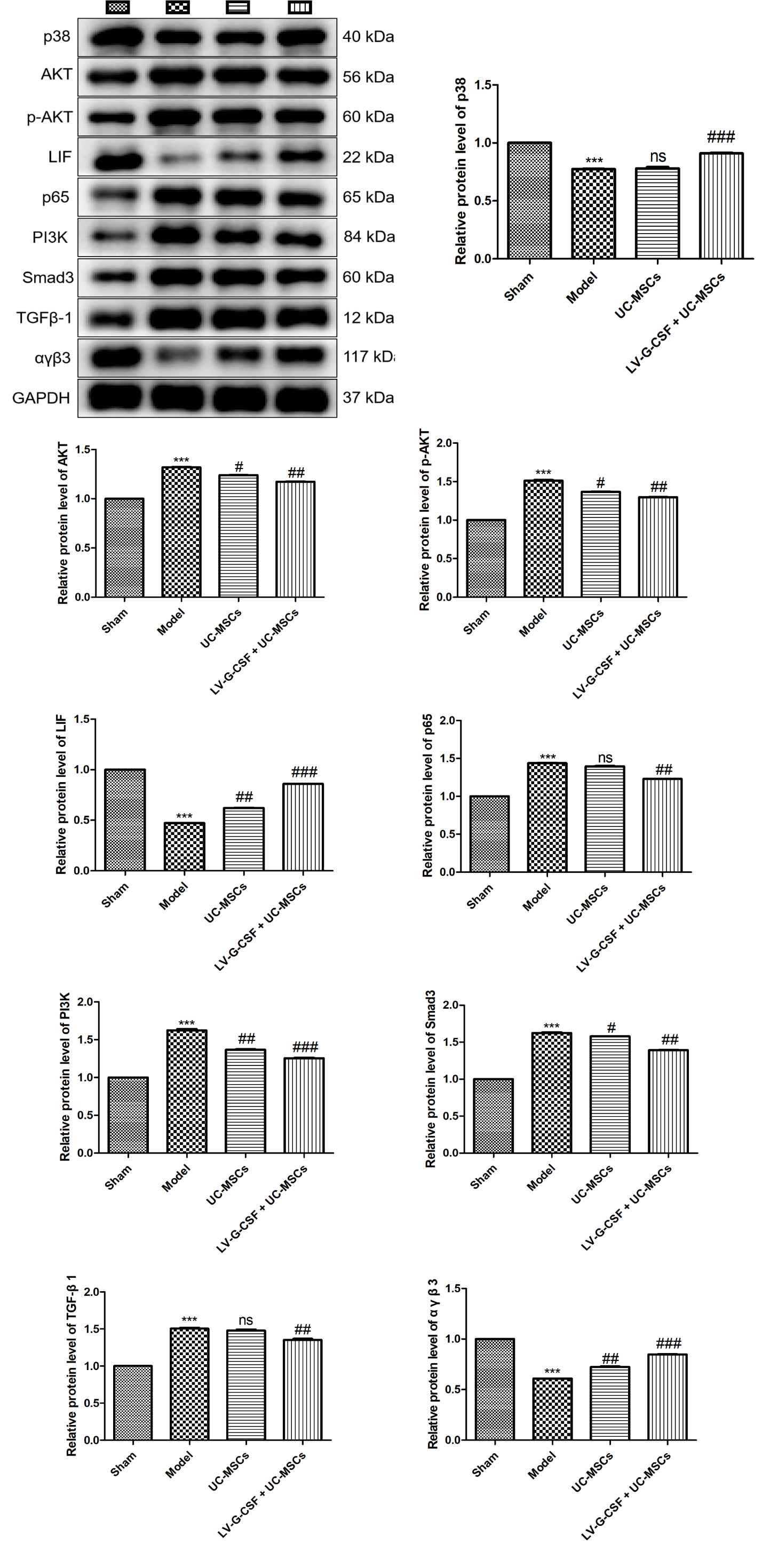
Figure 4. Effects of UC-MSCs overexpressing G-CSF on fibrosis-, angiogenesis-, inflammation-, apoptosis-, and endometrial receptivity-related pathways. The protein levels of p38, AKT, p-AKT, LIF, p65, PI3K, Smad3, TGF-β1, and αγβ3 in uterine tissues were measured by western blotting. ***P < 0.001 vs. sham; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. model. ns: no significance
Discussion
In general, stem cells can be categorized into embryonic stem cells (ESCs) and adult stem cells (ASCs) based on their developmental trajectory. Among the ASCs, MSCs are the most widely used that have been successfully isolated from menstrual blood, bone marrow, umbilical cord, and other tissues [29]. The clinical application is immensely limited due to the disadvantages of BM-MSCs (a decrease in differentiative potential with an increase in the donor’s age) and Men-MSCs (difficult preservation and high contamination rate) [30,31]. At present, human UC-MSCs, characterized by a shorter proliferation time, ease of extraction, multidirectional differentiation potential, prolonged post-transplantation survival, low immunogenicity, and other distinctive features, have emerged as the preferred choice for transplantation [32]. In this study, a novel therapy related to UC-MSCs on gynecology was preliminarily explored, indicating that UC-MSCs overexpressing G-CSF can attenuate endometrial injury in a rat model.
The International Society for Cellular Therapy (ISCT) has put forth a set of three criteria, as suggested by the Mesenchymal and Tissue Stem Cell Committee, in order to establish a clear definition for MSCs [26]. First, MSCs must exhibit adherent property when cultured under standard conditions. Second, MSCs should demonstrate in vitro differentiation potential towards adipocytes, osteoblasts, and chondroblasts. Third, MSCs must express adhesion molecules such as CD105, CD90, and CD73 while lacking expression of cell surface markers HLA-DR, CD45, CD34, CD19, and CD14. Hence, the characteristics of MSCs can be utilized to assess their purity following in vitro cultivation and purification. In our pre-experiments, the UC-MSCs that were cultured exhibited a spindle-shaped adherent growth pattern, capable of differentiating down the adipogenic, osteogenic and chondrogenic lineages (data were not shown). Meanwhile, the analysis of immunophenotyping was conducted on the isolated UC-MSCs, indicating that UC-MSCs exhibited high levels of expression for specific antigens such as CD105, CD90, and CD73, while lacking expression for HLA-DR, CD45, CD34, CD19, and CD14. All these results suggested that UC-MSCs were isolated successfully, adhering to the globally accepted criteria for characterizing UC-MSCs.
The regenerative potential of UC-MSCs in repairing endometrial damage has been extensively documented. For instances, a recent study showed the potential of transplantation of UC-MSCs to enhance the recovery of endometrial morphology and function [33]. Zhang, et al. injected UC-MSCs into the tail vein of the rat endometrial injury model and demonstrated that transplantation of UC-MSCs contributes to rejuvenation of the endometrium and enhancement of fertility through mitigating excessive fibrosis and inflammation, stimulating proliferation of endometrial cells, and promoting remodeling of blood vessels [14]. Our study demonstrated that compared to the model group, transplantation of UC-MSCs could restore the endometrial structure, promote angiogenesis and epithelial growth, and reduce fibrosis. However, the homing capacity, duration of local residence in the endometrium, and colonization ability of stem cells remain poorly understood. Considering the low local persistence and utilization of MSCs in the endometrium, Xin, et al. used a collagen scaffold (CS) loaded with UC-MSCs to transplant onto the damaged endometrial surface and uncovered that CS/UC-MSCs can promote the homing and colonization capacities of UC-MSCs, accelerating endometrial regeneration and restores fertility through the paracrine effects [34]. G-CSF, a crucial paracrine factor initially discovered in hematopoietic stem cells, has been documented to induce stem cell mobilization, which is extensively employed for clinical transplantation, including the treatment of endometrial injury [35,36]. Early in 2012, Yener, et al. has proposed that the combined utilization of UC-MSCs and G-CSF demonstrates superior efficacy compared to either treatment alone in mitigating renal damage induced by carbon tetrachloride [37]. Chen, et al. also reported that the combination of UC-MSCs transplantation with G-CSF promotes the proliferation and colonization of UC-MSCs in a rat model with acute liver failure, emphasizing the impact of G-CSF on mobilizing UC-MSCs [25]. In this study, we established a lineage of UC-MSCs that exhibited G-CSF overexpression. As expected, we further indicated that intravenous transplantation of UC-MSCs overexpressing G-CSF could attenuate endometrial injury more effectively than UC-MSCs alone through inducing angiogenesis and reducing excessive fibrosis.
Except for excessive fibrosis and endometrial angiogenesis inhibition, endometrial injury is also implicated in various pathological mechanism, such as apoptosis of endometrial stromal cells, inflammatory responses, and decrease in endometrial receptivity [38-40]. TGF-β1 plays a pivotal role in the activation of profibrotic mediators via TGF-β receptors, thereby initiating the classical TGF-β/Smad signaling pathway and exerting critical influence on fibroblast activation and extracellular matrix deposition [41]. Both αγβ3 and LIF are considered as two important markers of endometrial receptivity [42,43]. PI3K/AKT signaling, p65 NF-κB, and p38 MAPK have been reported to be a classical pathway that closely associated with angiogenesis, inflammation, and apoptosis, respectively [44-46]. Compared to the model group, we observed a decrease in AKT, p-AKT, PI3K, and Smad3 levels in the UC-MSCs group. Additionally, LIF and αγβ3 levels were increased while there were no significant changes in p38, p65 and TGF-β1 levels. These results suggested that treatment with UC-MSCs alone significantly enhances angiogenesis and improves endometrial receptivity in the damaged endometrium. Additionally, it partially inhibits fibrosis but does not appear to have any effects on suppressing inflammation or preventing apoptosis of endometrial stromal cells. More importantly, it was observed that treatment with UC-MSCs overexpressing G-CSF significantly regulated all these proteins, highlighting its therapeutic potential in modulating angiogenesis, fibrosis, inflammation, endometrial stromal cell apoptosis, and endometrial receptivity during the progression of endometrial injury.
The presence of certain limitations in this study should not be disregarded. Firstly, further in vitro investigation is required to explore the impact of UC-MSCs overexpressing G-CSF on the proliferation and apoptosis of human endometrial stromal cells. Additionally, more inflammation-related indicators are necessary to fully understand the effects of UC-MSCs overexpressing G-CSF on inflammatory responses. Lastly, while this study focuses on the ameliorating effects of UC-MSCs overexpressing G-CSF on animal models, its efficacy for patients with endometrial injury requires further verification.
Conclusion
The present study provides preliminary evidence for the therapeutic efficacy of UC-MSCs overexpressing G-CSF in mitigating endometrial injury, suggesting that this intervention effectively regulates key processes including apoptosis, angiogenesis, fibrosis, inflammation, and endometrial receptivity within the endometrial stromal cells. These findings offer valuable insights into potential clinical interventions for managing endometrial injury.
List of abbreviations
IUA: Intrauterine Adhesions; ESCs: Embryonic Stem Cells; ASCs: Adult Stem Cells; MSCs: Mesenchymal Stem Cells; UC: umbilical cords; UC-MSCs: Human Umbilical Cord-Derived MSCs; Men-MSCs: Menstrual Blood-Derived MSCs; BM-MSCs: Bone Marrow-Derived MSCs; G-CSF: Granulocyte-Colony Stimulating Factor; LV-G-CSF: Lentivirus-G-CSF; VEGF: Vascular Endothelial Growth Factor; mAbs: Monoclonal Antibodies; HE: Hematoxylin & Eosin; ISCT: The International Society for Cellular Therapy; CS: Collagen Scaffold.
Authors’ contributions
XZ made substantial contributions to the conception and design of the study. YL, LY and YW made substantial contributions to the acquisition, analysis and interpretation of the data. YL drafted the manuscript. All authors critically revised the manuscript for intellectual content. LY and YW confirmed the authenticity of all the raw data. All authors have read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Ethics approval and consent to participate
This study was approved by the Ethical Committee of Jinhua Maternal & Child Health Care Hospital (approval number: 2021-KY-010).
Consent for publication
Not applicable.
Funding
This work was supported by The Science and Technology Research Plan Project in Jinhua (No. 2021-3-133).
Acknowledgements
Not available.
Availability of data and materials
The data analyzed during the current study are available from the corresponding author on reasonable request.
References
- Xu L, Ding L, Wang L, Cao Y, Zhu H, et al. (2017) Umbilical cord-derived mesenchymal stem cells on scaffolds facilitate collagen degradation via upregulation of MMP-9 in rat uterine scars. Stem Cell Res Ther 8: 84. [Crossref]
- Gilman AR, Dewar KM, Rhone SA, Fluker MR (2016) Intrauterine adhesions following miscarriage: Look and learn. J Obstet Gynaecol Can 38: 453-457. [Crossref]
- Lee WL, Liu CH, Cheng M, Chang WH, Liu WM, et al. (2021) Focus on the primary prevention of intrauterine adhesions: Current concept and vision. Int J Mol Sci 22: 5175. [Crossref]
- Ma J, Zhan H, Li W, Zhang L, Yun F, et al. (2021) Recent trends in therapeutic strategies for repairing endometrial tissue in intrauterine adhesion. Biomater Res 25: 40. [Crossref]
- Ye Q, Zhang Y, Fu J, Zou Y, Zhao W, et al. (2019) Effect of ligustrazine on endometrium injury of thin endometrium rats. Evid Based Complement Alternat Med 2019: 7161906. [Crossref]
- Dreisler E, Kjer JJ (2019) Asherman’s syndrome: Current perspectives on diagnosis and management. Int J Womens Health 20: 191-198. [Crossref]
- Suginami K, Sato Y, Horie A, Matsumoto H, Kyo S, et al. (2017) Platelets are a possible regulator of human endometrial re‐epithelialization during menstruation. Am J Reprod Immunol 77: e12609. [Crossref]
- Zhang Y, Lin X, Dai Y, Hu X, Zhu H, et al. (2016) Endometrial stem cells repair injured endometrium and induce angiogenesis via AKT and ERK pathways. Reproduction 152: 389-402. [Crossref]
- Lin X, Wei M, Li TC, Huang Q, Huang D, et al. (2013) A comparison of intrauterine balloon, intrauterine contraceptive device and hyaluronic acid gel in the prevention of adhesion reformation following hysteroscopic surgery for Asherman syndrome: A cohort study. Eur J Obstet Gynecol Reprod Biol 170: 512-516. [Crossref]
- Nicolay NH, Perez RL, Debus J, Huber PE (2015) Mesenchymal stem cells–A new hope for radiotherapy-induced tissue damage?. Cancer Lett 366: 133-140. [Crossref]
- Trohatou O, Roubelakis MG (2017) Mesenchymal stem/stromal cells in regenerative medicine: Past, present, and future. Cell Reprogram 19: 217-224. [Crossref]
- Hu J, Song K, Zhang J, Zhang Y, Tan BZ (2019) Effects of menstrual blood-derived stem cells on endometrial injury repair. Mol Med Rep 19: 813-820. [Crossref]
- Wang J, Ju B, Pan C, Gu Y, Zhang Y, et al. (2016) Application of bone marrow-derived mesenchymal stem cells in the treatment of intrauterine adhesions in rats. Cell Physiol Biochem 39: 1553-1560. [Crossref]
- Zhang L, Li Y, Guan CY, Tian S, Lv XD, et al. (2018) Therapeutic effect of human umbilical cord-derived mesenchymal stem cells on injured rat endometrium during its chronic phase. Stem Cell Res Ther 9: 36. [Crossref]
- Zhao YX, Chen SR, Su PP, Huang FH, Shi YC, et al. (2019) Using mesenchymal stem cells to treat female infertility: An update on female reproductive diseases. Stem Cells Int 2019: 9071720. [Crossref]
- de Miguel‐Gomez L, Ferrero H, Lopez‐Martinez S, Campo H, Lopez‐Perez N, et al. (2020) Stem cell paracrine actions in tissue regeneration and potential therapeutic effect in human endometrium: A retrospective study. BJOG 127: 551-560. [Crossref]
- Bozkaya IO, Azik F, Tavil B, Koksal Y, Ozguner M, et al. (2015) The effect of granulocyte colony–stimulating factor on immune-modulatory cytokines in the bone marrow microenvironment and mesenchymal stem cells of healthy donors. Biol Blood Marrow Transplant 21: 1888-1894. [Crossref]
- Avila-Portillo LM, Aristizabal F, Riveros A, Abba MC, Correa D (2020) Modulation of adipose‐derived mesenchymal stem/stromal cell transcriptome by G‐CSF stimulation. Stem Cells Int 2020: 5045124. [Crossref]
- Miranda JP, Filipe E, Fernandes AS, Almeida JM, Martins JP, et al. (2015) The human umbilical cord tissue-derived MSC population UCX® promotes early motogenic effects on keratinocytes and fibroblasts and G-CSF-mediated mobilization of BM-MSCs when transplanted in vivo. Cell Transplant 24: 865-877. [Crossref]
- Zhao J, Xu B, Xie S, Zhang Q, Li YP (2016) Whether G-CSF administration has beneficial effect on the outcome after assisted reproductive technology? A systematic review and meta-analysis. Reprod Biol Endocrinol 14: 62. [Crossref]
- Li J, Mo S, Chen Y (2017) The effect of G-CSF on infertile women undergoing IVF treatment: A meta-analysis. Syst Biol Reprod Med 63: 239-247. [Crossref]
- Noël L, Fransolet M, Jacobs N, Foidart JM, Nisolle M, et al. (2020) A paracrine interaction between granulosa cells and leukocytes in the preovulatory follicle causes the increase in follicular G-CSF levels. J Assist Reprod Genet 37: 405-416. [Crossref]
- de Castro Rocha MN, de Souza Florêncio R, Alves RR (2020) The role played by granulocyte colony stimulating factor (G-CSF) on women submitted to in vitro fertilization associated with thin endometrium: Systematic review. JBRA Assist Reprod 24: 278. [Crossref]
- Eftekhar M, Miraj S, Mojtahedi MF, Neghab N (2016) Efficacy of Intrauterine infusion of granulocyte colony stimulating factor on patients with history of implantation failure: A randomized control trial. Int J Reprod Biomed 14: 687. [Crossref]
- Chen H, Tang S, Liao J, Liu M, Lin Y (2019) Therapeutic effect of human umbilical cord blood mesenchymal stem cells combined with G-CSF on rats with acute liver failure. Biochem Biophys Res Commun 517: 670-676. [Crossref]
- Dominici ML, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315-317. [Crossref]
- Lin N, Li XA, Song T, Wang J, Meng K, et al. (2012) The effect of collagen-binding vascular endothelial growth factor on the remodeling of scarred rat uterus following full-thickness injury. Biomaterials 33: 1801-1807. [Crossref]
- Li J, Huang B, Dong L, Zhong Y, Huang Z (2021) WJ‑MSCs intervention may relieve intrauterine adhesions in female rats via TGF‑β1‑mediated Rho/ROCK signaling inhibition. Mol Med Rep. 23: 8. [Crossref]
- Kim N, Cho SG (2015) New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int J Stem Cells 8: 54-68. [Crossref]
- Santamaria X, Cabanillas S, Cervello I, Arbona C, Raga F, et al. (2016) Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman's syndrome and endometrial atrophy: A pilot cohort study. Hum Reprod 31: 1087-1096. [Crossref]
- Tan J, Li P, Wang Q, Li Y, Li X, et al. (2016) Autologous menstrual blood-derived stromal cells transplantation for severe Asherman's syndrome. Hum Reprod 31: 2723-2729. [Crossref]
- Fan CG, Zhang QJ, Zhou JR (2011) Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev Rep. 7: 195-207. [Crossref]
- Zhang L, Li Y, Dong YC, Guan CY, Tian S, et al. (2022) Transplantation of umbilical cord-derived mesenchymal stem cells promotes the recovery of thin endometrium in rats. Sci Rep 12: 412. [Crossref]
- Xin L, Lin X, Pan Y, Zheng X, Shi L, et al. (2019) A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater 92: 160-171. [Crossref]
- Işık G, Oktem M, Guler I, Oktem E, Ozogul C, et al. (2021) The impact of granulocyte colony-stimulating factor (G-CSF) on thin endometrium of an animal model with rats. Gynecol Endocrinol 37: 438-445. [Crossref]
- Zhang Y, Chen X, Chen S, Wei C, Li B, et al. (2022) Intrauterine administration of G-CSF for promoting endometrial growth after hysteroscopic adhesiolysis: A randomized controlled trial. Hum Reprod 37: 725-733. [Crossref]
- Koc Y, Sokmen M, Unsal A, Cigerli S, Ozagari A, et al. (2012) Effects of human umbilical cord stem cells and granulocyte colony-stimulating factor (G-CSF) on carbon tetrachloride-induced nephrotoxicity. Nephrourol Mon 4: 545. [Crossref]
- Drizi A, Djokovic D, Laganà AS, van Herendael B (2020) Impaired inflammatory state of the endometrium: A multifaceted approach to endometrial inflammation. Current insights and future directions. Prz Menopauzalny 19: 90-100. [Crossref]
- Wang H, Zhao X, Ni C, Dai Y, Guo Y (2018) Zearalenone regulates endometrial stromal cell apoptosis and migration via the promotion of mitochondrial fission by activation of the JNK/Drp1 pathway. Mol Med Rep 17: 797-806. [Crossref]
- Celik O, Yurci A, Ersahin A, Gungor ND, Celik N, et al. (2023) Endometrial injury upregulates expression of receptivity genes in women with implantation failure. Int J Environ Res Public Health 20: 3942. [Crossref]
- Zhang Z, Li S, Deng J, Yang S, Xiang Z, et al. (2020) Aspirin inhibits endometrial fibrosis by suppressing the TGF-β1-Smad2/Smad3 pathway in intrauterine adhesions. Int J Mol Med 45: 1351-1360. [Crossref]
- Kim EY, Chung TW, Choi HJ, Jung YS, Lee SO, et al. (2019) Extracts from Paeonia lactiflora Pallas, Rehmannia Glutinosa var. Purpurea Makino, Perilla Frutescens var. Acuta Kudo may increase the endometrial receptivity through expression of leukemia inhibitory factor and adhesion molecules. J Tradit Chin Med 39: 15-25. [Crossref]
- Li C, Zhou L, Xie Y, Guan C, Gao H (2019) Effect of irisin on endometrial receptivity of rats with polycystic ovary syndrome. Gynecol Endocrinol 35: 395-400. [Crossref]
- Kim GD (2017) Myricetin inhibits angiogenesis by inducing apoptosis and suppressing PI3K/Akt/mTOR signaling in endothelial cells. J Cancer Prev 22: 219. [Crossref]
- González-Ramos R, Defrère S, Devoto L (2012) Nuclear factor–kappaB: A main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil Steril 98: 520-528. [Crossref]
- Pereira L, Igea A, Canovas B, Dolado I, Nebreda AR (2013) Inhibition of p38 MAPK sensitizes tumour cells to cisplatin‐induced apoptosis mediated by reactive oxygen species and JNK. EMBO Mol Med 5: 1759-1774. [Crossref]




