Abstract
This study is aimed to evaluate the effects of human umbilical cord-derived mesenchymal stem cells (UC-MSCs) combined with chitosan hydrogel loaded with transforming growth factor (TGF)-β3 on meniscus injury in rabbits. Different concentrations (2, 5 and 10 ng/mL) of TGF-β3 were used to treat UC-MSCs, followed by selecting the optimal concentration to induce the chondrogenic differentiation of UC-MSCs. New Zealand White rabbits underwent a longitudinal tear in the peripheral region of the medial meniscus and then a chitosan hydrogel scaffold of UC-MSCs recombination with TGF-β3 was engrafted. TGF-β3 (10 ng/mL) could effectively elevate the levels of collagen type II, aggrecan and ALP, but inhibit collagen type I level in UC-MSCs. Additionally, we found that UC-MSCs combined with chitosan hydrogel loaded with TGF-β3 could improve meniscus histology and GAG distribution effectively. Meanwhile, the engrafted chitosan hydrogel scaffold restored the levels of collagen type II, aggrecan and ALP, and suppressed collagen type I level in the injured meniscus of rabbit models. These findings supported that UC-MSCs combined with chitosan hydrogel loaded with TGF-β3 can regenerate and repair meniscus in tearing injury effectively, which provided a theoretical basis for clinical therapy in patients.
Keywords
umbilical cord-derived mesenchymal stem cells, chitosan hydrogel, TGF-β3, meniscus injury, rabbit
Introduction
Meniscus is a crescent-shaped fibrocartilage tissue located between the tibial plateau and the inner and outer femoral condyles [1]. It is an important anatomical structure to maintain the structure stability of knee joint, and act as a cushion when the articular cartilage is impacted by external forces [2]. Meniscus injury can lead to knee joint cartilage degeneration and osteoarthritis [3]. Due to the lack of blood vessels, high requirements for local microenvironment conditions, and slow metabolism of chondrocytes in the structure of meniscus cartilage tissue, the self-repair and regeneration ability of cartilage at the damaged site of meniscus tissue are limited [4,5]. Currently, the clinical means of treating meniscus injury are partial excision or meniscal suture under arthroscopy, while in serious cases, the meniscus is completely removed [6]. The early treatment effect is satisfactory, which can reduce the local pain and swelling of the knee joint of patients to a certain extent. However, it may cause joint stiffness, muscle atrophy and even lead to osteonecrosis and osteoarthritis in the long term, which brings great pain and life pressure to patients [6]. Therefore, how to achieve efficient repair and regeneration of the injured meniscus has become a major challenge in orthopedic disease research.
In the field of stem cell transformation and regeneration, mesenchymal stem cells (MSCs) have the characteristics of differentiating into various types of specialized cells such as adipocytes, chondrocytes and osteoblast [7]. Meanwhile, MSCs can secrete a series of trophic factors including exosomes, cytokines, chemokines and growth factors under the mediation of stimulus sources [8-10]. These bioactive macromolecules can promote the change of the microenvironment in the injury areas, accelerate cell regeneration and repair, while also reducing the inflammatory response caused by meniscus injury through immune regulation [11-13]. The above public data indicated the high research value of MSCs in repairing bone and cartilage damage. Chitosan is a natural macromolecular polysaccharide, with the biological characteristics of physiological neutrality, temperature sensitivity, injectability, degradability, and slow release to macromolecular drugs [14]. It has been reported that chitosan hydrogel porous scaffold shows the similarly mechanical properties with native cartilage, in the aspect of hydraulic permeability, aggregate modulus, tensile strain and tensile stress [15], which provides a suitable extracellular environment for MSCs to grow and differentiate. For example, Moradi, et al. [16] constructed a polyvinyl alcohol/chitosan hydrogel scaffold seeded by adipose-derived MSCs and articular chondrocytes and they demonstrated that this hybrid scaffold can promote meniscus regeneration in a rabbit model [16]. Additionally, some researches have reported that in the microenvironment where MSCs are directed differentiation into chondrocytes, multiple cytokines such as TGF-β3, BMP, Sox9, FGF and PDG are interacted [17]. Among these cytokines, TGF-β3 is a key factor that affects the differentiation and growth of chondrocytes, which can not only promote the synthesis of collagen and glycosaminoglycan (GAG), but also play an important role in various stages of cell adhesion, aggregation, proliferation, and developmental growth during chondrocyte differentiation [18-20].
In current study, a chitosan hydrogel scaffold seeded by human umbilical cord-derived mesenchymal stem cells (UC-MSCs) recombination with TGF-β3 was constructed. Its repair and regeneration effect on the injured meniscus of rabbit model was preliminarily evaluated. These results may provide a clinical therapeutic method for patients with meniscus injury.
Materials and methods
Cells and reagents
ScienCell (San Diego, CA, USA) provided UC-MSCs and MSC medium. Fetal bovine serum (FBS) was from Gibco (Rockville, MD, USA). From Golden-Shell Pharmaceutical Co., Ltd. (Yuhuan, China), chitosan (85% degree of deacetylation) was purchased. From Promega (Madison, WI, USA), total RNA Extraction Kit was acquired, and Qiagen (Dusseldorf, GER) provided First Strand Kit and QuantiFast SYBR® Green PCR Kit. The ECL Kit was got from Abbkine Scientific (Wuhan, China). Biosharp Life Sciences (Hefei, China) provided the BCA Kit. RIPA lysis buffer was obtained from BOSTER (Wuhan, China). Primary antibody aggrecan was from Abcam (Cambridge, UK); collagen type I, collagen type II, GAPDH primary antibodies and HRP-conjugated secondary antibodies were obtained from Proteintech (Wuhan, China). Jiancheng Bioengineering Institute (Nanjing, China) provided alkaline phosphatase (ALP) assay Kit. Hematoxylin and eosin (HE) Staining Kit, TGF-β3 and toluidine blue solution were procured from Solarbio Science and Technology (Beijing, China).
Culture and treatment of UC-MSCs
MSC medium containing 10% FBS was utilized for the cultivation of UC-MSCs. The cultures were maintained at the following conditions: 5% CO2, 37°C. UC-MSCs were induced by different concentrations of TGF-β3 (2, 5 and 10 ng/mL) for 24 h. UC-MSCs without TGF-β3 treatment were served as the blank group. The levels of collagen type I, collagen type II, aggrecan and ALP were determined to screen out the optimal concentration of TGF-β3.
Preparation of UC-MSCs combined with chitosan hydrogel loaded with TGF-β3
Chitosan hydrogel was prepared as previously described [21,22]. Then, the optimal concentration of TGF-β3 was added into chitosan hydrogel and adjust the pH value to 7.3 following complete mixing. UC-MSCs were digested using trypsin and then collected for centrifugation, followed by discarding the supernatant. Chitosan hydrogel containing TGF-β3 was added to the cell suspension slowly and then mixed uniformly to produce chitosan hydrogel scaffold of UC-MSCs recombination with TGF-β3, in which the final density of UC-MSCs is 5 × 106/mL.
Animal grouping and treatment
A total of 18 New Zealand White male rabbits (8-month-old) with body weight 3.5-4.0 kg was operated on. Based on the results of roentgenograms, these rabbits were skeletally mature with closed epiphyses. The rabbits were separated into three groups at random: the control, model and model + UC-MSCs + TGF-β3 groups. Before operating, rabbits were anesthetized by ketamine (35 mg/kg) and xylazine (5 mg/kg) via intramuscular injection. Subsequently, a medial parapatellar incision and arthrotomy were performed. The patella was dislocated laterally and made the knee placed in full flexion, rotated externally, turner over and then the medial meniscus was exposed. A 5 mm longitudinal tear in the anterior half of the medial meniscus was created with surgical blade (No. 11). After the meniscus scaffold was stabilized in the anatomically correct position, 5–0 monofilament nylon sutures were used to repaired the tear sites. The rabbits in the model group were received the same surgical procedure except seeding the meniscus scaffold, while the control rabbits did not receive any intervention. Following surgery, the rabbits were placed to cages and allowed to move freely. Six weeks later, the rabbits were euthanatized via an overdose of sodium pentobarbital (200 mg/kg) and the meniscus tissues were collected. All the experiments of this study were conducted by three experienced technicians who were blinded to experimental design and purpose. Experiments for animals were executed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Ethics Committee of Jinhua Fifth Hospital (approval number: 2021-03).
Histology examination and GAG distribution analysis
Meniscus tissues were fixed in neutral buffered formalin and then embedded in paraffin, followed by cutting into slices (5-μm thickness). The sections were executed for HE staining and toluidine blue staining and observed with the aid of a light microscope from OLYMPUS (Tokyo, Japan).
Immunohistochemistry
Meniscus tissues were fixed, paraffin-embedded, dewaxed, and rehydrated. After antigen retrieval, the samples were stained with primary antibodies collagen type I (1: 1,500) and collagen type II (1: 1,500) at 4°C overnight, followed by incubation with HRP-conjugated secondary antibody (1:3,000) for 1 h at 37°C. After that, each slice was stained with DAB, pictures were taken under a light microscope.
ELISA
According to manufacturer’s protocol, the concentration of ALP in UC-MSCs or meniscus tissues was determined via the commercial Kit.
Total RNA isolation and qRT-PCR
Total RNA from UC-MSCs or meniscus tissues was isolated via a total RNA Extraction Kit, followed by synthesizing cDNA with the aid of First Strand Kit. qRT-PCR analysis was carried out using the QuantiFast SYBR® Green PCR Kit in accordance with the instructions. The gene expression levels were calculated by applying the 2-ΔΔCt approach, and GAPDH was used for normalization. The used primers were listed in Table 1.
Gene |
Sequences |
GAPDH |
Forward |
5,- AAGCCTGCCGGTGACTAAC -3, |
Reverse |
5,- GCATCACCCGGAGGAGAAAT -3, |
Collagen Type I |
Forward |
5,- GGATGAGGAGACTGGCAACC -3, |
(COL1A2) |
Reverse |
5,- TGCCCTCAGCAACAAGTTCA -3, |
Collagen Type II |
Forward |
5,- GCCGTTTCGCTGCGCT -3, |
(COL2A1) |
Reverse |
5,- CCCAGTGTCACAGACACAGAT -3, |
Aggrecan (ACAN) |
Forward |
5,- AAGGGCGAGTGGAATGATGT -3, |
Reverse |
5,- CGTTTGTAGGTGGTGGCTGTG -3, |
Table 1. Real-time PCR Primer synthesis list
Western blotting assay
Total proteins were extracted from UC-MSCs or meniscus tissues using RIPA lysis buffer and then measured the concentration with a BCA Kit. The collected proteins were then separated by SDS polyacrylamide gel electrophoresis and transferred onto a PVDF membrane. The membrane was blocked by nonfat milk (5%), on which primary antibodies (collagen type I, 1:2,000; collagen type II, 1:1,000; aggrecan, 1:1,000; GAPDH, 1:50,000) and the corresponding secondary antibody (1:10,000) were incubated respectively. The signals were identified using an ECL Kit and immunoblots were quantified with Alpha Innotech software (Alpha Innotech, San Leandro, CA, USA). GAPDH was the normalization for proteins.
Statistical analysis
The variations among the data were gauged by applying the one-way ANOVA accordingly. Data analysis was performed in SPSS software v22.0. The data were indicated as the mean ± standard deviation. P < 0.05 was counted as statistically significant.
Results
TGF-β3 treatment induces the chondrogenic differentiation of UC-MSCs
Different concentrations of TGF-β3 were used to induce UC-MSCs to determine chondrogenic differentiation. We found that following treatment of TGF-β3, the mRNA expression of collagen type I, collagen type II and aggrecan was increased in a time-dependent manner (Figure 1A, P < 0.01). Meanwhile, different concentrations of TGF-β3 significantly increased the mRNA expression of collagen type I, collagen type II and aggrecan, especially for 10 ng/mL of TGF-β3 (P < 0.001). Western blotting indicated that the protein levels of collagen type I, collagen type II and aggrecan in UC-MSCs were distinctly increased, as the increased concentration of TGF-β3 (Figure 1B, P < 0.05). Additionally, the concentration of ALP in UC-MSCs following TGF-β3 treatment was also measured. It was shown that ALP concentration was remarkably elevated with an increase in TGF-β3 concentration (Figure 1C, P < 0.05). Therefore, 10 ng/mL of TGF-β3 was selected for succeeding experiments.
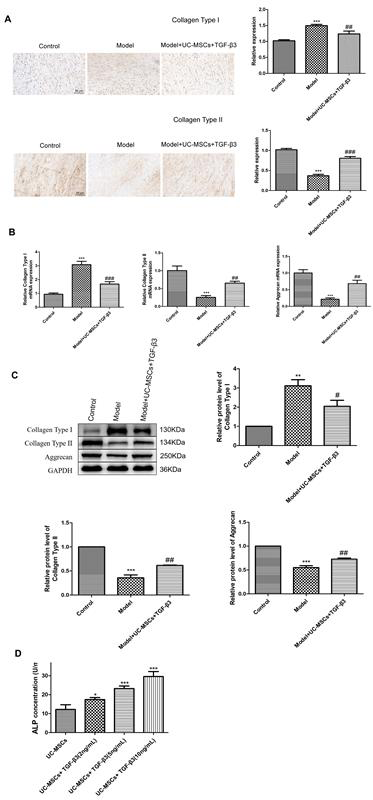
Figure 1. TGF-β3 treatment induces the chondrogenic differentiation of UC-MSCs. (A) The mRNA expression of collagen type I, collagen type II and aggrecan in TGF-β3-treated UC-MSCs was detected via qRT-PCR. (B) The protein levels of collagen type I, collagen type II and aggrecan in TGF-β3-treated UC-MSCs were measured by western blotting. (C) The concentration of ALP in TGF-β3-treated UC-MSCs was determined via ELISA. *P < 0.05, **P < 0.01, ***P < 0.001 vs. UC-MSCs
UC-MSCs combined with chitosan hydrogel loaded with TGF-β3 improves histology and GAG content in meniscus-injured rabbits
HE staining was executed to monitor the influences of UC-MSCs combined with chitosan hydrogel loaded with TGF-β3 on histology of injured meniscus. As shown in Figure 2A, the structure of meniscus tissue in the control group was intact and collagen fibers were arranged neatly. The tissue structure of meniscus in the model group was damaged seriously, with a large number of inflammatory cells infiltrating. In the treated group, the damaged meniscus structure and inflammation infiltrating were improved, to some extent. Toluidine blue staining visualized the spatial distribution of GAG. As illustrated in Figure 2B, the control group showed the uniform spatial distributions of GAG, while meniscus injury resulted in severe loss of staining. In the treated group, staining loss was restored.
UC-MSCs combined with chitosan hydrogel loaded with TGF-β3 promotes meniscus injury healing in rabbits
Immunohistochemical staining was used to calculate the expression of collagen type I and collagen type II in injured meniscus. As illustrated in Figure 3A, the expression of collagen type II was significantly decreased, while collagen type I expression was increased in meniscus-injured rabbits (P < 0.001), which were reversed following treatment of UC-MSCs combined with chitosan hydrogel loaded with TGF-β3 (P < 0.05). The mRNA expression of collagen type I, collagen type II and aggrecan in injured meniscus was also determined. We found that compared to the rabbits in the control group, the mRNA expression of collagen type II and aggrecan was dramatically reduced, but collagen type I mRNA expression was increased in those rabbits of model group (Figure 3B, P < 0.001). Treatment of UC-MSCs combined with chitosan hydrogel loaded with TGF-β3 significantly elevated the mRNA expression of collagen type II and aggrecan, and suppressed collagen type I mRNA expression (P < 0.01). Similar patterns were observed in the protein levels of collagen type I, collagen type II and aggrecan through western blotting (Figure 3C, P < 0.05). Meanwhile, UC-MSCs combined with chitosan hydrogel loaded with TGF-β3 could also restore ALP concentration in meniscus-injured rabbits effectively (Figure 3D, P < 0.05).
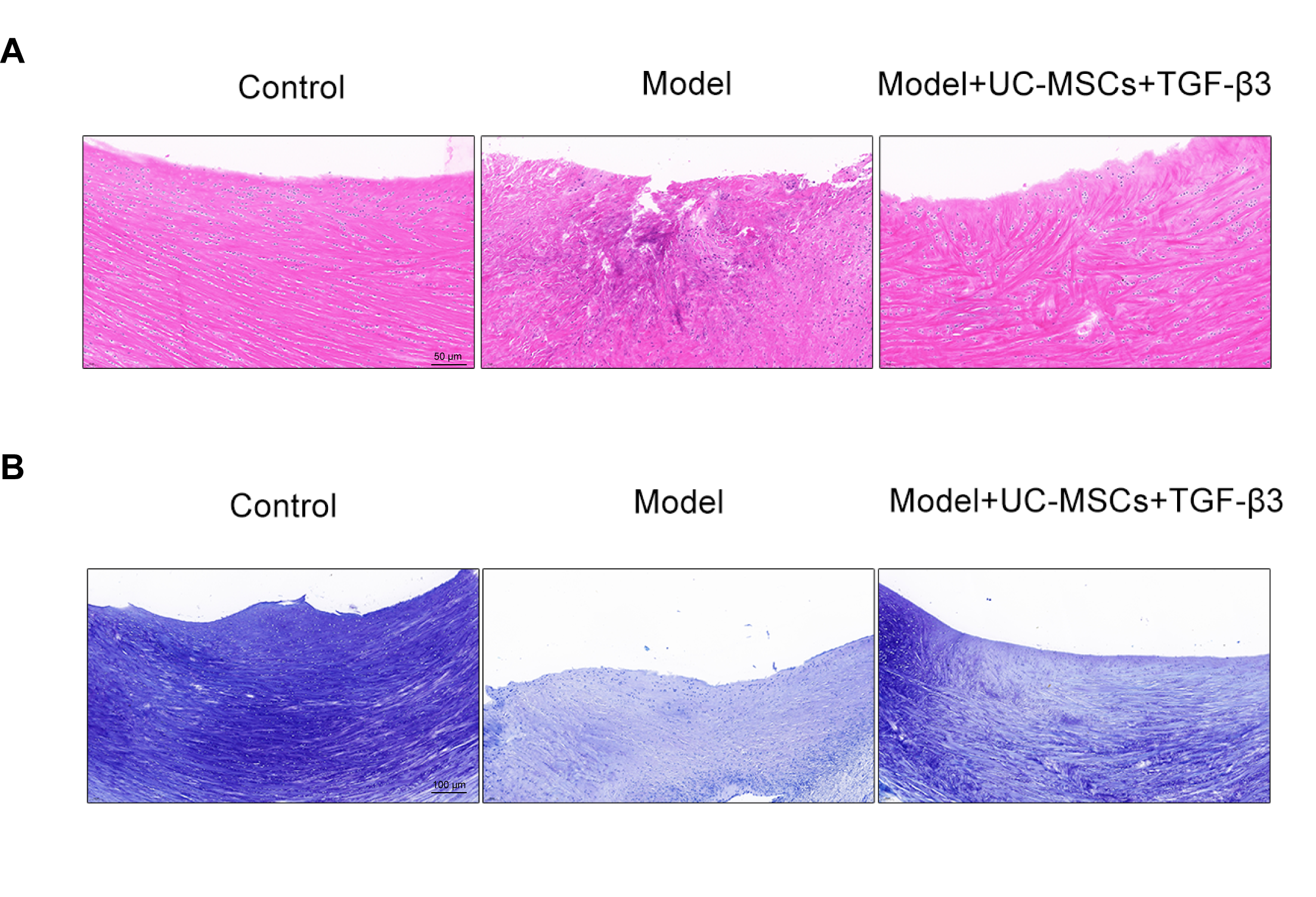
Figure 2. UC-MSCs combined with chitosan hydrogel loaded with TGF-β3 improves histology and GAG content in meniscus-injured rabbits. (A) The histology of the injured meniscus tissues was assessed by HE staining. Scale bar = 50 μm. (B) Toluidine blue staining visualized the spatial distribution of GAG. Scale bar = 100 μm
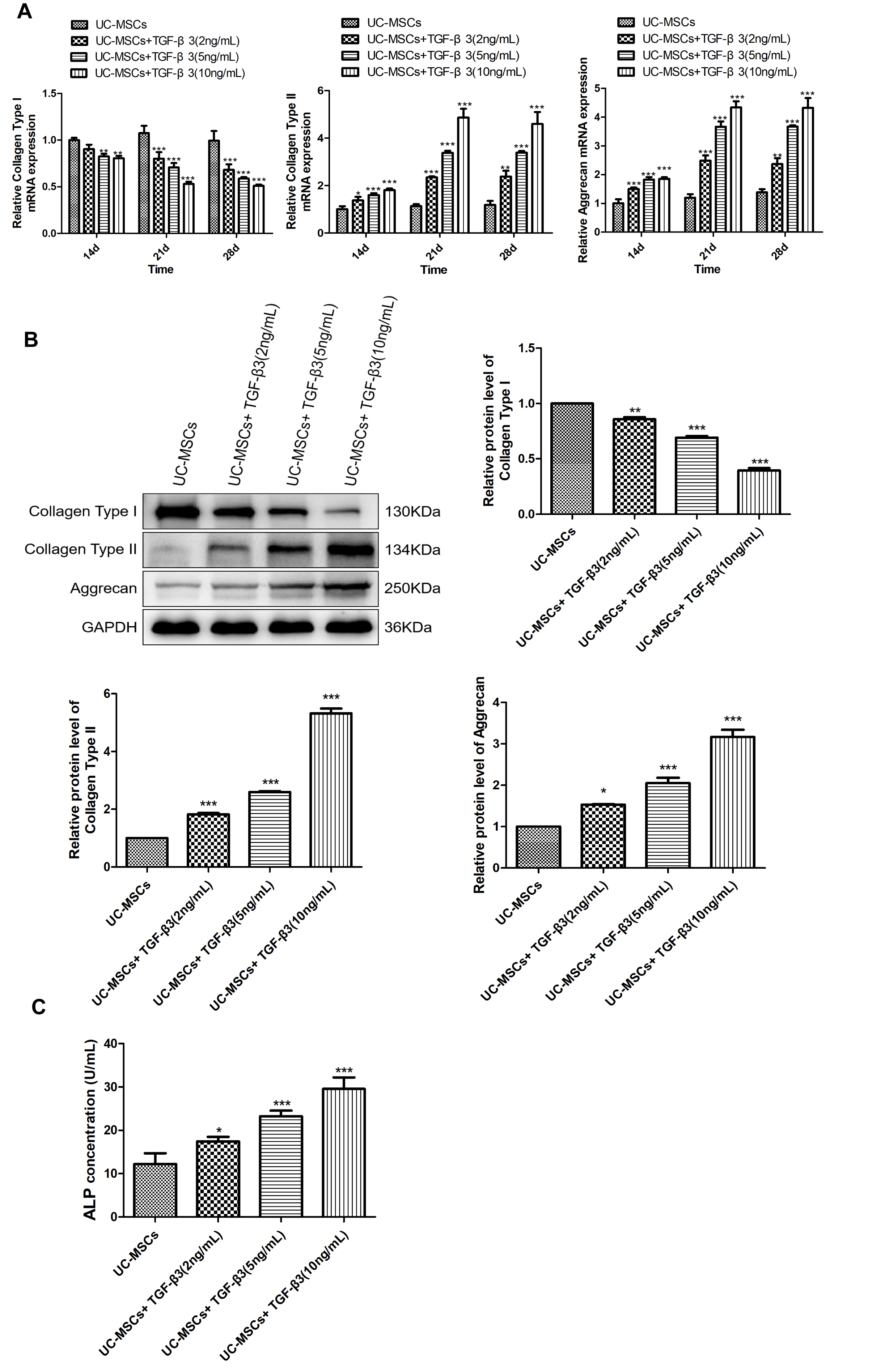
Figure 3. UC-MSCs combined with chitosan hydrogel loaded with TGF-β3 promotes meniscus injury healing in rabbits. (A) Immunohistochemical staining was used to calculate the expression of collagen type I and collagen type II in injured meniscus. Scale bar = 50 μm. (B) The mRNA expression of collagen type I, collagen type II and aggrecan in injured meniscus was detected via qRT-PCR. (C) The protein levels of collagen type I, collagen type II and aggrecan in injured meniscus were measured by western blotting. (D) The concentration of ALP in injured meniscus was determined via ELISA. **P < 0.01, ***P < 0.001 vs. control. #P < 0.05, ##P < 0.01, ###P < 0.001 vs. model
Discussion
Meniscus injury affects populations of all ages. In pediatric patients, the meniscus is generally vascularized, making it more amenable to repair [23]. Meniscus injury mostly occurs in adults engaged in a variety of sports such as football, soccer, basketball, and wrestling, with an incidence of 8.27 per 1000 person [24,25], while the incidence of meniscus injury in older populations is 31%, which are asymptomatic and degenerative tears [26]. At present, the clinical therapies to treat meniscus injury mainly include conservative management, meniscus repair and suture, partial meniscectomy and total meniscectomy. However, meniscectomy may lead to increased stress and pressure on the articular surface and affect the internal balance of the knee, eventually causing multiple clinical symptoms such as articular cartilage degeneration, joint deformity, narrowing of joint cavity and osteoarthritis [27]. To the best of our knowledge, this is the first study to investigate the therapeutic impact of UC-MSCs combined with chitosan hydrogel loaded with TGF-β3 for treatment of meniscus injury in a rabbit model.
Long term research data indicates that the structural and functional repair of the meniscus is a key factor in meniscus treatment. Cartilage tissue engineering technology has been a hot research field in the repair of meniscus injury in recent years. Its purpose is to develop substitutes with structures and physiological functions highly similar to autologous cartilages, thereby effectively alleviating symptoms of affected limbs, maximizing joint mobility and improving treatment effectiveness [18]. It is well-known that the reliable cell source is essential to successful cartilage tissue engineering and previous study has demonstrated that bone marrow-derived mesenchymal stem cells (BM-MSCs) can be cultured in vitro and used in tissue engineering [28]. However, compared to BM-MSCs, UC-MSCs not only possess the advantages of easily harvested via non-invasive procedure, but also have stronger immunosuppressive effects [29,30]. Additionally, some approaches such as adding members of TGF family are used to induce chondrogenic differentiation due to the low incidence of spontaneous chondrogenic differentiation of UC-MSCs [31]. Therefore, in this study, different concentrations of TGF-β3 were used to induce to chondrogenic differentiation of UC-MSCs. Collagen type I is an important marker for chondrocyte dedifferentiation, while increased level of collagen type II means chondrogenic differentiation and the formation mechanically normal cartilage extracellular matrix [32,33]. Meanwhile, aggrecan is considered as a type of cartilage-specific proteoglycan that involved in early chondrogenesis [34]. In the current study, we found that following treatment by TGF-β3, collagen type I level was decreased, whereas the levels of collagen type II and aggrecan were increased. Moreover, 10 ng/mL of TGF-β3 had the most obvious effects. Additionally, ALP is a well-known marker for osteogenic differentiation and increased ALP levels can validate the osteogenic phenotype of UC-MSCs [35]. Herein, we further demonstrated that ALP concentration was also elevated with the increased concentrations of TGF-β3. These results suggested that 10 ng/mL of TGF-β3 could induce the chondrogenic differentiation of UC-MSCs, to the maximum.
Studies have shown that chitosan hydrogel scaffolds possess superior biomimetic performance, which can provide a highly simulated normal physiological microenvironment for cell proliferation and tissue regeneration [21,36]. Therefore, a chitosan hydrogel scaffold of UC-MSCs recombination with TGF-β3 was engrafted in meniscus-injured New Zealand White rabbits. In this work, UC-MSCs combined with chitosan hydrogel loaded with TGF-β3 could improve the histology of injured meniscus effectively, indicating that this scaffold attenuated meniscus injury to some extent. Meanwhile, we found that the content of GAG, a major component of extracellular matrices of cartilage [37], was restored following treatment by chitosan hydrogel scaffold. Of course, UC-MSCs combined with chitosan hydrogel loaded with TGF-β3 also increased the levels of collagen type II, aggrecan and ALP, but decreased collagen type I level in meniscus-injured rats. These results implied that UC-MSCs combined with chitosan hydrogel loaded with TGF-β3 could promote the regeneration and repair of meniscus.
Conclusion
In a word, the current study uncovers the effects of UC-MSCs combined with chitosan hydrogel loaded with TGF-β3 on meniscus injury, indicating that this hydrogel scaffold can promote chondrogenic differentiation and thus be helpful for the regeneration and repair of meniscus. This may contribute information and insights on possible clinical therapeutic therapies for meniscus injury.
List of abbreviations
UC-MSCs: Umbilical Cord-Derived Mesenchymal Stem Cells; BM-MSCs: Bone Marrow-Derived Mesenchymal Stem Cells; TGF: Transforming Growth Factor; GAG: Glycosaminoglycan; FBS: Fetal Bovine Serum; ALP: Alkaline Phosphatase; HE: Hematoxylin and Eosin
Authors’ contributions
Conceptualization, Mingxing Ding; Methodology, Junhong Lin, Song Qiao, Zhongliang Zhang, Jianguang Wang, Chaofan Zhu and Jinyi Wu; Software, Junhong Lin, Song Qiao, Zhongliang Zhang, Jianguang Wang, Chaofan Zhu and Jinyi Wu; Validation, Mingxing Ding; Formal Analysis, Junhong Lin, Song Qiao, Zhongliang Zhang, Jianguang Wang, Chaofan Zhu and Jinyi Wu; Investigation, Junhong Lin; Resources, Mingxing Ding and Junhong Lin; Data Curation, Mingxing Ding and Junhong Lin; Writing-Original Draft Preparation, Junhong Lin; Writing-Review and Editing, all authors.; Visualization, Junhong Lin, Song Qiao, Zhongliang Zhang, Jianguang Wang, Chaofan Zhu and Jinyi Wu; Supervision, Mingxing Ding and Junhong Lin; Project Administration, Mingxing Ding and Junhong Lin.
Conflicts of interest
The authors declare no conflicts of interest.
Ethics approval and consent to participate
Experiments for animals were executed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Ethics Committee of Jinhua Fifth Hospital (approval number: 2021-03).
Consent for publication
Not available.
Funding
This work was supported by The Science and Technology Project of Jinhua City in China (Project no: 2021-3-145).
Acknowledgements
Not available.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
- Ashraf S, Wibberley H, Mapp PI, Hill R, Wilson D, et al. (2011) Increased vascular penetration and nerve growth in the meniscus: A potential source of pain in osteoarthritis. Ann Rheum Dis 70: 523-529. [Crossref]
- Fox AJ, Bedi A, Rodeo SA (2012) The basic science of human knee menisci: Structure, composition, and function. Sports Health 4: 340-351. [Crossref]
- Makris EA, Hadidi P, Athanasiou KA (2011) The knee meniscus: Structure–function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 32: 7411-7431. [Crossref]
- Osawa AK, D HARNER CH, Gharaibeh B, Matsumoto T, Mifune Y, et al. (2013) The use of blood vessel–derived stem cells for meniscal regeneration and repair. Med Sci Sports Exerc 45: 813. [Crossref]
- Travascio F, Jackson AR (2017) The nutrition of the human meniscus: A computational analysis investigating the effect of vascular recession on tissue homeostasis. J Biomech 61: 151-159. [Crossref]
- Vaquero J, Forriol F (2016) Meniscus tear surgery and meniscus replacement. Muscles Ligaments Tendons J 6: 71-89. [Crossref]
- Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, et al. (2017) Concise review: Multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med 6: 2173-2185. [Crossref]
- Amable PR, Teixeira MV, Carias RB, Granjeiro JM, Borojevic R (2014) Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res Ther 5: 53. [Crossref]
- Caplan AI, Correa D (2011) The MSC: An injury drugstore. Cell Stem Cell 9: 11-15. [Crossref]
- Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, et al. (2014) MSC-derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 28: 970-973. [Crossref]
- Liu Y (2017) Host immune and mesenchymal stem cells-based bone regeneration. Zhonghua kou Qiang yi xue za zhi 52: 620-624. [Crossref]
- Murphy MB, Moncivais K, Caplan AI (2013) Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 45: e54. [Crossref]
- Zhang S, Chuah SJ, Lai RC, Hui JH, Lim SK, et al. (2018) MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 156: 16-27. [Crossref]
- Neves SC, Teixeira LS, Moroni L, Reis RL, Van Blitterswijk CA, et al. (2011) Chitosan/Poly (ɛ-caprolactone) blend scaffolds for cartilage repair. Biomaterials 32: 1068-1079. [Crossref]
- Moutos FT, Freed LE, Guilak F (2007) A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat Mater 6: 162-167. [Crossref]
- Moradi L, Vasei M, Dehghan MM, Majidi M, Mohajeri SF, et al. (2017) Regeneration of meniscus tissue using adipose mesenchymal stem cells-chondrocytes co-culture on a hybrid scaffold: In vivo study. Biomaterials 126: 18-30. [Crossref]
- Asen AK, Goebel L, Rey‐Rico A, Sohier J, Zurakowski D, et al. (2018) Sustained spatiotemporal release of TGF‐β1 confers enhanced very early chondrogenic differentiation during osteochondral repair in specific topographic patterns. FASEB J 32: 5298-5311. [Crossref]
- Kwon H, Brown WE, Lee CA, Wang D, Paschos N, et al. (2019) Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat Rev Rheumatol 15: 550-570. [Crossref]
- Mendes LF, Katagiri H, Tam WL, Chai YC, Geris L, et al. (2018) Advancing osteochondral tissue engineering: Bone morphogenetic protein, transforming growth factor, and fibroblast growth factor signaling drive ordered differentiation of periosteal cells resulting in stable cartilage and bone formation in vivo. Stem Cell Res Ther 9:1-3. [Crossref]
- Yamasaki S, Mera H, Itokazu M, Hashimoto Y, Wakitani S (2014) Cartilage repair with autologous bone marrow mesenchymal stem cell transplantation: Review of preclinical and clinical studies. Cartilage 5: 196-202. [Crossref]
- Chen X, Cao X, Jiang H, Che X, Xu X, et al. (2018) SIKVAV-modified chitosan hydrogel as a skin substitutes for wound closure in mice. Molecules 23: 2611. [Crossref]
- Furuike T, Komoto D, Hashimoto H, Tamura H (2017) Preparation of chitosan hydrogel and its solubility in organic acids. Int J Biol Macromol 104: 1620-1625. [Crossref]
- Clark CR, Ogden JA (1983) Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury. J Bone Joint Surg Am 65: 538-547. [Crossref]
- Jones JC, Burks R, Owens BD, Sturdivant RX, Svoboda SJ, et al. (2012) Incidence and risk factors associated with meniscal injuries among active-duty US military service members. J Athl Train 47: 67-73. [Crossref]
- Mitchell J, Graham W, Best TM, Collins C, Currie DW, et al. (2016) Epidemiology of meniscal injuries in US high school athletes between 2007 and 2013. Knee Surg Sports Traumatol Arthrosc 24: 715-722. [Crossref]
- Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P, et al. (2008) Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med 359: 1108-1115. [Crossref]
- Bilgen B, Jayasuriya CT, Owens BD (2018) Current concepts in meniscus tissue engineering and repair. Adv Healthc Mater 7: 1701407. [Crossref]
- Gao J, Dennis JE, Solchaga LA, Awadallah AS, Goldberg VM, et al. (2001) Tissue-engineered fabrication of an osteochondral composite graft using rat bone marrow-derived mesenchymal stem cells. Tissue Eng 7: 363-371. [Crossref]
- Chan CK, Wu KH, Lee YS, Hwang SM, Lee MS, et al. (2012) The comparison of interleukin 6–associated immunosuppressive effects of human ESCs, fetal-Type MSCs, and adult-type MSCs. Transplantation 94: 132-138. [Crossref]
- Fan CG, Zhang QJ, Zhou JR (2011) Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev Rep 7: 195-207. [Crossref]
- Ju Y, Yi L, Li C, Wang T, Zhang W, et al. (2022) Comparison of biological characteristics of human adipose-and umbilical cord-derived mesenchymal stem cells and their effects on delaying the progression of osteoarthritis in a rat model. Acta Histochem 124: 151911. [Crossref]
- Marlovits S, Hombauer M, Truppe M, Vecsei V, Schlegel W (2004) Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J Bone Joint Surg Br 86: 286-295. [Crossref]
- Wang ZH, Li XL, He XJ, Wu BJ, Xu M, et al. (2014) Delivery of the Sox9 gene promotes chondrogenic differentiation of human umbilical cord blood-derived mesenchymal stem cells in an in vitro model. Braz J Med Biol Res 47: 279-286. [Crossref]
- Yao X, Huang H, Li Z, Liu X, Fan W, et al. (2017) Taurine promotes the cartilaginous differentiation of human umbilical cord-derived mesenchymal stem cells in vitro. Neurochem Res 42: 2344-2353. [Crossref]
- Chen X, Zhang F, He X, Xu Y, Yang Z, et al. (2013) Chondrogenic differentiation of umbilical cord-derived mesenchymal stem cells in type I collagen-hydrogel for cartilage engineering. Injury 44: 540-549. [Crossref]
- Gao J, Liu R, Wu J, Liu Z, Li J, et al. (2012) The use of chitosan based hydrogel for enhancing the therapeutic benefits of adipose-derived MSCs for acute kidney injury. Biomaterials 33: 3673-3681. [Crossref]
- Buschmann MD, Grodzinsky AJ (1995) A molecular model of proteoglycan-associated electrostatic forces in cartilage mechanics. J Biomech Eng 117: 179-192. [Crossref]



