Background: Injectable contraceptives are critical to contraceptive security in South Africa. The contract for supply of oral and injectable contraceptives to public sector facilities in South Africa ended in September 2017. As of that time no new contracts had been signed with manufacturers of injectable contraceptives. Stock outs of 2- and 3-monthly injectables began to emerge in early 2018 and continued through to at least June 2019. Stock outs of oral contraceptives were also reported for a short period at the end of 2017. Stock outs put women at risk of unwanted pregnancy.
Objectives: To determine the extent of reported shortages of injectable and oral contraceptives following the end of the public sector tender, and to explore how South Africa might further enhance contraceptive security.
Methods: Analysis of routine data collected from public sector depots and private sector wholesalers supplying public and private sectors. Statistical analysis carried out using the “R” package for Change-Point Analysis.
Results: Significant declines in public sector supply of the 2-monthly injectable followed the end of the public sector tender (p<0.05). Re-supply of the 2-monthly injectable into the public sector is associated with significant decline of supplies into the private sector (p<0.05). 3-monthly injectable supplies increased significantly (p<0.05) in the public and private sectors following the end of the public sector tender. No shortage of oral contraceptives was detected in the public sector despite media reports, although this may be due to gaps in the data used. In the public and private sectors oral contraceptives and emergency contraceptives show significant, if small, increases in volume.
Conclusion: The end of the public sector tender for injectable and combined oral contraceptives in September 2017 was associated with considerable disruption to supplies, impacting not only the public sector, but also the private sector. Contraceptive security can be enhanced by a broad method mix, a deliberate attempt to attract a range of suppliers of similar, if not identical, products, and ongoing support to women and the healthcare workforce regarding the availability and advantages of different methods.
family planning; South Africa; contraceptive security
In South Africa, public sector supply of contraceptive services and commodities is free or provided at a reduced cost relative to the private sector [1]. Private sector supply is, on the other hand, largely covered by insurance, the remainder being funded by out-of-pocket expenditure. Injectable contraceptives (2- or 3-monthly) are the most popular form of modern contraceptive. 47% of women that use a contraceptive use an injectable contraceptive, with condoms, oral contraceptives (OCs), sterilisation and implants being used by 25%, 10%, 9% and 7% of users respectively [1].
In September 2017 the contract for the supply of oral and injectable contraceptives to public sector facilities in South Africa ended [2]. As of that time no new contracts had been signed with manufacturers of injectable contraceptives. Neither of the two existing suppliers of the 3-monthly injectable bid for the public sector contract [3]. One, Fresenius Kabi, agreed to manufacture the contraceptive until April 2018, when it withdrew from the market [3]. Ad hoc arrangements were then put in place for provincial depots to order direct from the one other manufacturer of the 3-monthly contraceptive registered and selling product in the country. Media reports indicate that supplies of the 2-monthly injectable were not resumed until May 2018 [3], the delay being attributed to prolonged pricing negotiations [4]. Stock outs of OCs were reported immediately following the end of the tender, these coinciding with reports of short-term issues with supply from the main supplier [4]. Stock outs of both 2- and 3-monthly injectables began to emerge in early 2018 [5,6], and continued through to at least June 2019 [3,7], although reports of shortage are much higher for the 2-monthly injectable than the 3-monthly.
Stockouts put women at risk of unwanted pregnancy, already estimated in 2016 to be 20% of all births in South Africa [1] and as high as 71% in HIV-infected parturient women [8]. Stock outs are also thought to increase the likelihood of contraceptive discontinuation [9].
To determine the extent of the reported shortages of injectable and oral contraceptives following the end of the public sector contract for oral and injectable contraceptives in September 2017, and to explore how South Africa might enhance contraceptive security in the future.
Study design and data source
Retrospective information relating to the volumes of oral and injectable contraceptives sold by private sector wholesalers supplying the public and private sectors across all provinces in South Africa were provided by IQVIA, a leading global provider of advanced analytics, technology solutions and contract research services to the life sciences industry. IQVIA also provided information relating to volumes issued by provincial depots serving public sector facilities in 4 provinces and direct deliveries to public sector facilities from some, but not all, manufacturers.
An analysis of the accuracy of IQVIA’s estimates of private sector volumes is carried out each year [10]. During the validation process manufacturers are requested to provide their estimates of volumes at pack level, and these estimates are then compared to those made by IQVIA. In 2018, 30 manufacturers participated in the comparison, providing information on 1062 different packs, these constituting approximately 24% of the market by value. The results of the comparison suggest that in 2018, IQVIA’s published figures under-estimated the private sector market, on average, by -5.9%, with 85.8% of comparisons lying between -4.6% and -7.3% of manufacturer estimates.
No information is made public by the Department of Health on public sector consumption. As such there is no information that can be used to assess the validity of IQVIA’s public sector data in the 4 provinces from which it collects depot data. However, IQVIA’s panel of depots, wholesalers and manufacturers remained constant over the study period. As such it was felt appropriate to use these data to analyse trends in public sector supply, noting the absolute numbers may not be representative.
Data used ran from the first quarter of 2014 to the last quarter of 2019. Public sector data are available on a quarterly basis. Private sector data are available for each month. As noted above, the public sector tender for oral and injectable contraceptives lapsed in September 2017.
Contraceptive volumes were converted to Couple Years of Protection (CYP), as per the CYP conversion factors described by the United States Agency for International Development (USAID) [11].
Statistical analysis
Statistical analysis was carried out using the “R” package for Change-Point Analysis. Change-Point has proven to be a useful tool to identify trends and change in time series datasets. It has been widely used in medical and public health analyses as well as in analyses of climate and financial data [12]. Change-Point Analysis detects shifts over time and can be used on data that are not normally distributed. Change-Point Analysis produces a confidence level for every change in the mean detected. Confidence levels are calculated using cumulative sum and bootstrapping. First the cumulative sum of the differences between individual data values and the mean is calculated. Bootstrapping then generates many random iterations of the data set. For each randomized data set, the corresponding cumulative sums are determined, along with the ranges for the sums. The percent of times that the cumulative sum range for the original data exceeds the cumulative sum range for the randomized bootstrap data is the confidence level [13]. Changes with a confidence level beyond 95% were considered significant.
We present two sets of results relating to trends in the supply of oral and injectable contraceptives at and around the time that the public sector contract for these commodities ended. The first describes trends in public sector supply, the second trends in private sector supplies over the same period.
Public sector supply
Significant changes in the supply of injectables and oral contraceptives contraception were seen at or around the end of the public sector contract for oral and injectable contraceptives. These results are depicted graphically in Figure 1.
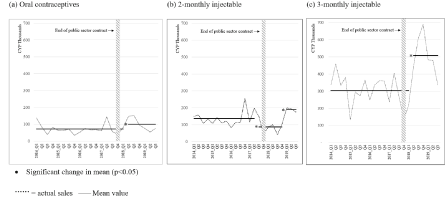
Figure 1. Public sector supplies of oral contraceptives, 2-monthly and 3-monthly injectables.
The end of the contract sees a significant decline (-36%, p<0.05) in the supply of the 2-monthly injectable. This is followed by a sharp recovery some months after the manufacturer had committed to resupply (February 2018 [4]). At or around the same period (Quarter 2, 2018), supplies of the 3-monthly injectable saw a significant increase (+67%, p<0.05), this despite the withdrawal from the market of the current supplier. The increase in supply of the 3-monthly injectable is surprising given the ongoing reports of stockouts of the 3-monthly injectable, although it is clear that reports of stock outs of the 3-monthly injectable are less frequent than those seen for the 2-monthly injectable (19 reports versus 93 reports over the study period [14]).
The end of the public sector contract also coincided with a significant increase in the supply of oral contraceptives. This increase in oral contraceptive supply from the first quarter of 2018 contrasts with the media reports of oral contraceptive stock outs. Further investigation reveals 78% of reported stockouts between July 2017 and December 2019 came from provinces from which IQVIA does not collect public sector data [14]. In other words, the data from the 4 provinces where IQVIA collects more complete data from the public sector may not be typical of all provinces. Media reports also suggest that the shortages of oral contraceptives were relatively short-term which may also mean that such shortages are not easily detected in IQVIA’s quarterly data supply.
Increases were also seen in the supply of emergency contraceptives to the public sector over the same period (data not shown), but this increase came after a series of significant increases seen in prior years. As such the increase in emergency contraception seen around the end of the public sector contract may not be related to the end of the contract.
Private sector supply
The private sector also saw a significant decline in supply of the 2-monthly injectable, but this decline was associated with the date of resupply to the public sector (-60%, p<0.05) (Figure 2 (a)). Significant declines in 2-monthly injectable supply into the private sector were noted across all but one of the provinces at approximately the same time (data not shown). The only province not to see a significant decline was North West Province. This is almost certainly because usage of injectables in this province is already relatively very low.
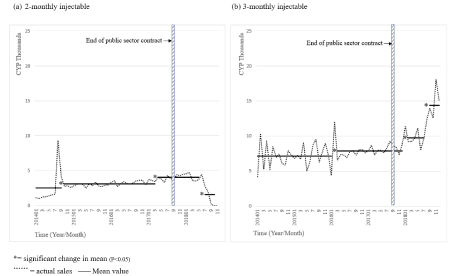
Figure 2. Private sector supply, 2-monthly and 3-monthly injectables.
Sales of the 3-monthly injectable into the private sector saw significant increases in the period following contract end (+24%, p<0.05) (Figure 2 (b)) and took a further dramatic turn upwards towards the end of 2018, significantly increasing by 48% (p<0.05). The unexpectedly high growth in the 3-monthly injectable in the private sector suggests challenging supply conditions for the 2-monthly injectable had positive effects on the use of the 3-monthly injectable.
Significant, if smaller, increases were also seen in oral contraceptive and emergency contraceptive supplies (+8% and +17%, p<0.05) (Figure 3 (a) and Figure 3 (b)).
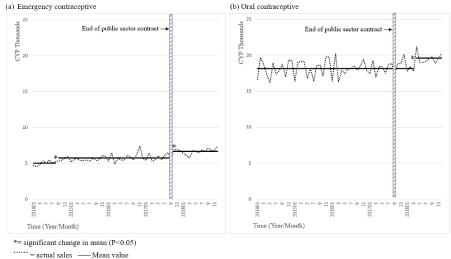
Figure 3. Private sector supplies of emergency contraceptives and oral contraceptives.
The impact of the end of the public sector tender in September 2017 is clear. A decline in the supply of the 2-monthly injectable in the public sector was mirrored by increases in the supply of 3-monthly injectables and oral contraceptives. Re-supply of the 2-monthly injectable into the public sector is associated with significant declines in supplies into the private sector. These changes indicate that some women were willing to follow the Department of Health’s advice and turn to other methods and/or use the private sector to source additional supplies of modern contraceptives.
Regional differences in media reports of public sector contraceptive stock outs point to the apparent complexity of securing supplies of reproductive health commodities in South Africa. It is nevertheless notable that supplies of the 3-monthly injectable increased over the study period, despite the failure of the tender and the eventual withdrawal of one of the two manufacturers from the market. This suggests a willingness of manufacturers and health officials to work together to overcome what could have been an even more challenging situation.
The challenges faced by South Africa are common to many countries in sub-Saharan Africa as they wrestle with the relatively small number of manufacturers willing to bid on tenders, or even register product for sale in the country. The South African Department of Health generally attempts to mitigate supply risks by awarding contracts to several suppliers. In South Africa, for example, two suppliers of the monophasic and triphasic OCs were selected, one being awarded 90% of volume, the other 10%. However, in the case of the 2-monthly and 3-monthly injectable contraceptives, the Department of Health was only able to attract one supplier of each. This is despite there being at least two United States Food and Drug Administration (US FDA) approved 3-monthly injectable suppliers globally at this time, another that achieved World Health Organisation (WHO) prequalification in 2018, and a further three known regional suppliers.
These facts suggest the challenge for public sector procurement agencies in countries like South Africa is not a limited number of qualified suppliers of contraceptives but rather that qualified suppliers are unwilling to bid for public sector contracts and/or register products in such countries. As might be expected, the attractiveness of public sector contracts appears to depend more on price than on volume. For example, an analysis of the tenders awarded for drugs used in the treatment of tuberculosis between 2013 and 2019 [15] indicates that nine different packs were to be supplied by more than one manufacturer. Of these nine, the minimum quantity to be supplied was just over 130,000 packs, a quantity that is dwarfed by the quantity of 2- and 3-monthly injectables awarded in the 2015-17 tender (17 million).
South Africa’s contraceptive supply is largely dependent upon the injectable contraceptive. We estimate, for example, that injectables constituted 70% of the CYP delivered to public sector facilities in 2018 in those provinces where IQVIA collects data from the public sector.
The dominance of injectables may be attributed to the relatively low acceptance of the contraceptive implant in South Africa following on from the 2014 publication of the Department of Health’s circular, suggesting that the effectiveness of the implant may reduce when taken with certain enzyme inducing drugs [16].
To reinforce supply security of generics in situations where supply is concentrated to just one or two manufacturers, we suggest that countries could investigate whether it is possible to (i) encourage manufacturers to register more than one factory as the source of supply so as to insure against disruptions to a single source of supply; and (ii) find ways to incentivise other manufacturers of reproductive health commodities to export into sub-Saharan Africa. In the case of injectables in South Africa, this might, for example, include those suppliers of combination injectable contraceptives that are included on the WHO Model Essential Medicines List [17], but which are not yet sold in South Africa. Regulatory and/or marketing support could also be considered under certain circumstances, as was provided to SAI Pharmaceuticals for their range of oral contraceptives in Kenya [18].
It is recognised of course that it is not a simple task for countries to expand the contraceptive method mix or the range of commodities offered. Expansion involves changes to the logistics management system, management of differences in storage conditions or shelf-life, and of course, training of health care staff. Guidance and support are needed for health facilities and women in order to ensure both are familiar with the full range of contraceptive method options, and that health facility staff are able to advise, and where appropriate administer, the full range of options. Such support needs to be planned and sustained over time.
The key weakness of this study is the absence of comprehensive public sector data. The South African Department of Health publishes the results of its tenders which give an indication of total volumes over the tender period, but no public information is available on actual volumes supplied to public health facilities. IQVIA’s public sector data is also incomplete. As noted above, as IQVIA’s sample remained constant, the analysis focused on changes in trend rather than absolute numbers.
The end of the public sector contract for injectable and combined oral contraceptives in September 2017 was associated with considerable disruption to supplies, impacting public and private sectors. Contraceptive security can be enhanced by a broad contraceptive method mix, a deliberate attempt to attract a range of suppliers of similar, if not identical, products, and ongoing support to women and the healthcare workforce regarding the availability and advantages of different methods.
The authors would like to thank Denise Harrison and Jennifer Mason of the Office of Population and Reproductive Health at USAID, and Morgan Simon and Priyanka Mysore of the USAID Global Health Supply Chain-Procurement and Supply Management (GHSC-PSM) Project, for their comments on the manuscript.
This study was funded through the USAID Global Health Supply Chain – Procurement and Supply Management Program (GHSC-PSM) project. GHSC-PSM had no involvement in the design of the study, collection and analysis of the data, or in the drafting of the manuscript.
IQVIA is a leading global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry. It receives funding from biotech, medical device and pharmaceutical companies, medical researchers, government agencies, payers and other healthcare stakeholders.
- National Department of Health, Statistics South Africa (Stats SA), South African Medical Research Council (SAMRC), and ICF. 2019. South Africa Demographic and Health Survey 2016.
- Department of Health, Republic of South Africa. HP03=2015CHM. Supply and delivery of contraceptive and hormone modulating agents to the Department of Health for the period 01 October 2015 to 30 September 2017.
- van Dyk J (2019) This popular birth control shot is out of stock for the second year running Available from: https://bhekisisa.org/features/2019-03-01-00-birth-control-injection-south-africa-unplanned-pregnancy-tender-delay-bayer/
- Pilane P (2018) Are strong-armed tactics by Big Pharma behind the country’s birth control shortage. Available from: https://bhekisisa.org/article/2018-07-20-00-bitter-pill-inside-sas-national-birth-control-shortage/
- The Stop Stockouts Project. Reproductive health rights of women in South Africa under threat, as supply shortages leave health facilities across the country without supplies of contraceptives. Available from: https://stockouts.org/Content/Files/FINAL%20SSP%20Press%20statement%20contraceptives.pdf
- The Daily News (2018) Unavailable contraceptive injection causing stress for women. Available from: https://www.iol.co.za/dailynews/unavailable-contraceptive-injection-causing-stress-for-women-14350621
- The Stop Stockouts Project (2020) SSP alarmed at second line ARV and contraceptive shortages across South Africa. Available from: https://stockouts.org/Content/Files/Stockouts%20of%20Dumiva%20and%20contraceptive%20(1).pdf
- Adeniyi OV, Ajay A, Moyaki MG, Ter Goon D, Avramovic G et al. (2018) High rate of unplanned pregnancy in the context of integrated family planning and HIV care services in South Africa. BMC Health Serv Res 18: 140. [Crossref]
- Douglas-Durham E, Blanchard K and Higgins S (2015) Contraceptive stockouts: A Review of the Published and Grey Literature. Available from: https://noemptyshelves.org/wp-content/uploads/2015/09/Contraceptive_Stockouts_Lit_review.pdf
- IQVIA Quality Assurance program. Available from: https://www.iqvia.com/library/publications/acts-2019-33rd-edition-quality-assurance-report-of-iqvia
- USAID (2021) Couple Years of Protection. Available from: https://www.usaid.gov/global-health/health-areas/family-planning/couple-years-protection-cyp
- Arif S, Mohamad Mohsin, MF, Abu Bakar A, Hamdan A, Syed Abdullah S (2017) Change point analysis: A statistical approach to detect potential abrupt change. Jurnal Teknologi 79: 147-159.
- Gavit P, Baddour Y, and Tholmer R (2009) Use of Change-Point Analysis for Process Monitoring and Control. BioPharm Int 22: 40-45.
- The Stop Stockouts Project. Available from: https://stockouts.org/Survey/SurveysAndResearch#
- Author’s (PS) analysis of the following Department of Health Circulars (i) HP01-2013TB Supply and delivery of anti-tuberculosis medicines to the Department of Health for the period 1 August 2013-31 July 2015; (ii) HP01-2013TB/1: Supply and delivery of anti-tuberculosis medicines to the Department of Health for the period up to 31 July 2015; (iii) HP01-2013TB/02: Supply and delivery of anti-tuberculosis agents to the Department of Health for the period up to 31 July 2015; (iv) HP01-2015TB: The supply and delivery of anti-tuberculosis medicines to the Department of Health for the period up to 01 October 2015-30 September 2017; (v) HP01-2019TB: Supply and delivery of anti-tuberculosis agents to the Department of Health for the period up to 01 October 2017-30 September 2021.
- Department of Health. Circular: Changes in the prescription of progestin subdermal implants (Implanon) in women who are taking enzyme-inducing drugs such as efavirenz for HIV, rifampicin for TB and certain drugs used for epilepsy (carbamazepine, phenytoin, and phenobarbital). 13th October 2014.
- World Health Organisation Model List of Essential Medicines List, 21st List, 2019. Available from: https://apps.who.int/iris/bitstream/handle/10665/325771/WHO-MVP-EMP-IAU-2019.06-eng.pdf?ua=1
- Klein K, Wood R, Cisek C and Koseki S. From Policy to Practice. Defining Health Market Interventions within a Total Market Approach. HP+ Policy Brief June 2019. Available from: http://www.healthpolicyplus.com/ns/pubs/11329-11601_MarketInterventions.pdf



