Background: The aim of this study was to show whether endoscopic biopsy specimens are adequate quality to enable reliable classification of gastric cancer, about differentiation, the presence of signet ring cells, or histologic type, and a histological difference with Dysplasia.
Method: In the present study, 154 patients with gastric carcinoma, with biopsy and surgical specimens were analyzed; and compared with the biopsy for suspected gastric carcinoma. In the gastric carcinoma biopsy specimens, (154 cases) the sensitivity and specificity were 86% and 82% respectively for intestinal type, and 87% and 90% respectively for the diffuse gastric carcinoma. The results of the pathology between endoscopic biopsy and surgical specimen analysis were 70%.
Conclusion: In gastric carcinoma, the degree of misclassification is largely independent of whether the source of the material is a biopsy or surgical specimens, and this is important in the preoperative planning of the surgical strategy.
Endoscopic biopsy, gastric cancer, Lauren classification.
In clinical practice, the Lauren classification seems to be of significance as a prognostic indicator and as a basis for decision with regard to surgical strategy [1,2]. The advent of a flexible endoscope and its worldwide use in gastroenterology practice has made use in gastroenterology practice has made a major impact in the management of gastric cancer. Whereas the intestinal type of gastric cancer spreads only a few millimeters into grossly normal stomach wall, tumor cells can be found several centimeters beyond the macroscopically visible margins of the diffuse type of tumor, recasting wider surgical margins in the later type [2]. Signet ring cell cancer corresponds largely to the diffuse type of gastric cancer, having similar prognosis [3,4]. Because the tissue available for histology and classification is less in biopsy specimens than in surgical specimens, we evaluate the importance of the histology based on biopsy by the Lauren method; and the prevalence of signet ring cells carcinoma.
An experienced pathologist classified and reviewed 154 biopsy specimens and surgical specimens from tumors in accordance with Lauren types. In all cases were assessed the grade of differentiation and the presence of signet ring cells carcinoma.
The biopsy specimens were stained using the hematoxylin-eosin procedure. The standard for classification the results after histology were on the basis of surgical specimens. For all endoscopic biopsy materials, the histopathology estimated the type after Lauren classification, the presence of signet ring cells, and the tumor grade of differentiation. In Lauren P definition [5] the intestinal type is characterized by large cells with a definitive glandular pattern, and the “diffuse” type has poorly differentiated cells and rarely forms glandular structures. The rapport from histology shown that in biopsy specimens the image of histology was uniform, and in surgical specimens, the heterogeneity made classification difficult, but not in all cases. For signet ring cells carcinoma, we used the description from Herman K [6], characterized by a large volume of intra cytoplasmic mucin sufficient to compress the nucleus against the periphery of the cell. In the histopathology we introduced the Japanese classification of gastric cancer after publication from M. Rugge et al. [7] (Table 1).
Table 1. Japanese classification of gastric cancer [7]
Category |
Definition |
Histologic description
|
Group I |
Normal mucosa, with no atypia |
Normal and benign lesion with no atypia; including intestinalized epithelium and hiperplastic epithelia |
Group II |
Lesion showing atypia but diagnosed as benign |
- Native or intestinalized epithelium with atypia, frequently associated with inflamation
- Hyperplastic polyps showing atypia caused by erosion
|
Group III |
Borderline lesion between benign and malign |
It aplies frequently to adenomatous lessions and rarey to lesions difficult to diagnosed as either benign or malignout. |
Group IV |
Lession strangely suspected for carcinoma |
Lession strangely suspected for carcinoma |
Group V |
Carcinoma |
Adenocarcinoma |
In the present analysis, a table of 154 histologically confirmed gastric adenocarcinoma was the study base. In all cases were verified by both gastric biopsy and resection specimens, and those are the form of present analysis. The histopathology in the biopsy and surgical specimens are given in Table 2. The result of the present study shown that there were no major differences between the histological types between the biopsy and surgical specimens. The concordance between diagnosis based on biopsy and surgical specimens in gastric carcinoma with Lauren classification shown 78% agreement (Table 3).
Table 2. The histologic classification in 154 adenocarcinoma of the stomac lauren classification, signet ring carcinoma in biopsy and surgical specimens
Classification |
|
Biopsy specimen % |
Surgical specimen % |
|
Lauren classification |
Intestinal |
|
61,0 |
59,1 |
Diffuse |
|
31,6 |
40,2 |
Mixed |
|
3,2 |
8,0 |
Unclassified |
|
4,2 |
2,7 |
Total |
|
100,0 |
100,0 |
|
Prevalence of signet ring carcinoma |
0% |
|
59,1 |
54,9 |
1-24% |
|
18,9 |
22,0 |
25-49% |
|
11,0 |
13,1 |
50-74% |
|
8,4 |
5,9 |
75-100% |
|
3,0 |
2,2 |
Unclassified |
|
3,6 |
2,0 |
Total |
|
100,0 |
100,0 |
|
Differentiation |
High |
|
7,0 |
10,0 |
Medium |
|
30,9 |
28,9 |
Low |
|
5,0 |
57,6 |
Undifferentiated |
|
51,9 |
1,0 |
Unclassifiable |
|
3,4 |
2,9 |
Total |
|
100,0 |
100,0 |
Table 3. Concordance between biopsy and surgical specimens in gastric carcinoma regard to Lauren classification
Biopsy and surgical specimens |
|
Intestinal |
Diffuse |
Sensitivity |
85% |
87% |
Specificity |
81,1% |
91% |
False positive |
13% |
21% |
False negative |
15% |
12,9% |
The concordance between diagnosed based on biopsy and surgical specimens regarding histological differentiation of gastric carcinoma is shown in Table 4. In the suspicions for invasive gastric carcinoma in some specimens, clearly neoplastic epithelium is present, but invasion cannot be clearly identified (Figure 1).
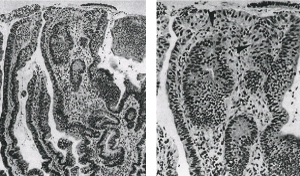
Figure 1a.A) atypical epithelium, B) The presence of positive invasion of the lamina propria and the definition “suspicion for invasion” is adequate in such situation.
Table 4. Differentiation concordance in gastric carcinoma between biopsy and surgical specimens
Biopsy and surgical specimens with concordance differentiation |
|
Moderate |
Poor |
Undifferentiated |
Sensitivity |
45% |
63% |
82% |
Specificity |
95% |
87% |
77% |
False positive |
53% |
35% |
17% |
False negative |
55% |
37% |
19% |
Despite almost universal trends of declining mortality rates, gastric carcinoma remains a major health problem. Surgery is recognized as the only treatment offering cure of the disease, but in most cases, it does not accomplish the task, as reflected by the fact that 5-year survival rates in most countries remain under 20% [8-10].
The advent of the flexible endoscope and its worldwide use in clinical practice has made a major impact in the management of gastric cancer. This impact has been most prominent in Japan, were close to 50% of gastric cancer are diagnosed in their early stage, limited to the mucosal and submucosal layers [7,11,12]. In the present study in 42% of the tumors, the biopsy specimens were the only ones available of the incident cases. In this study, the sensitivity of biopsy specimens was 85% in intestinal and diffuse Lauren type of gastric carcinoma. In previous studies, the authors have found 72-75% agreement between biopsy and surgical specimens with regard to the histological classification of Lauren [1,13-15].
An Italian study found a sensitivity of 85% and 64% and a specificity of 77% and 91% for intestinal and diffuse type carcinoma. The Lauren classification may also be of clinical importance there are reports of better survival among patients with the intestinal type compared with the diffuse type of gastric cancer [1,16-21]. For the surgery preoperatively, it is difficult to discern the border between tumor-infiltrated and normal gastric wall macroscopically. Because it is very difficult to discern the real invasion of the tumor cells, intramurally, in the resected specimens for the diffuse type of gastric carcinoma, the cancer infiltration of the resected margins in higher frequency [22-25].
These observations justify the importance of preoperative information about the Lauren type, as adequate resection margins are required for the intestinal or diffuse carcinoma [26,27]. For Hornig et al (28), in the diffuse type, a proximal resection margin of 10 cm in situ is described, whereas 4 cm in situ is considered enough in the intestinal tumors [28-30].
In the present study, signet ring cells were identified in 42% of all resected specimens, lower than the 60% found in a small American Study [31]. A recent, essentially population-based American Study found signet ring cell cancer in 8% [16]. Histopathologic subtyping of biopsy specimens obtained at gastroscopy is possible with acceptable reliability with regard to the Lauren classification. This observation is of importance in preoperative planning of surgical strategy. The morphology of gastric carcinoma shows a substantial variation of histopathological differentiation. Among the various classification systems that were developed in the past, the most widely used are those proposed by Lauren, and the World Health Organization (WHO).
The extent of luminal resection depends on tumor size, location, depth of invasion, and histological type as reflected by Lauren classification. If a carcinoma biopsy is falsely interpreted as being the intestinal type, the extent of resection would be too small, in the reciprocal case with an inadequate diagnosis of a diffuse type cancer) perhaps too extensive. On the other hand, if an intestinal type carcinoma diagnosed in the biopsy shows a diffuse growth pattern in the resected specimens, a subtotal gastrectomy, and a reduced safety margin would embrace the risk of a local recurrence, with the higher risk of metastases.
Therefore, the reliability of a preoperative Lauren classification of biopsied gastric cancer is a relevant problem in surgical oncology. Jonasson reported a disagreement between histological diagnosis based on preoperative biopsies and resection in 65 of 382 patients (17%). Davesarr reported an overall histological diagnostic disagreement between the pre and postoperative classification in 28%, and Amarosi in 23%. For Jonasson, the agreement for diffuse carcinomas in biopsies was only in 75% [32].
The diagnosis of a diffuse-type carcinoma is a reliable result of the histopathological evaluation of biopsy specimens, whereas the diagnosis of an intestinal type has to be judged critically. Especially if only a small number of biopsies could be investigated, biopsies should be performed.
- Davessar K, Pezzulo JC, Kessimian N, Hale JH, Jauregui HO (1990) Gastric adenocarcinoma: prognostic significance of several pathologic parameters and histologic classification. Hum Patol 21: 325-332.
- Nakajima T (2002) Gastric cancer treatment guidelines in Japan. Gastric cancer 5: 1-5.
- Baba M, Hokita S, Natsugoe S (2000) Paraaortic lymphadenectomy in patients with advanced gastric carcinoma of the upper third of the stomach. Hepatogastroenterology 47: 586-589.
- Kunisaki C, Shimada H, Takahashi M, Nomura M, Matsuda G, et al. (2003) Implication of extended lymph node dissection stratified for advanced gastric cancer. Anticancer Res 23: 4181-4186. [Crossref]
- Udagawa H, Ueno M, Shinohara H, Haruta S, Kaida S, et al. (2012) The importance of grouping of lymph node stations and rationale of three-field lymphoadenectomy for thoracic esophageal cancer. J Surg Oncol 106: 742-747. [Crossref]
- Darling G (2009) The role of lymphadenectomy in esophageal cancer. J Surg Oncol 99: 189-193. [Crossref]
- Saito H, Osaki T, Murakami D, Sakamoto T, Kanaji S, et al. (2006) Macroscopic tumor size as a simple prognostic indicator in patients with gastric cancer. Am J Surg 192: 296-300. [Crossref]
- Shao LF, Gao ZG, Yang NP, Wei GQ, Wang YD, et al. (1989) Results of surgical treatment in 6,123 cases of carcinoma of the esophagus and gastric cardia. J Surg Oncol 42: 170-174. [Crossref]
- Yoshikawa T, Sasao M, Sano T (2006) Stage migration caused bu D2 dissection with paraaortic lumphadenectomy for gastric cancer form the result of a prospected randomized controlled trial. Br J Surg 93: 1526-1529.
- Han S, Sakinci U, Dural K (2005) Left thoracophrenotomy and cervical approach in the surgery of distal third oesophageal and cardia tumours. ANZ J Surg 75: 1045-1048. [Crossref]
- Han S, Sakinci U, Dural K (2005) Left thoracophrenotomy and cervical approach in the surgery of distal third oesophageal and cardia tumours. ANZ J Surg 75: 1045-1048. [Crossref]
- Japanese Research Society for Gastric Cancer (1995) Japanese Clasificatio of Gastric Carcinoma, Ist English ed. Tokyo: Kanehara&co, Ltd.
- Pisani P, Parkin DM, Munoz N, Ferlay J (1997) Cancer and infection: estimates of the attributable fraction in 1990. Cancer epidemiol Biomarkers prev 6: 387-400.
- Palli D, Bianchi S, Cipriani F, Duca P, Amorosi A, et al. (1991) Reproducibility of histologic classification of gastric cancer. Br J Cancer 63: 765-768. [Crossref]
- Ishwaran H, Blackstone EH, Apperson Hansen C, Rice TW (2009) A novel approach to cancer staging: application to oesophageal cancer. Biostatistics 10: 603-620.
- Komatsu S, Ichikawa D, Kurioka H, Kan K, Shioaki Y, et al. (2005) Prognostic and clinical evaluation of patients with T2 gastric cancer. Hepatogastroenterology 52: 965-968. [Crossref]
- Wanebo HJ, Kennedy BJ, Chmiel J, Steele G Jr, Winchester D, et al. (1993) Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg 218: 583-592. [Crossref]
- Scott A, Hundahl SA, Phillips JL, Menck HR (2000) The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the "different disease" hypothesis. Cancer 88: 921-932. [Crossref]
- Piso P, Werner U, Lang H, Mirena P, Klempnauer J (2000) Proximal versus distal gastric carcinoma--what are the differences? Ann Surg Oncol 7: 520-525.
- Fleming ID, Cooper JS, Henson DE, Hutter RVP, Kennedy BJ, et al. (1997) American Joint Committee on Cancer staging manual. 5th edition. Philadelphia: Lippincott-Racen.
- Roder JD, Böttcher K, Busch R, Wittekind C, Hermanek P, et al. (1998) Classification of regional lymph node metastasis from gastric carcinoma. German Gastric Cancer Study Group. Cancer 82: 621-631. [Crossref]
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, et al. (2010) Oesophagus and Oesophago-Gastric junction. AJCC Cancer Staging Manual. 7th edn New York, NY:Springer 9: 103-111.
- Haugstvedt TK, Viste A, Eide GE, Maartmann-Moe H, Myking A, et al. (1992) Is Lauren's histopathological classification of importance in patients with stomach cancer? A national experience. Norwegian Stomach Cancer Trial. Eur J Surg Oncol 18: 124-130. [Crossref]
- Japanese Society for Endoscopic Surgery (2008) The 9th nationwide survey of endoscopic surgery in Japan. J Jpn Soc Endosc Surg 13: 499-611.
- Pugliese R, Maggioni D, Sansonna F, Scandroglio I, Ferrari GC, et al. (2007) Total and subtotal laparoscopic gastrectomy for adenocarcinoma. Surg Endosc 21: 21-27.
- Kawamura H, Okada K, Isizu H, Masuko H, Yamagami H, et al. (2008) Laparoscopic gastrectomy for early gastric cancer targeting as a less invasive procedure. Surg Endosc 22: 81-85. [Crossref]
- Huscher CG, Mingoli A, Sgarzini G, Brachini G, Binda B, et al. (2007) Totally laparoscopic total and subtotal gastrectomy with extended lymph node dissection for early and advanced gastric cancer: early and long-term results of a 100-patient series. Am J Surg 194: 839-844. [Crossref]
- Topal B, Leys E, Ectors N, Aerts R, Penninckx F (2008) Determinants of complications and adequacy of surgical resection in laparoscopic versus open total gastrectomy for adenocarcinoma. Surg Endosc 22: 980-984. [Crossref]
- Mochiki E, Toyomasu Y, Ogata K, Andoh H, Ohno T, et al. (2008) Laparoscopically assisted total gastrectomy with lymph node dissection for upper and middle gastric cancer. Surg Endosc 22: 1997-2002. [Crossref]
- Shiraishi N, Yasuda K, Kitano S (2006) Laparoscopic gastrectomy with lymph node dissection for gastric cancer. Gastric cancer 9: 167-176.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69-90. [Crossref]
- Antonioli DA, Goldman H (1982) Changes in the location and type of gastric adenocarcinoma. Cancer 50: 775-81.

